A Simple Method for Imaging and Quantifying Respiratory Cilia Motility in Mouse Models
Abstract
1. Introduction
2. Experimental Design
Materials
3. Procedure
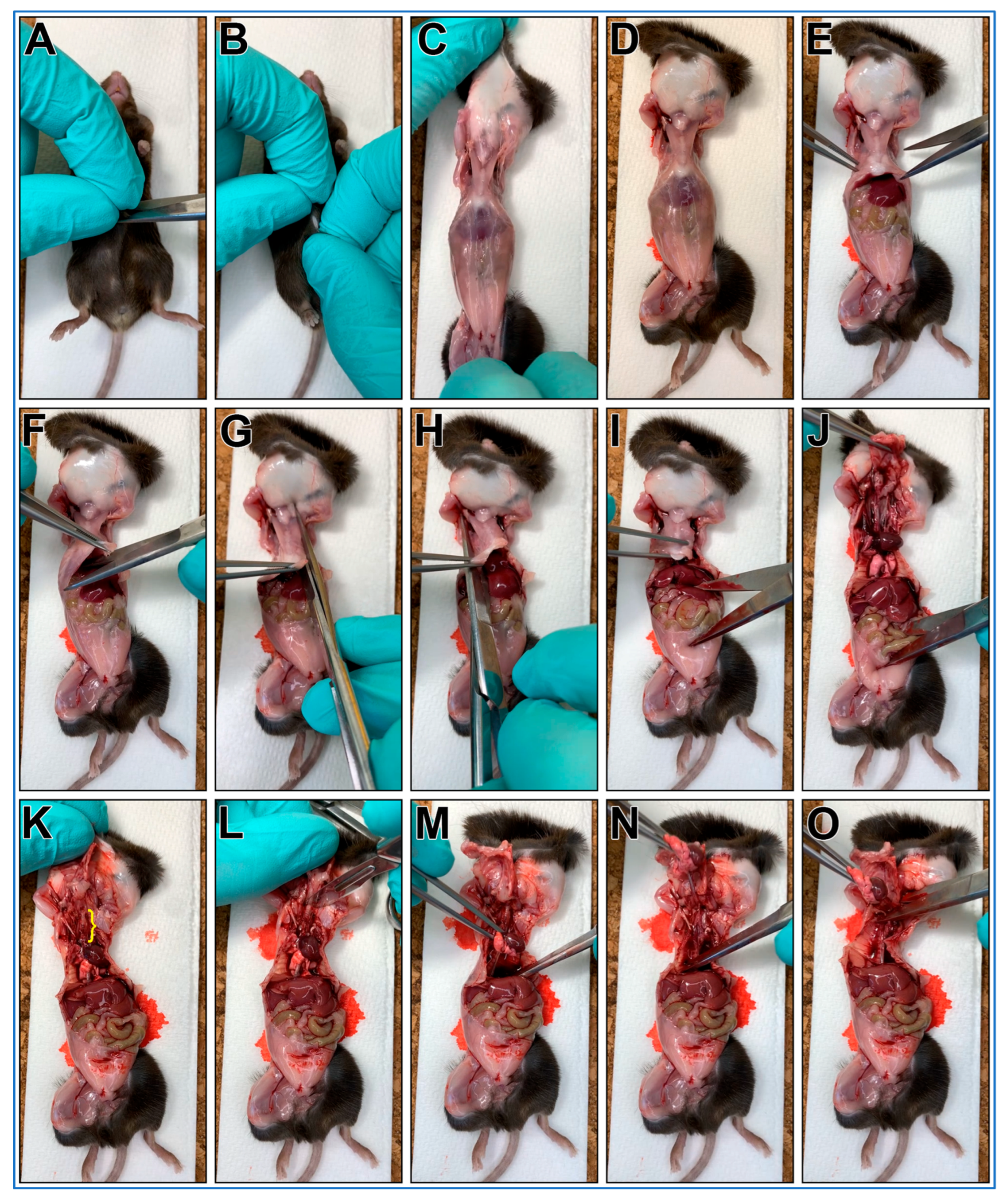
3.1. Gross Necropsy of Mouse Pluck (~30 min per Mouse)
- A mouse is euthanized via CO2 euthanasia and the carcass is placed on ice for transport to the laboratory.
CRITICAL STEP Cervical dislocation should not follow CO2 euthanasia as it may cause damage to the trachea;
- The mouse is placed posterior side down onto a cork dissecting board;
- Dissecting scissors are used to make a small cut through the skin over the abdomen;
- The skin on each side of the cut are grasped between thumbs/forefingers, then the skin is pulled off the mouse abdomen and chest;
- OPTIONAL STEP. Mouse may be pinned to the dissecting board through each paw. This is recommended for people new to this protocol;
- Dissecting scissors are used to cut through the abdominal wall using a transverse cut just below the diaphragm;
- The xiphoid process is secured using blunt forceps to better secure chest for following steps;
- Dissecting scissors are used to make a transverse cut through diaphragm;
- One blade of the dissecting scissors is inserted into the chest cavity lateral to the sternum (left side) then advanced up and under the clavicle then cut;
- One blade of the dissecting scissors is inserted into the chest cavity lateral to the sternum (right side) then advanced up and under the clavicle then cut;
- The xiphoid process is secured with blunt forceps, then while pushing down on lower body, the forceps are pulled up removing the anterior portion of the rib cage. This should reveal the trachea up to the larynx (Figure 1K, yellow bracket);
- A transverse cut through the larynx is made with a scalpel;
- The heart/lungs are secured using blunt forceps, then pulled up removing the pluck up and out of the chest;
- Being careful not to cut into the trachea, any remaining small tissue connections are cut with scissors to facilitate pluck removal;
- The mouse pluck is then placed into a 50 mL centrifuge tube with a small amount of DPBS (~3–4 mL).
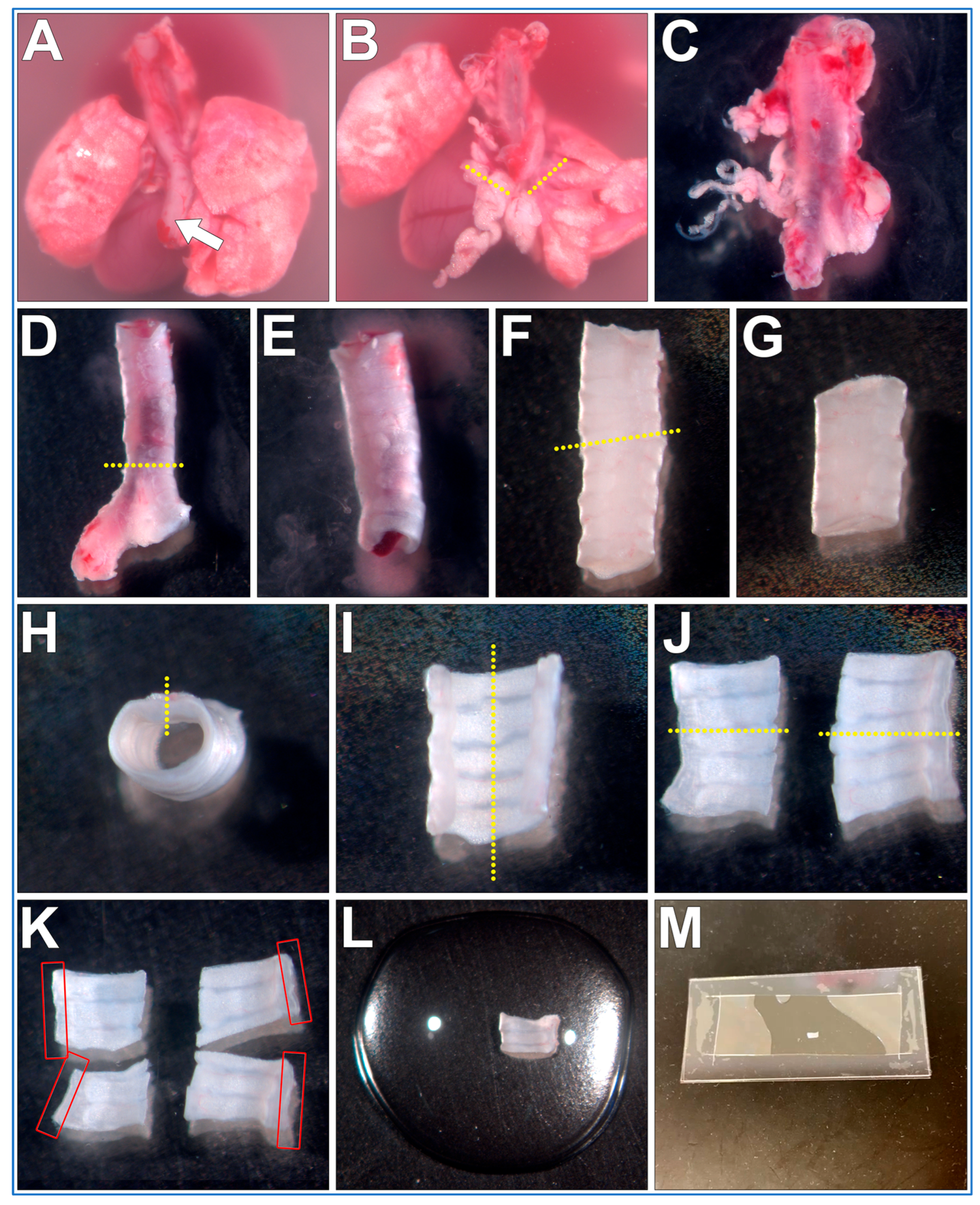
3.2. Fine Dissection of Trachea Sections from the Mouse Pluck
- The mouse pluck is placed posterior side up into a 35 mm culture dish filled with DPBS;
- Micro tweezers are used to peel the oesophagus up and off the trachea;
- Micro scissors are used to make transverse cuts through both bronchi, then the heart and lungs are removed;
- The isolated trachea is now moved into a new 35 mm culture dish filled with fresh DPBS (old dish inevitability being contaminated with excess blood obscuring details);
- Using blunt dissection and the micro tweezers/scissors, all fat/miscellaneous tissue is removed from the trachea surface;
- Micro scissors are used to make a transverse cut just above the carina of the trachea, bronchi are removed and discarded;
- A P1000 pipette and P1000 tip is used to gently flush the trachea lumen to remove any blood/mucus;
- Micro scissors are used to make a transverse cut through the trachea ~5–6 cartilaginous rings above the carina. The cranial trachea section is removed and discarded;
- Micro scissors are used to make a sagittal cut through the trachealis muscle (Figure 2H, yellow dotted line);
- Micro scissors are used to make a second sagittal cut through the middle of the cartilaginous rings (Figure 2I, yellow dotted line);
- Tissue can be now mounted and imaged (see below). Cilia are best visualized along the length of the curled trachealis muscle (Figure 2K, red boxes);
- OPTIONAL STEP. Trachea halves can be further sectioned into smaller samples for different experimental treatments (Figure 2K);
PAUSE STEP. Trachea sections can be stored in DPBS at 4 °C for up to 12 h before mounting and imaging [12].
3.3. Trachea Sample Mounting (~5 min per Sample)
- A rectangular glass coverslip (24 mm × 50 mm) is covered with ~0.25 mm thick silicone sheet, no adhesive is required, pressing coverslips and gasket together is sufficient;
- A sharp scalpel is used to remove a small rectangular section of silicone from the middle of the silicone sheet covering the coverslip, excess silicone is also trimmed from the edges of the coverslip as needed;
- OPTIONAL STEP. If silicone sheet is not available, other material of similar thickness may be used to form a gasket (e.g., 1+ layers of electrical tape);
- A second rectangular glass coverslip (24 mm × 50 mm) is placed onto the stage of a dissection microscope;
- A drop (~100–200 µL) of DPBS containing 1 μm Carboxylate Microspheres (~1 drop of microspheres per 4 mL of DPBS) is placed onto the middle of the second coverslip;
- The trachea sample is placed into the middle of the DPBS drop (Figure 2L);
- The first silicone covered coverslip is gently lowered at a 45° angle onto the bottom coverslip containing the DPBS drop and trachea sample, then gently pressed into place (no adhesive is required, pressing coverslips and gasket together is sufficient); Care is taken so that the silicone layer is between the two coverslips;
- A Kimwipe or paper towel is used to remove any leaked media from coverslip edges;
CRITICAL STEP Once sample is enclosed within the glass coverslips it should be imaged immediately. The small volume of media and enclosed environment may cause tissue to become hypoxic if left too long, which may impact cilia motility;
3.4. Cilia Imaging (~5 min per Sample)
- The trachea sample mounted as above is now placed onto the stage of an inverted bright-field microscope, preferably with Differential Interference Contrast (DIC) filters for optimal cilia visualization and a high magnification/NA water/glycerol or oil immersion objectives (e.g., 63×/1.4);
- OPTIONAL STEP. As the coverslip sample mounts are fragile, a microscope with adjustable slide holder is recommended (e.g., Thorlabs, MZS500P2—MZS500-Compatible Slide/Petri Dish Holder). Samples can then be simply lowered onto the objective without using clips to secure sample in place;
- OPTIONAL STEP. A heated microscope incubation chamber is preferable for these studies, but cells kept at room temperature are still usable for imaging ciliary motility. The major drawback of this would be that cilia in colder temperatures display decreased motility;
- Standard microscopy techniques are used to move/scan and image cilia within the mounted samples;
- A microscope camera capable of high-speed imaging (>200 fps) is used to image cilia motility to ensure accuracy of measurements. Camera speeds ~300 fps are recommended, especially if samples are imaged at 37 °C;
- One second movies are collected at >200 fps for quantification of CBF;
- Ten second movies are collected at 30 fps for tracking microsphere movement to quantify cilia generated flow.
- OPTIONAL STEP. This protocol may be coupled with fluorescent microscopy and/or other live cell imaging dyes (e.g., fluorescent microspheres for flow assessment). However, care should be taken to minimize bright excitation light exposure which may damage and impair cilia motility (personal observation);
3.5. Data Analysis (~10–20 min per Sample)
- Cilia movies are analyzed using ImageJ (FIJI 2.3.0/1.53q);
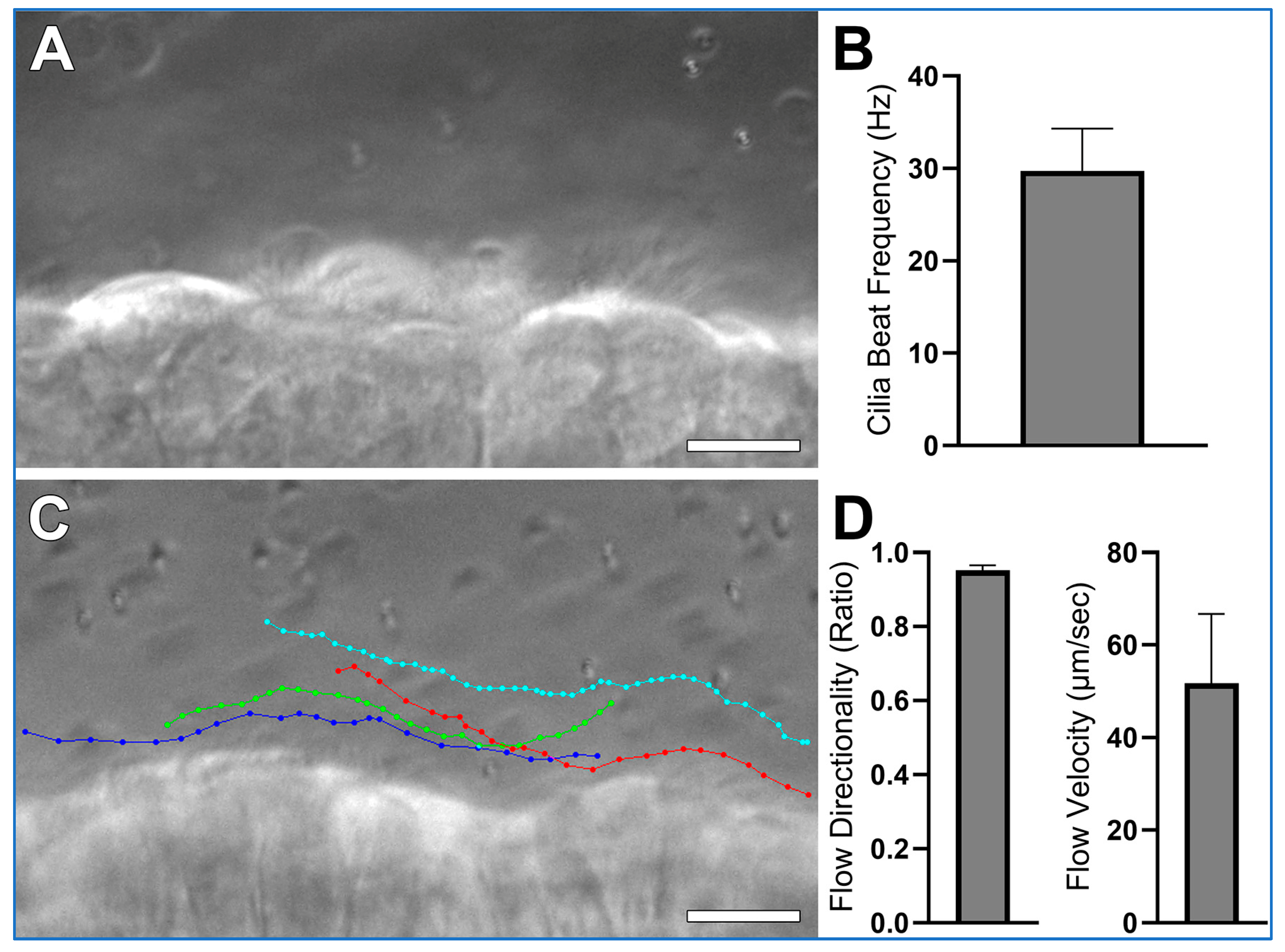
- CBF is quantified from the one second movies (>200 fps) as outlined in Figure 4. The ImageJ straight-line tool is used to draw a line through the moving cilia of one epithelial cell (Figure 4A). The ImageJ Reslice tool is then used to generate a kymograph image (Figure 4B). As the movies are one second in length, simple counting of waves yields cilia beat frequency for each ciliated cell analyzed;
- Cilia generated flow is quantified from the ten second movies (30 fps) by tracking the 1 µm microspheres suspended within the media by using the Manual Tracking plugin for ImageJ (FIJI 2.3.0/1.53q; https://imagej.net/plugins/manual-tracking; accessed on 5 August 2025);
- Microsphere directionality (ratio representing liner flow) is calculated from the flow data using Microsoft excel by dividing net microsphere displacement by total distance travelled.
4. Expected Results
5. Discussion
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBF | Cilia Beat Frequency |
| DIC | Differential Interference Contrast |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPBS | Dulbecco′s Phosphate Buffered Saline |
| fps | Frames Per Second |
| HBSS | Hank’s Balanced Salt Solution |
| L15 | Leibovitz’s L-15 Medium |
| M199 | Medium 199 |
| RPMI | Roswell Park Memorial Institute Medium |
References
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Primers 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Bricmont, N.; Bonhiver, R.; Benchimol, L.; Louis, B.; Papon, J.F.; Monseur, J.; Donneau, A.F.; Moermans, C.; Schleich, F.; Calmes, D.; et al. Temporal Stability of Ciliary Beating Post Nasal Brushing, Modulated by Storage Temperature. Diagnostics 2023, 13, 2974. [Google Scholar] [CrossRef] [PubMed]
- Behr, W.; Li, H.; Birk, R.; Nastev, A.; Kramer, B.; Klein, S.; Stuck, B.A.; Birk, C.E. Impact of Bepanthen((R)) and dexpanthenol on human nasal ciliary beat frequency in vitro. Eur. Arch. Otorhinolaryngol. 2023, 280, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, L.K.; Turgutoglu, N.; Allan, K.M.; Belessis, Y.; Widger, J.; Jaffe, A.; Waters, S.A. Comparing Cytology Brushes for Optimal Human Nasal Epithelial Cell Collection: Implications for Airway Disease Diagnosis and Research. J. Pers. Med. 2023, 13, 864. [Google Scholar] [CrossRef]
- Delmotte, P.; Sanderson, M.J. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef]
- Awatade, N.T.; Reid, A.T.; Nichol, K.S.; Budden, K.F.; Veerati, P.C.; Pathinayake, P.S.; Grainge, C.L.; Hansbro, P.M.; Wark, P.A.B. Comparison of commercially available differentiation media on cell morphology, function, and anti-viral responses in conditionally reprogrammed human bronchial epithelial cells. Sci. Rep. 2023, 13, 11200. [Google Scholar] [CrossRef] [PubMed]
- Fradique, R.; Causa, E.; Delahousse, C.; Kotar, J.; Pinte, L.; Vallier, L.; Vila-Gonzalez, M.; Cicuta, P. Assessing motile cilia coverage and beat frequency in mammalian in vitro cell culture tissues. R. Soc. Open Sci. 2023, 10, 230185. [Google Scholar] [CrossRef]
- Thomas, B.; Rutman, A.; O’Callaghan, C. Disrupted ciliated epithelium shows slower ciliary beat frequency and increased dyskinesia. Eur. Respir. J. 2009, 34, 401–404. [Google Scholar] [CrossRef]
- You, Y.; Richer, E.J.; Huang, T.; Brody, S.L. Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L1315–L1321. [Google Scholar] [CrossRef]
- Christopher, A.B.; Ochoa, S.; Krushansky, E.; Francis, R.; Tian, X.; Zahid, M.; Munoz, R.; Lo, C.W. The effects of temperature and anesthetic agents on ciliary function in murine respiratory epithelia. Front. Pediatr. 2014, 2, 111. [Google Scholar] [CrossRef]
- Scopulovic, L.; Francis, D.; Pandzic, E.; Francis, R. Quantifying cilia beat frequency using high-speed video microscopy: Assessing frame rate requirements when imaging different ciliated tissues. Physiol. Rep. 2022, 10, e15349. [Google Scholar] [CrossRef]
- Francis, R. Assessment of liquid media requirements for storing and evaluating respiratory cilia motility. PeerJ 2025, 13, e19191. [Google Scholar] [CrossRef]
- Sisson, J.H.; Stoner, J.A.; Ammons, B.A.; Wyatt, T.A. All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 2003, 211, 103–111. [Google Scholar] [CrossRef]
- Bricmont, N.; Alexandru, M.; Louis, B.; Papon, J.F.; Kempeneers, C. Ciliary Videomicroscopy: A Long Beat from the European Respiratory Society Guidelines to the Recognition as a Confirmatory Test for Primary Ciliary Dyskinesia. Diagnostics 2021, 11, 1700. [Google Scholar] [CrossRef]
- Reula, A.; Pitarch-Fabregat, J.; Milara, J.; Cortijo, J.; Mata-Roig, M.; Milian, L.; Armengot, M. High-Speed Video Microscopy for Primary Ciliary Dyskinesia Diagnosis: A Study of Ciliary Motility Variations with Time and Temperature. Diagnostics 2021, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A. Airway Epithelial Differentiation and Mucociliary Clearance. Ann. Am. Thorac. Soc. 2018, 15, S143–S148. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, G.C.; Hoffmann, K.; Loges, N.T.; Birker, D.; Rossier, C.; de Santi, M.M.; Olbrich, H.; Fliegauf, M.; Failly, M.; Liebers, U.; et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum. Mutat. 2008, 29, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.; Lo, C. Ex vivo method for high resolution imaging of cilia motility in rodent airway epithelia. J. Vis. Exp. JoVE 2013, 78, 50343. [Google Scholar] [CrossRef]
- Saito, H.; Matsukawa-Usami, F.; Fujimori, T.; Kimura, T.; Ide, T.; Yamamoto, T.; Shibata, T.; Onoue, K.; Okayama, S.; Yonemura, S.; et al. Tracheal motile cilia in mice require CAMSAP3 for the formation of central microtubule pair and coordinated beating. Mol. Biol. Cell 2021, 32, ar12. [Google Scholar] [CrossRef]
- Song, E.; Iwasaki, A. Method for Measuring Mucociliary Clearance and Cilia-generated Flow in Mice by ex vivo Imaging. Bio-Protocol 2020, 10, e3554. [Google Scholar] [CrossRef]
- Vo, Q.; Benam, K.H. Protocol for characterizing airway epithelial ciliary beating and mucociliary transport using image processing and particle imaging velocimetry. STAR Protoc. 2025, 6, 103674. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.M.; Tomei, A.A.; Swartz, M.A. Physiological 3D tissue model of the airway wall and mucosa. Nat. Protoc. 2006, 1, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, M.L.; Gabriel, S.; Burns, K.A.; Yankaskas, J.R.; Randell, S.H. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005, 107, 183–206. [Google Scholar] [CrossRef] [PubMed]


| Source | Identifier | |
|---|---|---|
| Tools | ||
| Cork Dissecting Board | Agar Scientific Ltd. (Stansted, United Kingdom) | AGL4121 |
| #10 Scalpel Blades | Roboz Surgical Instrument Co. (Gaithersburg, MD, USA) | RS-9801 |
| No 3. Scalpel Handle | Roboz Surgical Instrument Co. (Gaithersburg, MD, USA) | 65-9843 |
| Blunt Dressing Forceps | Roboz Surgical Instrument Co. (Gaithersburg, MD, USA) | RS-8100 |
| Dissecting Scissors; Straight; 5” Length | Roboz Surgical Instrument Co. (Gaithersburg, MD, USA) | RS-6808 |
| Micro Scissors, Straight | Roboz Surgical Instrument Co. (Gaithersburg, MD, USA). | RS-5602 |
| #4 Inox Dumont Tweezers | Roboz Surgical Instrument Co. (Gaithersburg, MD, USA). | RS-4904 |
| Equipment | ||
| Basic Dissection Microscope. Allowing up to ~8–10× magnification. e.g., Leica EZ4 (Leica, Wetzlar, Germany); Zeiss Stemi 305 (Zeiss, Oberkochen, Germany); Olympus SZX7 (Olympus, Tokyo, Japan); Nikon SMZ800N (Nikon, Tokyo, Japan). | ||
| Inverted Bright-Field Microscope, preferably with Differential Interference Contrast (DIC) filters (e.g., Leica DMi8 (Leica, Wetzlar, Germany); Zeiss Axiovert 5 (Zeiss, Oberkochen, Germany); Nikon Eclipse Ts2 (Nikon, Tokyo, Japan)). High magnification/NA (≥40×/>1 NA) water/glycerol or oil immersion objective. Heated incubation chamber (37.5 °C) optional, but lower temperatures will result in slower cilia [10]. | ||
| Microscope camera capable of high-speed imaging †. Examples: 1.5 MP USB3.0 Mono Microscope camera E3CMOS01500KMA (ProSciTech Pty Ltd., Thuringowa, Australia); 3.2 MP FLIR Grasshopper3 USB Camera GS3-U3-32S4M-C (Teledyne Vision Solutions, Waterloo, ON, Canada); Phantom Miro C321 (Vision Research Inc., Wayne, NJ, USA). | ||
| Reagents | ||
| Kimwipes (12 × 21 cm) | Thermo Fisher Scientific (Waltham, MA, USA) | 25509-KL |
| Dulbecco′s Phosphate Buffered Saline ‡ | Sigma-Aldrich (St. Louis, MO, USA) | D8662 |
| 35 mm culture dishes | Sigma-Aldrich (St. Louis, MO, USA) | CLS430165 |
| 50 mL centrifuge tubes | Sigma-Aldrich (St. Louis, MO, USA) | CLS430829 |
| Rectangular glass coverslips, No. 1, 24 mm × 50 mm | Sigma-Aldrich (St. Louis, MO, USA) | CLS2975245 |
| P1000 Micropipette | Sigma-Aldrich (St. Louis, MO, USA) | FA10006M |
| 1000 μL filtered pipet tips | Sigma-Aldrich (St. Louis, MO, USA) | CLS4809 |
| Immersion oil | Sigma-Aldrich (St. Louis, MO, USA) | 56822 |
| 1 μm Carboxylate Microspheres | Polysciences (Warrington, PA, USA) | 08226-15 |
| 0.254 mm thick silicone sheet | AAA Acme Rubber Co. (Tempe, AZ, USA) | 010X36-64904 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, R. A Simple Method for Imaging and Quantifying Respiratory Cilia Motility in Mouse Models. Methods Protoc. 2025, 8, 113. https://doi.org/10.3390/mps8050113
Francis R. A Simple Method for Imaging and Quantifying Respiratory Cilia Motility in Mouse Models. Methods and Protocols. 2025; 8(5):113. https://doi.org/10.3390/mps8050113
Chicago/Turabian StyleFrancis, Richard. 2025. "A Simple Method for Imaging and Quantifying Respiratory Cilia Motility in Mouse Models" Methods and Protocols 8, no. 5: 113. https://doi.org/10.3390/mps8050113
APA StyleFrancis, R. (2025). A Simple Method for Imaging and Quantifying Respiratory Cilia Motility in Mouse Models. Methods and Protocols, 8(5), 113. https://doi.org/10.3390/mps8050113








