Development and Validation of an HPLC–UV/PDA Method for the Determination of Cannflavins in Different Cannabis sativa Chemovars
Abstract
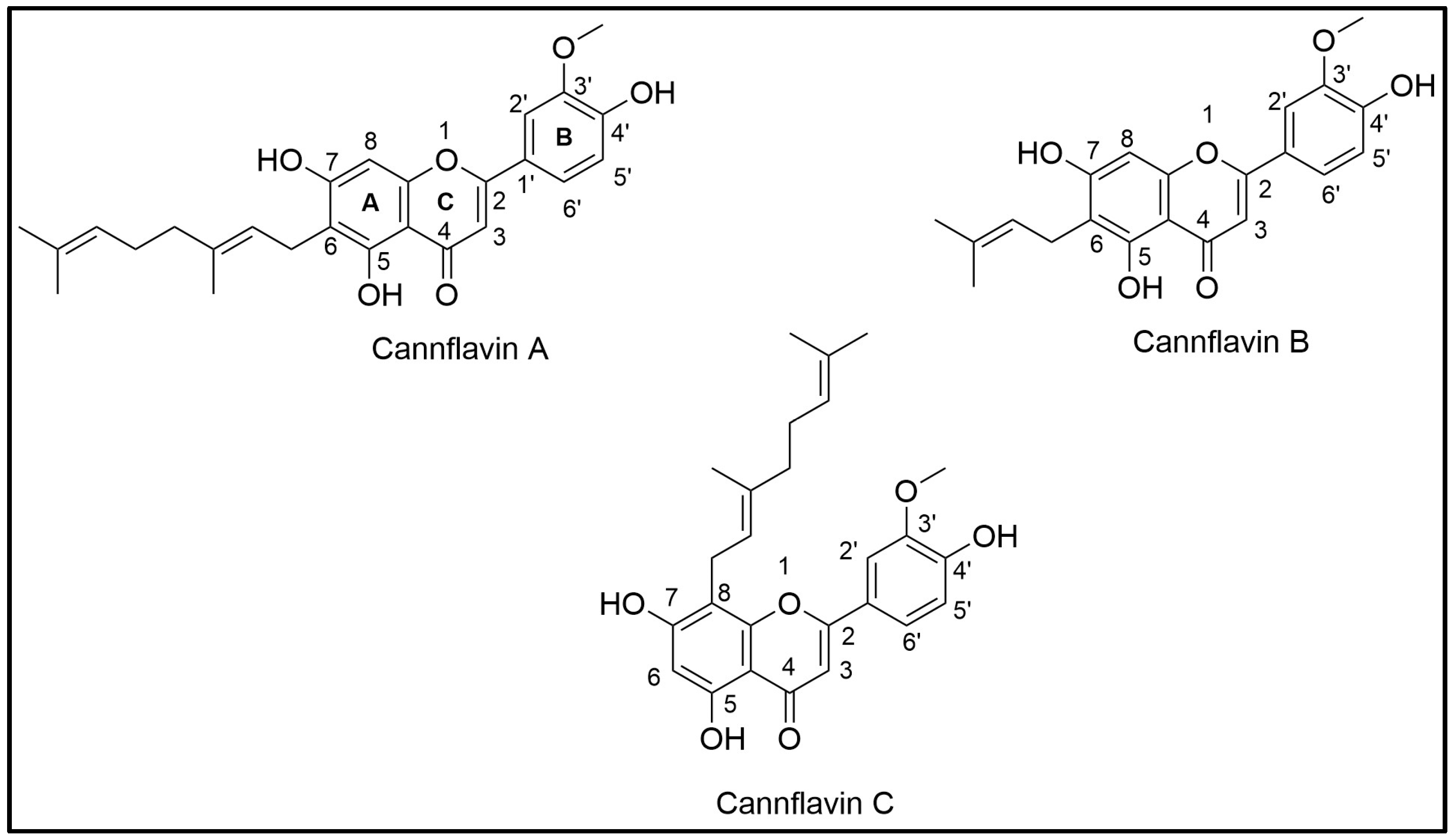
1. Introduction
2. Experimental Design
2.1. Standards and Reagents
2.2. Instrumentation
2.3. Preparation of Stock Standard Solutions
2.4. Method Validation Procedure
2.5. Cannabis Plant Material
2.6. Sample Preparation for the Decarboxylation of Cannabis Plant Material
2.7. Optimization of Cannflavin Extraction and Analysis
2.8. Statistics
3. Results
3.1. Optimization of the HPLC-PDA Conditions
3.2. Optimization of Cannflavin Extraction
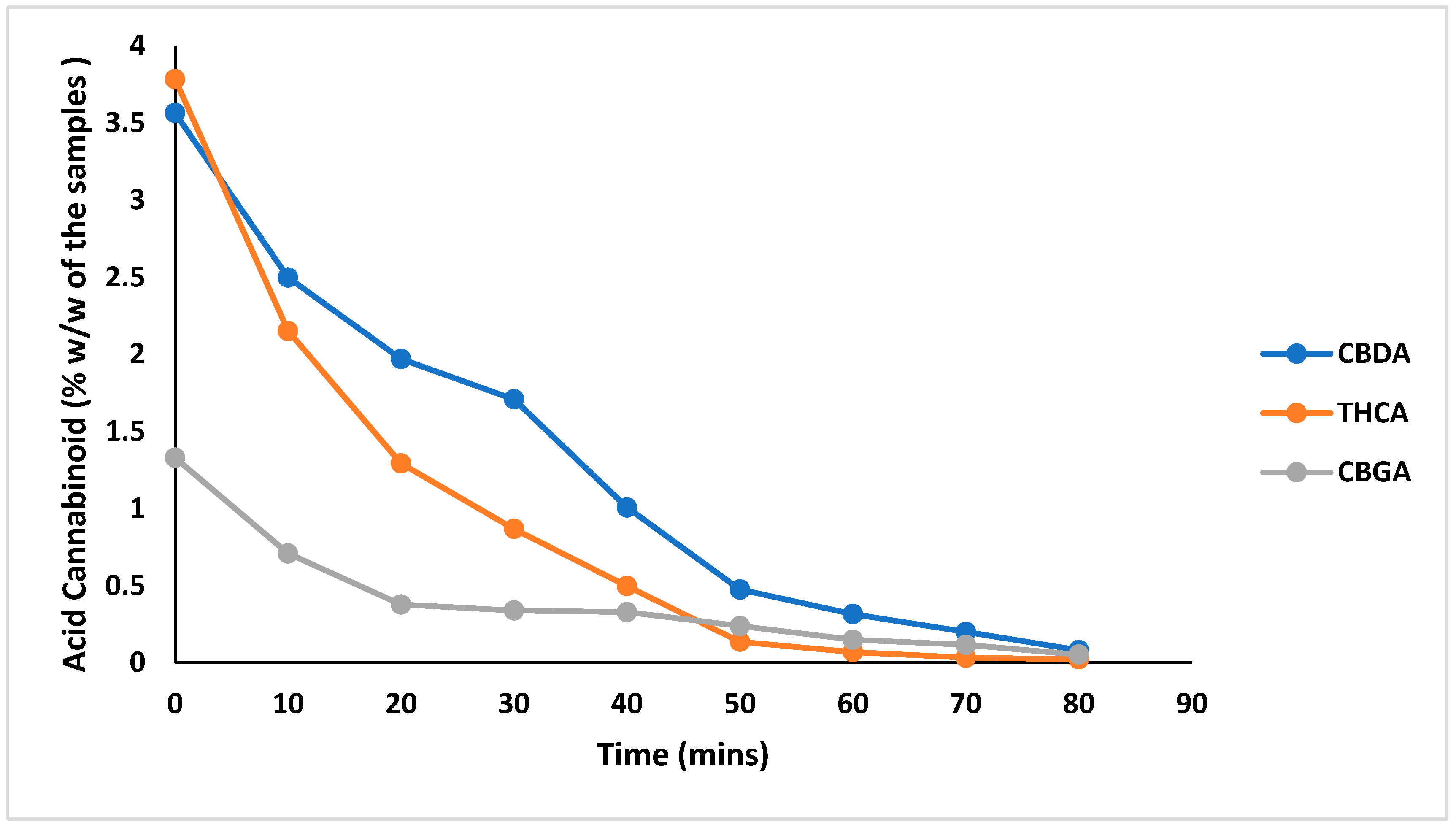
3.3. Optimization of the Decarboxylation Process
3.4. Method Validation Results
3.4.1. Linearity and Range
3.4.2. LOD and LOQ
3.4.3. Accuracy and Precision
3.4.4. Selectivity
3.4.5. Method Robustness
- Mobile Phase Composition: The polarity and strength of the mobile phase directly affect analyte interaction with the stationary phase. A higher organic content speeds up elution, reducing retention time but may lower resolution, especially for CF-B and CF-C. Conversely, a lower organic content enhances separation but extends the run time. We chose a mobile phase of acetonitrile and water (65:35, v/v), both containing 0.1% formic acid, at a flow rate of 1 mL/min, with the detector set at 342.4 nm to balance these effects and ensure baseline resolution of CF-B, CF-C, and CF-A.
- Flow Rate: Increasing the flow rate shortens retention times and can decrease peak width, but it may also cause coelution or loss of resolution. Lower flow rates improve separation but increase analysis time. Our flow rate was optimized to maintain peak symmetry and reproducibility.
- Column Temperature: Although our method was performed under ambient conditions, temperature can influence analyte volatility and interaction with the stationary phase. Elevated temperatures typically reduce retention times and may affect peak shape, especially for thermally sensitive compounds like cannflavins.
- Injection Volume and Solvent Strength: Large injection volumes or strong solvents can distort peak shapes and reduce efficiency. We used minimal injection volumes and matched the solvent strength to the initial mobile phase to preserve chromatographic integrity.
- Stationary Phase Selection: The choice of column chemistry (e.g., C18 reversed phase) determines selectivity and retention behavior. CF-A and CBDA exhibited similar retention under our conditions, necessitating decarboxylation to resolve the coelution.
3.5. Analysis of Cannflavins in Six Cannabis Chemovars
3.6. Analysis of Cannflavins in Samples from Different Parts of the Female Cannabis Plant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ∆9-THC | Δ9-tetrahydrocannabinol |
| bLOD | Below the Limit of Detection |
| bLOQ | Below the Limit of Quantification |
| C. sativa | Cannabis sativa |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic Acid |
| CBG | Cannabigerol |
| CBGA | Cannabigerolic Acid |
| CF-A | Cannflavin-A |
| CF-B | Cannflavin-B |
| CF-C | Cannflavin-C |
| HCBD | High CBD Chemovar |
| HCBG | High CBG Chemovar |
| HPLC-PDA | High Performance Liquid Chromatography–Photodiode Array Detector |
| HTHCV | High THC/THCV Chemovar |
| IM | High THC/CBD Intermediate Chemovar |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| ppm | Parts Per Million |
| RSD | Relative Standard Deviation |
| SD | Standard Deviation |
| THC | Tetrahydrocannabinol |
| THCA | Tetrahydrocannabinolic Acid |
| THCV | Tetrahydrocannabivarin |
References
- Li, H.-L. An archaeological and historical account of cannabis in China. Econ. Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Touw, M. The religious and medicinal uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P. Cannabis as an adjunct to or substitute for opiates in the treatment of chronic pain. J. Psychoact. Drugs 2012, 44, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Scharf, E.L.; Hurt, R.T. Medical cannabis. Mayo Clin. Proc. 2018, 93, 1842–1847. [Google Scholar] [CrossRef]
- Tramèr, M.R.; Carroll, D.; Campbell, F.A.; Reynolds, D.J.M.; Moore, R.A.; McQuay, H.J. Cannabinoids for control of chemotherapy induced nausea and vomiting: Quantitative systematic review. BMJ 2001, 323, 16. [Google Scholar] [CrossRef]
- Mattes, R.D.; Engelman, K.; Shaw, L.M.; Elsohly, M.A. Cannabinoids and appetite stimulation. Pharmacol. Biochem. Behav. 1994, 49, 187–195. [Google Scholar] [CrossRef]
- Alexander, S.P. Therapeutic potential of cannabis-related drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Lazarini-Lopes, W.; Do Val-da Silva, R.A.; da Silva-Júnior, R.M.; Leite, J.P.; Garcia-Cairasco, N. The anticonvulsant effects of cannabidiol in experimental models of epileptic seizures: From behavior and mechanisms to clinical insights. Neurosci. Biobehav. Rev. 2020, 111, 166–182. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.S.; Radwan, M.M.; Metwaly, A.M.; Eissa, I.H.; Hazekamp, A.; ElSohly, M.A. Chemistry and biological activities of cannflavins of the cannabis plant. Cannabis Cannabinoid Res. 2023, 8, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Berim, A.; Gang, D.R. Methoxylated flavones: Occurrence, importance, biosynthesis. Phytochem. Rev. 2016, 15, 363–390. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Crombie, L.; Crombie, W.M.L.; Jamieson, S.V. Extractives of Thailand cannabis: Synthesis of canniprene and isolation of new geranylated and prenylated chrysoeriols. Tetrahedron Lett. 1980, 21, 3607–3610. [Google Scholar] [CrossRef]
- De Vita, S.; Finamore, C.; Chini, M.G.; Saviano, G.; De Felice, V.; De Marino, S.; Lauro, G.; Casapullo, A.; Fantasma, F.; Trombetta, F. Phytochemical analysis of the methanolic extract and essential oil from leaves of industrial hemp Futura 75 cultivar: Isolation of a new cannabinoid derivative and biological profile using computational approaches. Plants 2022, 11, 1671. [Google Scholar] [CrossRef]
- Salem, M.M.; Capers, J.; Rito, S.; Werbovetz, K.A. Antiparasitic Activity of C-Geranyl Flavonoids from Mimulus bigelovii. Phytother. Res. 2011, 25, 1246–1249. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Abegaz, B.M.; Dongo, E.; Tamboue, H.; Fogue, K. Geranylated and prenylated flavonoids from the twigs of Dorstenia mannii. Phytochemistry 1998, 48, 349–354. [Google Scholar] [CrossRef]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.; Amaducci, S. High added-value compounds from Cannabis threshing residues. Ind. Crops Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Eggers, C.; Fujitani, M.; Kato, R.; Smid, S. Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid β-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone. Biochem. Pharmacol. 2019, 169, 113609. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Conte, F.M.; Russo, M.; Tedesco, I.; Moccia, S.; Sicuranza, M.; Scotti, R.; Russo, G.L. A Phenolic Extract from Extra Virgin Olive Oil Induces Authophagy and Apopotosis in Human Bladder Cancer Cell Lines Depending on Tumor Progression. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 871. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.M.; Whynot, E.G.; Dupré, D.J. Anti-cancer properties of cannflavin A and potential synergistic effects with gemcitabine, cisplatin, and cannabinoids in bladder cancer. J. Cannabis Res. 2022, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Holborn, J.; Robeson, H.; Chartley, E.; Gluscevic, T.; Borenstein, A.; Perrin, C.; Alural, B.; Perera, H.; Akhtar, T.A.; Jones, N. Preclinical Study of Cannflavins A and B Action Against Glioblastoma Cells. bioRxiv 2025. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287. [Google Scholar] [CrossRef]
- Rea, K.A.; Casaretto, J.A.; Al-Abdul-Wahid, M.S.; Sukumaran, A.; Geddes-McAlister, J.; Rothstein, S.J.; Akhtar, T.A. Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry 2019, 164, 162–171. [Google Scholar] [CrossRef]
- Chuanphongpanich, S.; Racha, S.; Saengsitthisak, B.; Pirakitikulr, P.; Racha, K. Computational assessment of Cannflavin A as a TAK1 inhibitor: Implication as a potential therapeutic target for anti-inflammation. Sci. Pharm. 2023, 91, 36. [Google Scholar] [CrossRef]
- Vanhoenacker, G.; Van Rompaey, P.; De Keukeleire, D.; Sandra, P. Chemotaxonomic features associated with flavonoids of cannabinoid-free cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.). Nat. Prod. Lett. 2002, 16, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; ElSohly, M.A.; Sultana, G.N.; Mehmedic, Z.; Hossain, C.F.; Chandra, S. Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 45–48. [Google Scholar]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Choi, Y.H.; Hazekamp, A.; Peltenburg-Looman, A.M.; Frédérich, M.; Erkelens, C.; Lefeber, A.W.; Verpoorte, R. NMR assignments of the major cannabinoids and cannabiflavonoids isolated from flowers of Cannabis sativa. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Vochyanova, Z.; Pokorna, M.; Rotrekl, D.; Smekal, V.; Fictum, P.; Suchý, P.; Gajdziok, J.; Šmejkal, K.; Hošek, J. Prenylated flavonoid morusin protects against TNBS-induced colitis in rats. PLoS ONE 2017, 12, e0182464. [Google Scholar] [CrossRef]
- Walle, T. Methylation of dietary flavones greatly improves their hepatic metabolic stability and intestinal absorption. Mol. Pharm. 2007, 4, 826–832. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of phenolic compounds in commercial Cannabis sativa L. inflorescences using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Peschel, W.; Politi, M. 1H NMR and HPLC/DAD for Cannabis sativa L. chemotype distinction, extract profiling and specification. Talanta 2015, 140, 150–165. [Google Scholar] [CrossRef]
- Pannico, A.; Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Carillo, P.; Corrado, G.; Ritieni, A.; Rouphael, Y.; De Pascale, S. Hemp microgreens as an innovative functional food: Variation in the organic acids, amino acids, polyphenols, and cannabinoids composition of six hemp cultivars. Food Res. Int. 2022, 161, 111863. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [PubMed]
- Conor, O.C.; Tyson, S.L.; Michael, D.; Raimar, L.; Neal, M.D. A validated method for detection of cannflavins in hemp extracts. J. Pharm. Biomed. Anal. 2023, 235, 115631. [Google Scholar] [CrossRef] [PubMed]
- Elhendawy, M.A.; Radwan, M.M.; Ibrahim, E.A.; Wanas, A.S.; Chandra, S.; Godfrey, M.; ElSohly, M.A. Validation and Quantitation of Fifteen Cannabinoids in Cannabis and Marketed Products Using High-Performance Liquid Chromatography-Ultraviolet/Photodiode Array Method. Cannabis Cannabinoid Res. 2024, 9, e1091–e1107. [Google Scholar] [CrossRef] [PubMed]
- Kuber, B.R.; Jamuna, A. Novel Stability Indicating Analytical Method Development and Validation for the Estimation of Canniflavin in Bulk By Uplc. Int. J. Pharm. Res. Technol. (IJPRT) 2023, 13, 64–72. [Google Scholar]
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology. Q2 (R1) 2005, 1, 5. [Google Scholar]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
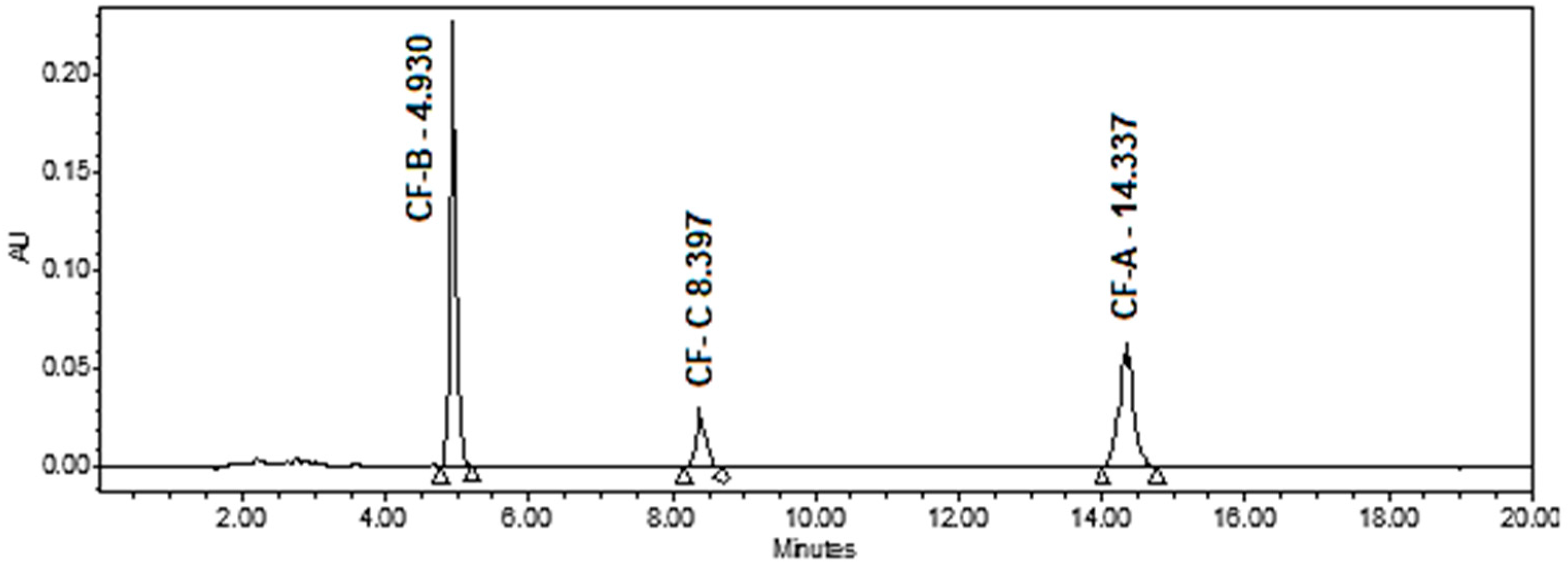
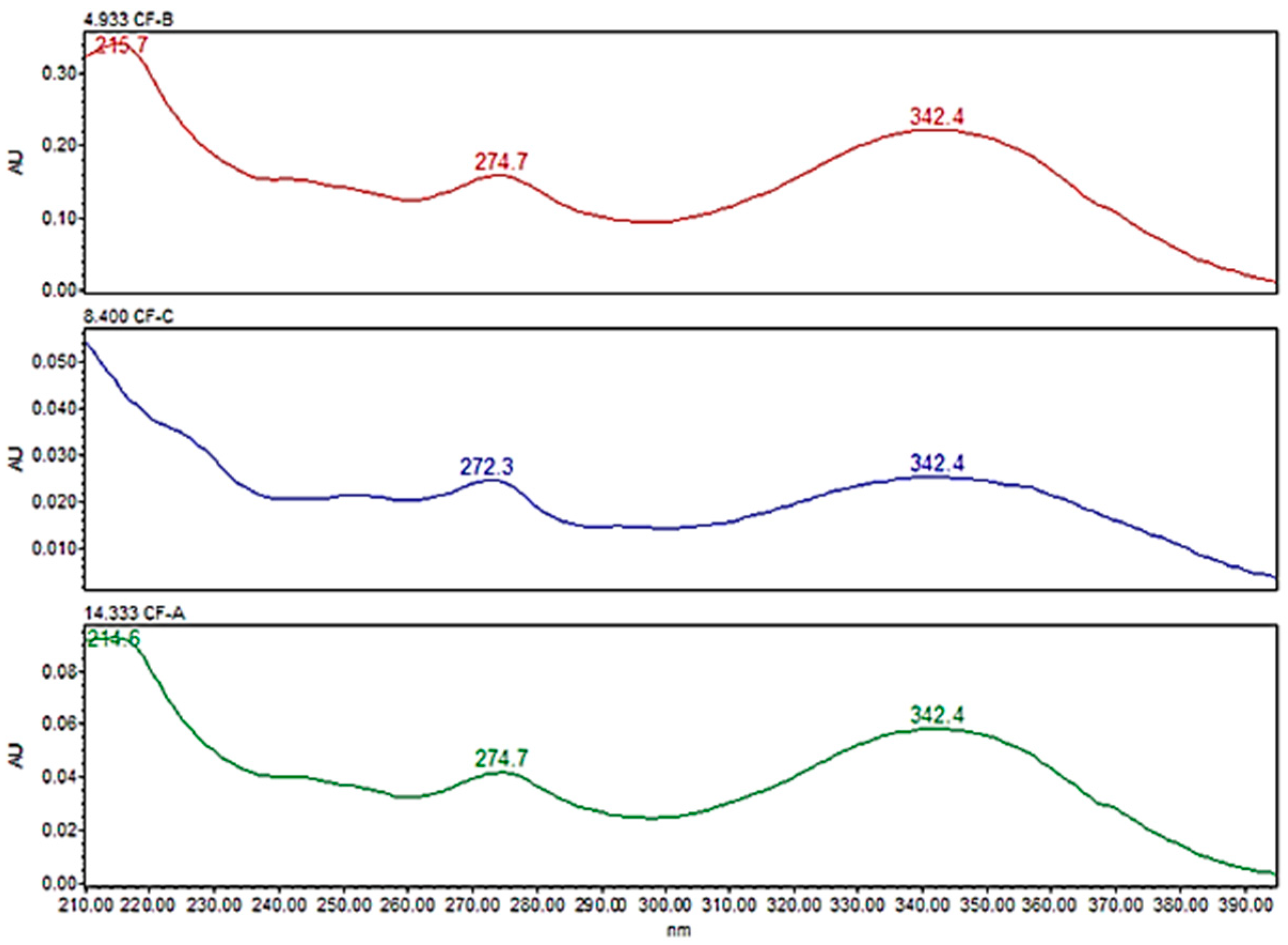
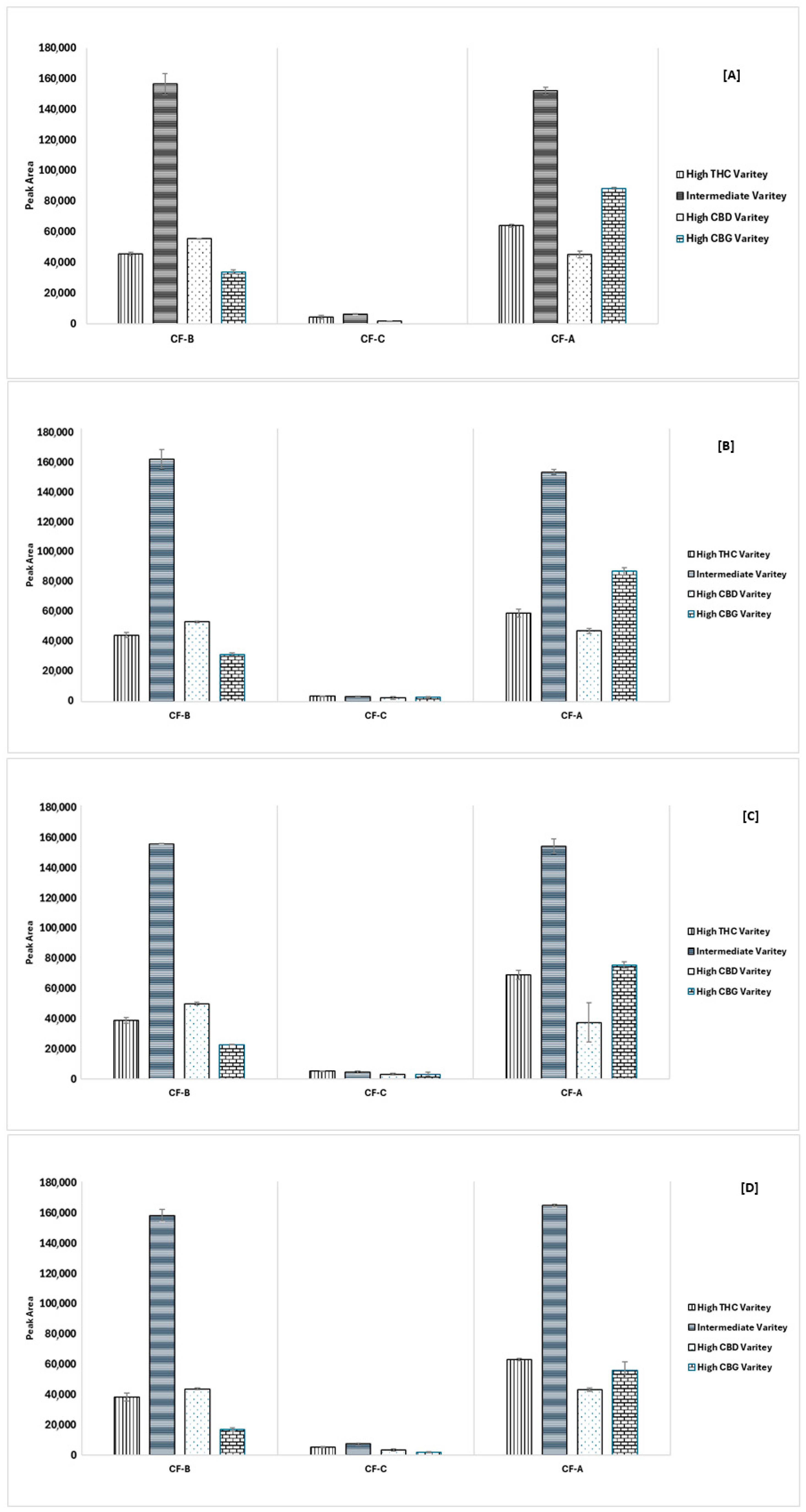
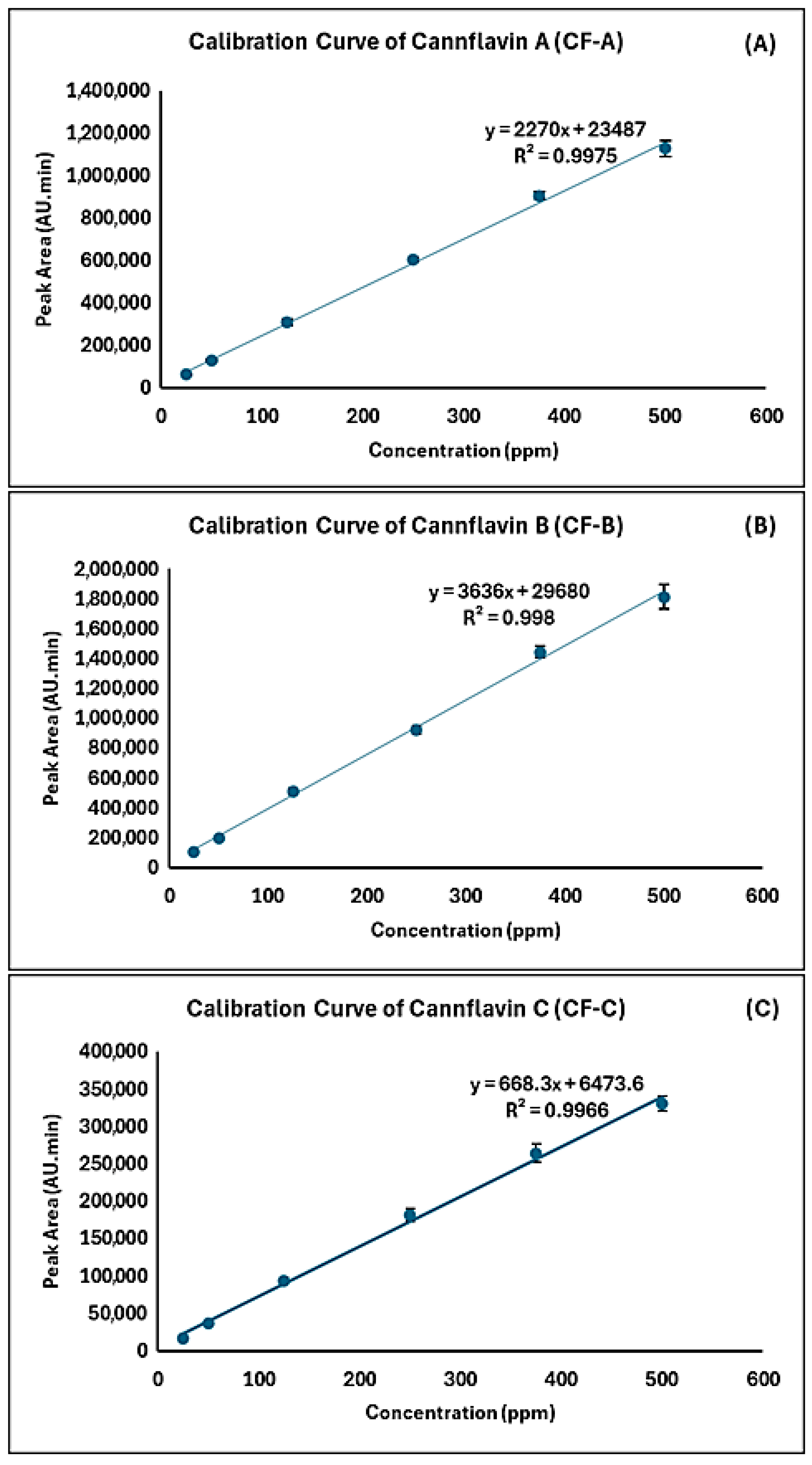

| No | Cannflavins | Rt (min.) | Regression Equation | R2 | Linearity Range (ppm) | LOD (ppm) | LOQ (ppm) |
|---|---|---|---|---|---|---|---|
| 1 | CF-A | 14.337 | y = 2270x + 23,487 | 0.9975 | 5–500 | 1.5 | 5.0 |
| 2 | CF-B | 4.930 | y = 3636x + 29,680 | 0.9980 | 5–500 | 1.2 | 4.0 |
| 3 | CF-C | 8.397 | y = 668.3x + 6473.6 | 0.9966 | 5–500 | 1.0 | 3.3 |
| Time | Day 1 | Day 2 | Day 3 | Day 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannflavins | CF-A | CF-B | CF-C | CF-A | CF-B | CF-C | CF-A | CF-B | CF-C | CF-A | CF-B | CF-C |
| 487.20 | 499.00 | 473.80 | 488.20 | 490.70 | 465.90 | 485.40 | 491.30 | 472.00 | 480.90 | 481.80 | 465.00 | |
| 500 ppm | 484.70 | 496.70 | 481.80 | 482.70 | 495.60 | 480.10 | 478.10 | 487.10 | 467.50 | 473.00 | 477.30 | 451.40 |
| 482.70 | 487.60 | 479.10 | 491.10 | 492.20 | 486.10 | 477.70 | 488.80 | 463.30 | 470.60 | 476.80 | 459.40 | |
| Average | 484.87 | 494.43 | 478.23 | 487.33 | 492.83 | 477.37 | 480.40 | 489.07 | 467.60 | 474.83 | 478.63 | 458.60 |
| SD | 2.25 | 6.03 | 4.07 | 4.27 | 2.51 | 10.37 | 4.33 | 2.11 | 4.35 | 5.39 | 2.75 | 6.84 |
| % RSD | 0.46 | 1.22 | 0.85 | 0.88 | 0.51 | 2.17 | 0.90 | 0.43 | 0.93 | 1.13 | 0.58 | 1.49 |
| % Recovery | 96.97 | 98.89 | 95.65 | 97.47 | 98.57 | 95.47 | 96.08 | 97.81 | 93.52 | 94.97 | 95.73 | 91.72 |
| 214.80 | 224.70 | 210.00 | 220.60 | 225.00 | 214.40 | 216.30 | 219.80 | 213.60 | 215.10 | 217.80 | 201.90 | |
| 250 ppm | 218.30 | 223.10 | 214.80 | 219.60 | 224.70 | 218.90 | 213.20 | 219.50 | 216.70 | 213.90 | 216.80 | 210.40 |
| 217.60 | 223.40 | 216.70 | 219.50 | 223.40 | 216.90 | 208.60 | 216.60 | 209.20 | 215.80 | 217.20 | 210.40 | |
| Average | 216.90 | 223.73 | 213.83 | 219.90 | 224.37 | 216.73 | 212.70 | 218.63 | 213.17 | 214.93 | 217.27 | 207.57 |
| SD | 1.85 | 0.85 | 3.45 | 0.61 | 0.85 | 2.25 | 3.87 | 1.77 | 3.77 | 0.96 | 0.50 | 4.91 |
| % RSD | 0.85 | 0.38 | 1.61 | 0.28 | 0.38 | 1.04 | 1.82 | 0.81 | 1.77 | 0.45 | 0.23 | 2.36 |
| % Recovery | 86.76 | 89.49 | 85.53 | 87.96 | 89.75 | 86.69 | 85.08 | 87.45 | 85.27 | 85.97 | 86.91 | 83.03 |
| 100 ppm | 90.50 | 92.70 | 91.40 | 86.80 | 89.70 | 89.90 | 84.00 | 90.00 | 88.60 | 83.60 | 89.20 | 92.10 |
| 89.50 | 93.20 | 95.40 | 85.40 | 90.40 | 87.90 | 84.10 | 87.50 | 80.50 | 83.40 | 89.40 | 83.80 | |
| 87.70 | 92.10 | 90.00 | 82.60 | 89.60 | 89.00 | 81.90 | 87.50 | 87.10 | 82.40 | 87.80 | 84.20 | |
| Average | 89.23 | 92.67 | 92.27 | 84.93 | 89.90 | 88.93 | 83.33 | 88.33 | 85.40 | 83.13 | 88.80 | 86.70 |
| SD | 1.42 | 0.55 | 2.80 | 2.14 | 0.44 | 1.00 | 1.24 | 1.44 | 4.31 | 0.64 | 0.87 | 4.68 |
| % RSD | 1.59 | 0.59 | 3.04 | 2.52 | 0.48 | 1.13 | 1.49 | 1.63 | 5.05 | 0.77 | 0.98 | 5.40 |
| % Recovery | 89.23 | 92.67 | 92.27 | 84.93 | 89.90 | 88.93 | 83.33 | 88.33 | 85.40 | 83.13 | 88.80 | 86.70 |
| Level | High Concentration | Medium Concentration | Low Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 ppm | 250 ppm | 100 ppm | ||||||||||
| Cannflavins | Average | SD | % RSD | % Recovery | Average | SD | % RSD | % Recovery | Average | SD | % RSD | % Recovery |
| CF-A | 481.86 | 3.23 | 0.67 | 96.37% | 216.11 | 2.68 | 1.24 | 86.44% | 85.16 | 3.19 | 3.74 | 85.16% |
| CF-B | 488.74 | 7.10 | 1.45 | 97.75% | 221.00 | 3.58 | 1.62 | 88.40% | 89.93 | 1.94 | 2.16 | 89.93% |
| CF-C | 470.45 | 8.62 | 1.83 | 94.09% | 212.83 | 4.76 | 2.24 | 85.13% | 88.33 | 2.72 | 3.08 | 88.33% |
| Chemovar | CF-A | CF-B | CF-C | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| High CBD chemovar | HCBD1 | 60.10 | 0.65 | 17.24 | 0.23 | 16.39 | 0.86 |
| HCBD2 | 49.52 | 1.05 | 15.79 | 0.28 | 13.17 | 0.95 | |
| HCBD3 | 37.48 | 1.05 | 14.87 | 0.18 | 29.62 | 0.46 | |
| HCBD4 | 54.15 | 3.56 | 16.05 | 0.01 | 18.34 | 3.59 | |
| HCBD5 | 55.82 | 0.41 | 14.34 | 0.19 | 7.38 | 0.59 | |
| THC/CBD Intermediate chemovar | IM1 | 58.45 | 0.70 | 38.95 | 0.11 | 9.60 | 0.77 |
| IM2 | 141.78 | 1.93 | 66.71 | 4.21 | 18.51 | 2.27 | |
| IM3 | 387.44 | 0.65 | 140.82 | 1.21 | 61.85 | 0.54 | |
| IM4 | 21.42 | 0.42 | 11.46 | 0.26 | 5.29 | 0.89 | |
| IM5 | 99.47 | 2.91 | 55.47 | 0.38 | 12.41 | 1.07 | |
| High THC chemovar | HTHC1 | 142.42 | 0.92 | 40.74 | 0.07 | 25.70 | 2.61 |
| HTHC2 | 38.87 | 0.85 | 11.20 | 0.07 | 12.45 | 1.85 | |
| HTHC3 | 128.62 | 2.06 | 53.40 | 1.98 | 25.11 | 1.29 | |
| HTHC4 | 94.76 | 2.38 | 35.21 | 2.00 | 17.75 | 0.02 | |
| HTHC5 | 162.63 | 1.09 | 50.15 | 3.41 | 28.58 | 1.05 | |
| High CBG chemovar | HCBG1 | 15.89 | 0.45 | bLOQ | bLOD | 3.39 | 0.04 |
| HCBG2 | 148.34 | 0.78 | 17.40 | 0.75 | 13.95 | 1.10 | |
| HCBG3 | 88.48 | 0.50 | 13.69 | 0.81 | bLOD | bLOD | |
| HCBG4 | 92.50 | 0.51 | 11.89 | 0.72 | bLOD | bLOD | |
| HCBG5 | 108.05 | 1.52 | 12.85 | 0.21 | bLOD | bLOD | |
| THC/THCV chemovar | HTHCV1 | 150.54 | 3.11 | 38.38 | 0.11 | 15.88 | 0.19 |
| CBD/CBDV chemovar | HCBDV1 | 181.68 | 2.07 | 101.55 | 1.21 | 9.60 | 0.06 |
| Vegetative Stage | Flowering Stage | ||||||
|---|---|---|---|---|---|---|---|
| Chemovar | High CBG Chemovar | ||||||
| Plant Part | CF-A | CF-B | CF-C | Plant Part | CF-A | CF-B | CF-C |
| Leaves | 133.76 | 27.52 | 10.26 | Leaves | 51.82 | 4.99 | bLOD |
| Stem | bLOD | bLOD | bLOD | Stem | bLOD | bLOD | bLOD |
| Roots | bLOD | bLOD | bLOD | Roots | bLOD | bLOD | bLOD |
| Buds | 478.38 | 75.39 | 49.34 | ||||
| Chemovar | High CBD Chemovar | ||||||
| Plant Part | CF-A | CF-B | CF-C | Plant Part | CF-A | CF-B | CF-C |
| Leaves | 27.79 | 3.84 | bLOD | Leaves | 15.20 | bLOD | bLOD |
| Stem | bLOD | bLOD | bLOD | Stem | bLOD | bLOD | bLOD |
| Roots | bLOD | bLOD | bLOD | Roots | bLOD | bLOD | bLOD |
| Buds | 18.47 | 28.63 | bLOD | ||||
| Male Plant | |||||||
| Plant Part | CF-A | CF-B | CF-C | ||||
| Leaves | bLOD | 5.95 | bLOD | ||||
| Stem | bLOD | bLOD | bLOD | ||||
| Roots | bLOD | bLOD | bLOD | ||||
| pollen | 117.95 | 14.98 | 12.85 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhendawy, M.A.; Radwan, M.M.; Ibrahim, E.A.; Wanas, A.S.; Marzouk, A.A.; Chandra, S.; Godfrey, M.; ElSohly, M.A. Development and Validation of an HPLC–UV/PDA Method for the Determination of Cannflavins in Different Cannabis sativa Chemovars. Methods Protoc. 2025, 8, 100. https://doi.org/10.3390/mps8050100
Elhendawy MA, Radwan MM, Ibrahim EA, Wanas AS, Marzouk AA, Chandra S, Godfrey M, ElSohly MA. Development and Validation of an HPLC–UV/PDA Method for the Determination of Cannflavins in Different Cannabis sativa Chemovars. Methods and Protocols. 2025; 8(5):100. https://doi.org/10.3390/mps8050100
Chicago/Turabian StyleElhendawy, Mostafa A., Mohamed M. Radwan, Elsayed A. Ibrahim, Amira S. Wanas, Adel A. Marzouk, Suman Chandra, Murelle Godfrey, and Mahmoud A. ElSohly. 2025. "Development and Validation of an HPLC–UV/PDA Method for the Determination of Cannflavins in Different Cannabis sativa Chemovars" Methods and Protocols 8, no. 5: 100. https://doi.org/10.3390/mps8050100
APA StyleElhendawy, M. A., Radwan, M. M., Ibrahim, E. A., Wanas, A. S., Marzouk, A. A., Chandra, S., Godfrey, M., & ElSohly, M. A. (2025). Development and Validation of an HPLC–UV/PDA Method for the Determination of Cannflavins in Different Cannabis sativa Chemovars. Methods and Protocols, 8(5), 100. https://doi.org/10.3390/mps8050100








