Efficient Collection of Skin Biopsies Using the Tissue Sampling Unit® for Subsequent Cryopreservation and Culture of Fibroblasts
Abstract
1. Introduction
2. Experimental Design
2.1. Materials: Reagents and Reagents Set Up
| M199 (high glucose, no L-glutamine, ThermoFisher #11960-044) | 40.695 mL |
| 15% FBS (Fetal Bovine Serum, ThermoFisher #10437-028) | 7.5 mL |
| Collagenase (10,000 units) | 80 µL |
| DNAase I (1250 Kunitz) | 0.625 mL |
| Antibiotics (1 μg/mL gentamicin, 10mg/mL stock, Sigma G1272) | 100 µL |
| Antibiotic-Antimycotic (100×, ThermoFisher #15240062) | 1 mL |
| M199 | 33 mL |
| 15% FBS | 15 mL |
| 1% (1X) GlutaMAX (ThermoFisher #35050-061) | 1 mL |
| Antibiotic-Antimycotic | 1 mL |
2.2. Equipment
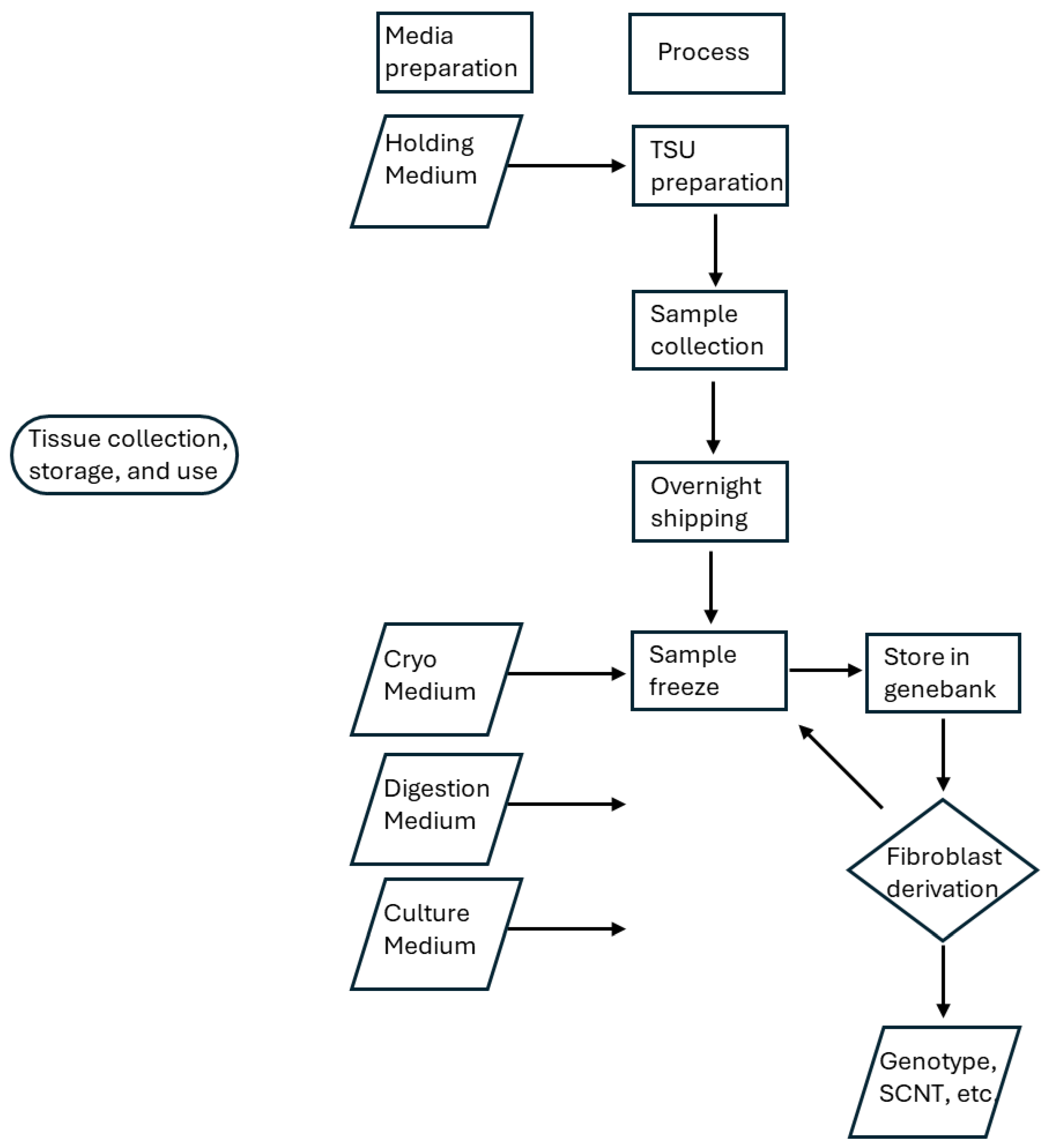
3. Procedure—Collection, Shipping, Cryopreservation, Thawing, Digestion, Culture, Counting
3.1. Tissue Sampling Unit (TSU) Preparation
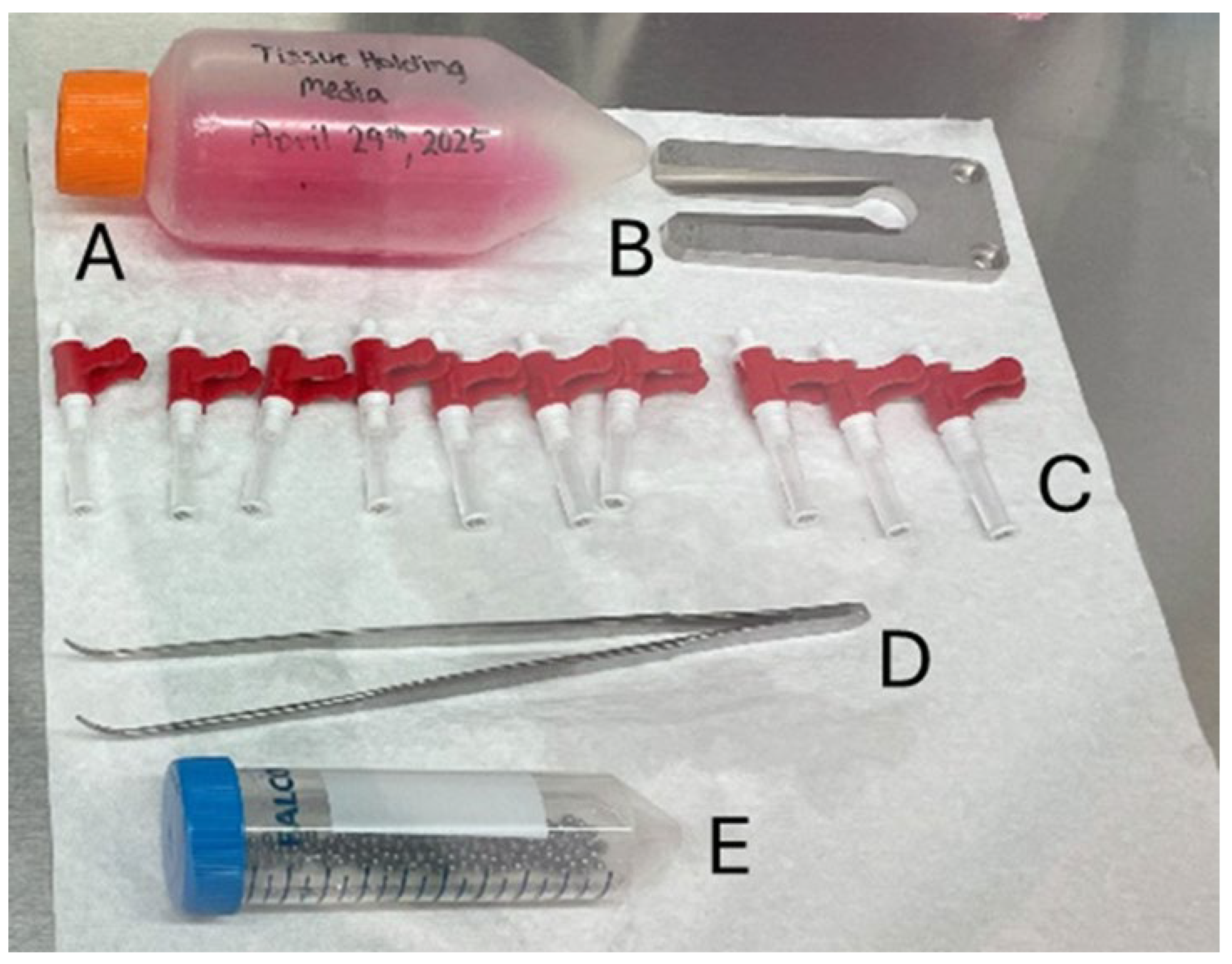
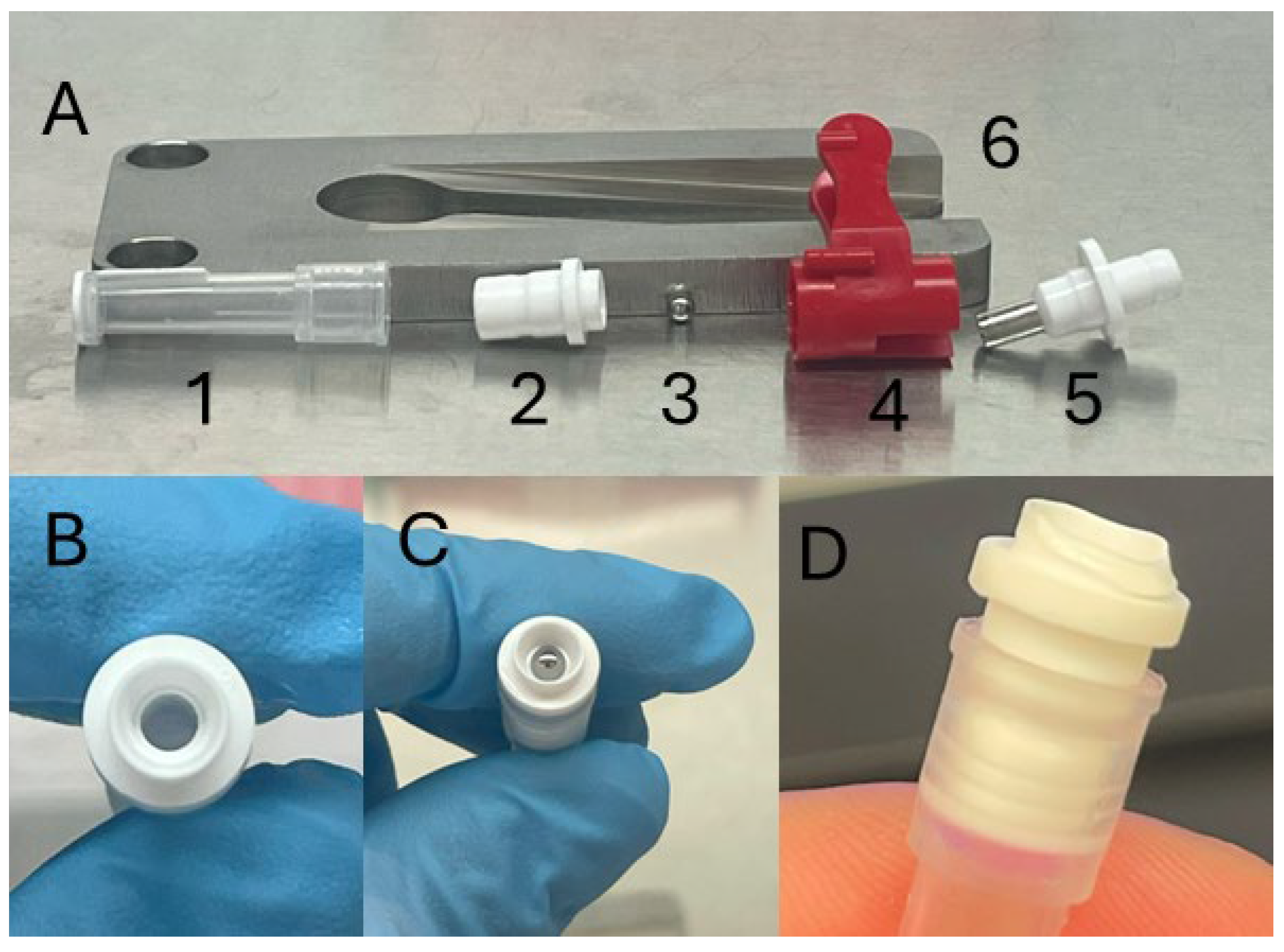
- CRITICAL STEP: All steps within this area of preparation should be conducted using sterile technique in a biosafety cabinet. Refer to Figure 1 for examples of the parts.
- Place 3 mm ball bearings in 70% ethanol.
- CRITICAL STEP: Ball bearings are not needed if the standard type TSUs are used that were originally intended for genotyping and contained the proprietary medium from Allflex are used. In this instance the sealing ball is included with the TSU, and it is critical to empty the proprietary medium, wash and rinse the TSU with distilled and deionized water, and finally sanitize the TSU components with 70% ethanol.
- Remove TSU blade (Figure 2A(4 and 5)) from TSU and place in 70% ethanol.
- After 10 min, remove all parts from ethanol and allow them to completely dry.
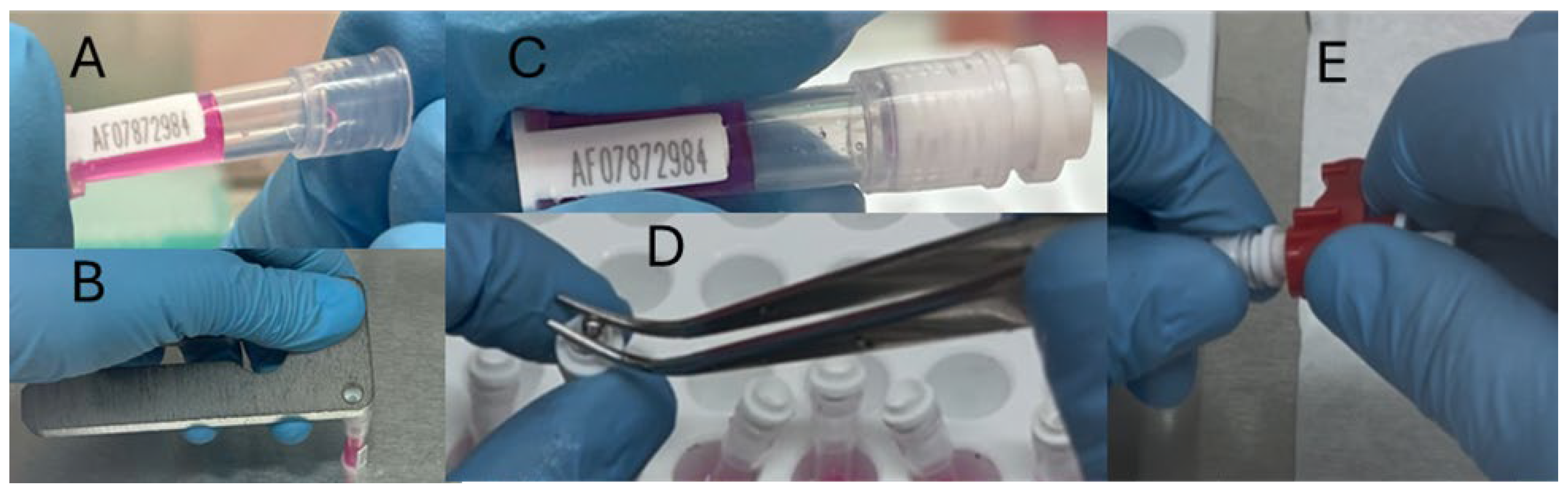
- Add 200 µL of Holding Medium to each of the tissue collectors (Figure 3A).
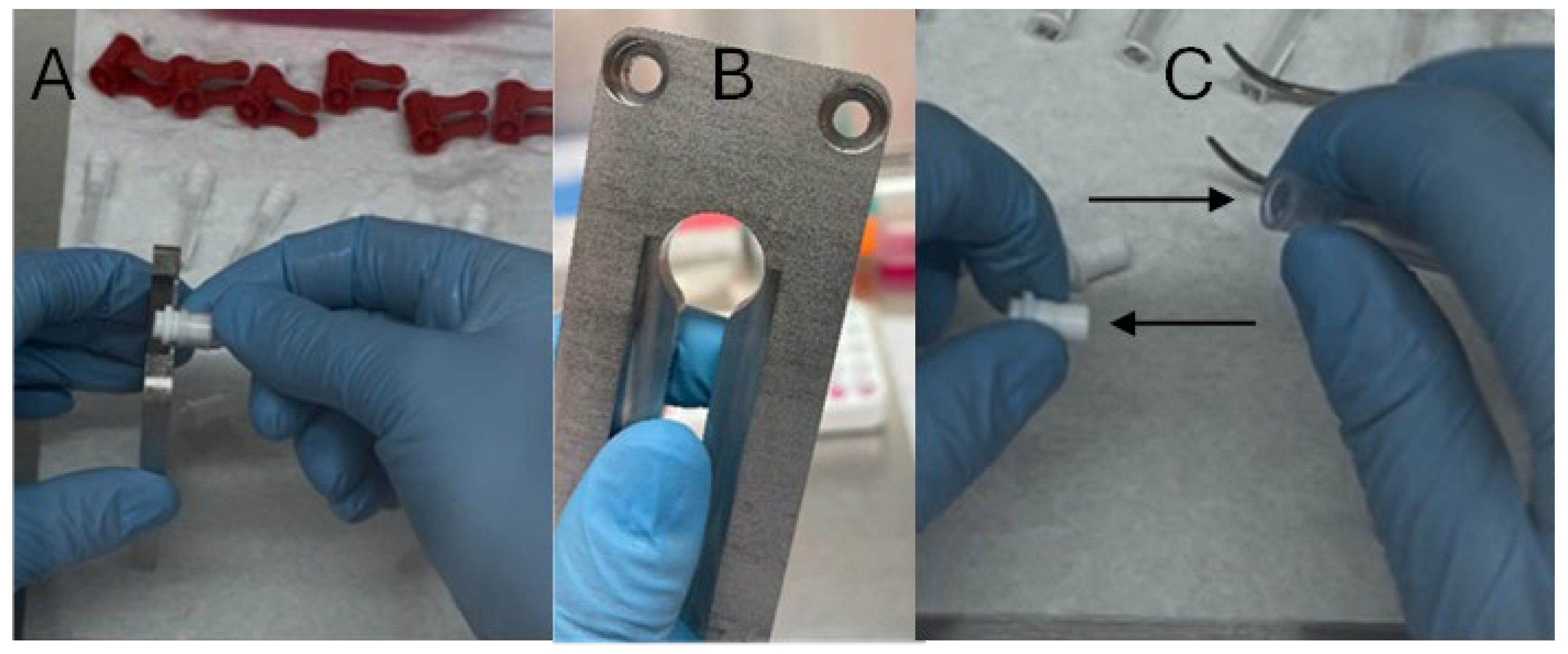
- Seal the tissue collector with the connector (Figure 4B–D).
- Insert a single ball bearing into the opening of the TSU connector and ensure it is firmly secured using the blade holder (Figure 2A(4)). Repeat for all connectors prior to subsequent steps.
3.2. Animal Preparation and Collection
- Trim the hair or wool with fine electric clippers and ideally shave the location for the biopsy from both sides of the ear where the sample will be collected.
- Wash both sides of the ear with 10% betadine and then 70% ethanol. Allow the ear to dry.
- Collect the samples per the TSU instructions (Tissue Sampling Unit—Allflex).
- Store the samples at 5 °C.
3.3. Overnight Transportation
- Expedited, overnight shipping from the collection location to the processing laboratory is ideal. However, samples can be held for up to 3 d at 5 °C following collection with the TSU method.
- ○
- CRITICAL STEP: Samples have been held for longer than 3 d, but the quality declines rapidly the longer the samples are stored prior to cryopreservation or tissue culture.
- For transportation, place the samples in a shipping cooler with multiple reusable ice packs ensuring the internal temperature will remain at 5 °C during transportation.
- ○
- CRITICAL STEP: Pack the shipping cooler so that the samples do not come in direct contact with the reusable ice packs. Samples should be separated using multiple layers of insulated material or bubble wrap.
3.4. Cryopreservation
- Place cryovials labeled with animal identification numbers into a rack in a Styrofoam box containing liquid nitrogen.
- ○
- CRITICAL STEP: Each tube must contain at least 1.5 mL of liquid nitrogen.
- Samples diluted in Holding Medium and cooled should be warmed to room temperature (22 to 23 °C).
- Transfer the individual tissue samples to 0.5 mL Cryopreservation Medium at 22 °C and ensure the tissue is fully submerged.
- ○
- CRITICAL STEP: To increase workflow and productivity, Cryopreservation Medium can be placed in the wells of a 48-well plate. Each well can then be used for a single sample.
- ○
- CRITICAL STEP: Only transfer the tissue and not the Holding Medium.
- Incubate the samples for 5 min at 22 °C.
- Remove the tissue from the Cryopreservation Medium and blot dry.
- Drop the samples into the liquid nitrogen in the respective, labeled cryovials.
- ○
- CRITICAL STEP: Only 1 sample is vitrified in a cryovial to avoid samples sticking together during the process. However, multiple samples can be stored in the same cryovial after vitrification, if desired.
- Liquid nitrogen should be poured off from the sample and the tube capped to avoid liquid nitrogen expansion and maintain sample integrity.
- ○
- CRITICAL STEP: Samples should not be out of liquid nitrogen vapor for longer than 6 s.
- Store samples in liquid nitrogen or in a liquid nitrogen vapor tank.
3.5. Thawing
- Remove the cryovials containing the samples from liquid nitrogen storage and place the cryovial in a 22 °C water bath for 10 min.
- ○
- CRITICAL STEP: ensure only the tube, and not the actual samples contact water.
- ○
- CRITICAL STEP: agitate samples in water bath to create a uniform thaw.
- ○
- CRITICAL STEP: sanitize the tube with 70% ethanol before placing it in the biosafety cabinet.
- Remove the sample from the cryovial and incubate the tissue in 5 mL of M199 for 5 min at 22 °C.
3.6. Tissue Culture
- The tissue culture protocol was adapted from Progeria Research Foundation https://www.progeriaresearch.org/fibroblast-cell-culture-protocols/, accessed on 10 September 2025)
- Tissue culture must be performed in a biosafety cabinet using sterile technique.
- Place the sample in a disposable Petri dish and mince the tissue with a scalpel until the tissue resembles a pulp.
- Dilute sample with 5 mL of Digestion Medium and transfer to a 15 mL tube.
- Rinse the Petri dish with 5 mL of fresh Digestion Medium and transfer to the 15 mL tube containing the sample.
- Incubate at 37 °C for 4–6 h.
- Agitate the sample often using a vortex mixer at room temperature and return to the incubator as quickly as possible.
- Centrifuge the samples at 1000× g.
- Remove the supernatant.
- Wash the sample 3× with 3 mL of Culture Medium for each wash.
- Remove the final supernatant.
- Dilute the final pellet with Culture Medium.
- ○
- CRITICAL STEP: the final pellet can be diluted with either 0.7 or 5 mL of Culture Medium. The smaller volume can be cultured in a 24-well plate whereas the larger volume can be cultured in a 5 mL vented flask.
- Culture the samples at 37 °C in 5% CO2 in humidified air for 10 d or until the fibroblasts reach 80% confluence.
- Culture Medium (one-third of the total volume of the well or flask) should be refreshed every 24–48 h.
- At 80% confluence, remove the Culture Medium and wash the fibroblasts with HBSS in the well/flask.
- Add Trypsin EDTA to the well/flask (0.3 mL/1 mL) and incubate (37 °C in 5% CO2) for 3–5 min.
- Tilt the plate/flask to loosen the fibroblasts. Gentle pipetting is also acceptable.
- Transfer the sample to a centrifuge tube (2 mL or 10 mL depending on culture volume) and dilute with Culture Medium.
- Rinse the plate/flask with additional Culture Medium and combine with the sample so that the final volume is 1.8 or 10 mL (plate or flask).
- Wash the samples 3 X at 1000× g using fresh Culture Medium each time.
- Suspend the pellet in a small volume of Culture Medium.
- Count the fibroblasts using a hemocytometer or flow cytometer to determine the final quantity.
- Successful derivations can now be used for SCNT or frozen for later use.
4. Expected Results
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groeneveld, E.; Tinh, N.H.; Kues, W.; Vien, N.T. A protocol for the cryoconservation of breeds by low-cost emergency cell banks—A pilot study. Animal 2008, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zieger, M.A.; Tredget, E.E.; Sykes, B.D.; McGann, L.E. Injury and protection in split-thickness skin after very rapid cooling and warming. Cryobiology 1997, 35, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Prather, R.S. A method for producing cloned pigs by using somatic cells as donors. In Methods in Molecular Biolology; Humana Press: Totowa, NJ, USA, 2004; Volume 254, pp. 149–164. [Google Scholar]
- Jansen van Vuuren, A.; Bolcaen, J.; Engelbrecht, M.; Burger, W.; De Kock, M.; Durante, M.; Fisher, R.; Martínez-López, W.; Miles, X.; Rahiman, F.; et al. Establishment of Primary Adult Skin Fibroblast Cell Lines from African Savanna Elephants (Loxodonta africana). Animals 2023, 13, 2353. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.A.; Lira, G.P.D.O.; Nascimento, L.E.; Santos, M.V.D.O.; Oliveira, M.F.D.; Silva, A.R.; Pereira, A.F. Isolation, characterization, and cryopreservation of collared peccary skin-derived fibroblast cell lines. PeerJ 2020, 8, e9136. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. User’s Guide: Statistics, Version 5 Edition; SAS Institute: Cary, NC, USA, 1985. [Google Scholar]
- Khan, M.; Gasser, S. Generating primary fibroblast cultures from mouse ear and tail tissues. J. Vis. Exp. 2016, 10, 53565. [Google Scholar]

| Animal | Days in Holding | Days in Culture | Passages | Cells (×106) |
|---|---|---|---|---|
| Adult ram 1 | 1 | 14 | 2 | 1.05 |
| Adult ram 2 | 1 | 14 | 3 | 2.82 |
| Adult ram 2 | 2 | 15 | 0 | 0 |
| Adult ram 3 | 1 | 14 | 2 | 1.31 |
| Adult ram 3 | 1 | 15 | 0 | 0 |
| Adult ram 4 | 1 | 14 | 3 | 2.64 |
| Adult ram 4 | 1 | 14 | 0 | 0 |
| Adult ram 5 | 1 | 17 | 2 | 1.04 |
| Adult ram 6 | 3 | 17 | 1 | 0.93 |
| Adult ram 7 | 2 | 17 | 2 | 1.18 |
| Adult ram 8 | 3 | 14 | 0 | 0 |
| Adult ram 9 | 2 | 15 | 0 | 0 |
| Ram lamb 1 | 1 | 14 | 0 | 0 |
| Ram lamb 1 | 3 | 15 | 0 | 0 |
| Ram lamb 2 | 3 | 15 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purdy, P.H.; Redel, B.; Chen, P.; Rahe, A.J.; Jivan, A.; Spiller, S.F. Efficient Collection of Skin Biopsies Using the Tissue Sampling Unit® for Subsequent Cryopreservation and Culture of Fibroblasts. Methods Protoc. 2025, 8, 114. https://doi.org/10.3390/mps8050114
Purdy PH, Redel B, Chen P, Rahe AJ, Jivan A, Spiller SF. Efficient Collection of Skin Biopsies Using the Tissue Sampling Unit® for Subsequent Cryopreservation and Culture of Fibroblasts. Methods and Protocols. 2025; 8(5):114. https://doi.org/10.3390/mps8050114
Chicago/Turabian StylePurdy, Phillip H., Bethany Redel, Paula Chen, Ashley J. Rahe, Aashi Jivan, and Scott F. Spiller. 2025. "Efficient Collection of Skin Biopsies Using the Tissue Sampling Unit® for Subsequent Cryopreservation and Culture of Fibroblasts" Methods and Protocols 8, no. 5: 114. https://doi.org/10.3390/mps8050114
APA StylePurdy, P. H., Redel, B., Chen, P., Rahe, A. J., Jivan, A., & Spiller, S. F. (2025). Efficient Collection of Skin Biopsies Using the Tissue Sampling Unit® for Subsequent Cryopreservation and Culture of Fibroblasts. Methods and Protocols, 8(5), 114. https://doi.org/10.3390/mps8050114






