A Simple Way to Quantify Plastic in Bats (Mammalia: Chiroptera) Using an Ultraviolet Flashlight
Abstract
1. Introduction
2. Experimental Design
2.1. Bat Sampling
2.2. Fecal Collection
2.3. Digestive Solution by Alkaline Hydrolysis
- (i)
- Precisely weighing 10 g of KOH on an analytical balance;
- (ii)
- Adding 100 mL of distilled and filtered water into a beaker or glass jar, and gradually incorporating the KOH.
3. Procedure
3.1. Extraction of the Biological Tissues
3.2. Digestion of the Tissues and Feces
3.3. Visual Analysis of Plastic Waste
- (a)
- Residues that resembled fibers with structures similar to animal joints were not considered;
- (b)
- Only residues that maintained a consistent shape, color, and pattern along their entire structure were included;
- (c)
- Particles smaller than 50 μm were excluded;
- (d)
- Confirmation of plastic residues was conducted using the hot needle test [34].
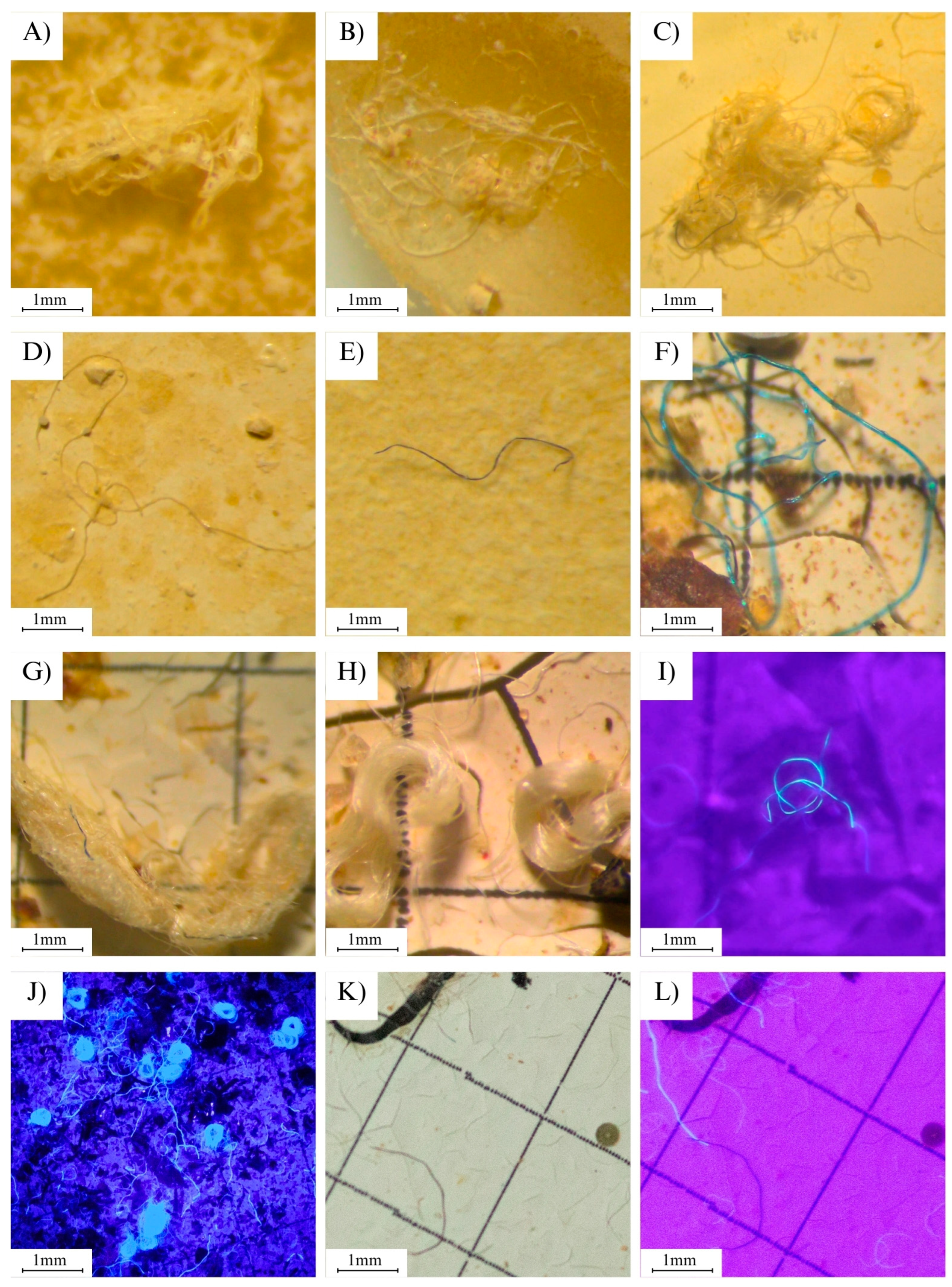
3.4. Detection of MPs Using UV Light
3.5. Quality Assurance and Quality Control (QA/QC)
4. Expected Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Fenton, M.B. Bat Ecology, 1st ed.; University of Chicago Press: Chicago, IL, USA, 2003; ISBN 0226462064. [Google Scholar]
- Fleming, T.H.; Muchhala, N. Nectar-feeding bird and bat niches in two worlds: Pantropical comparisons of vertebrate pollination systems. J. Biogeogr. 2008, 35, 764–780. [Google Scholar] [CrossRef]
- Trejo-Salazar, R.E.; Gámez, N.; Escalona-Prado, E.; Scheinvar, E.; Medellín, R.A.; Moreno-Letelier, A.; Aguirre-Planter, E.; Eguiarte, L.E. Historical, temporal, and geographic dynamism of the interaction between agave and leptonycteris nectar-feeding bats. Am. J. Bot. 2023, 110, e16222. [Google Scholar] [CrossRef] [PubMed]
- Zardo, R.N.; Henriques, R.P.B. Growth and fruit production of the tree caryocar brasiliense in the cerrado of central Brazil. Agrofor. Syst. 2011, 82, 15–23. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; Vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef]
- Wilson, D.R.; Godley, B.J.; Haggar, G.L.; Santillo, D.; Sheen, K.L. The influence of depositional environment on the abundance of microplastic pollution on beaches in the Bristol channel, UK. Mar. Pollut. Bull. 2021, 164, 111997. [Google Scholar] [CrossRef]
- Terra Lucio, F.; Marques Magnoni, D.; Elisa Pimenta Vicentini, V.; Conte, H. Availability and influence of microplastics on living organisms and environment: A review. Bol. Poluição Mar. 2019, 14, 47–55. (In Portuguese) [Google Scholar]
- Della Torre, C.; Riccardi, N.; Magni, S.; Modesto, V.; Fossati, M.; Binelli, A. First comparative assessment of contamination by plastics and non-synthetic particles in three bivalve species from an Italian sub-alpine lake. Environ. Pollut. 2023, 330, 121752. [Google Scholar] [CrossRef]
- Correia, L.L.; Ribeiro-Brasil, D.R.G.; Garcia, M.G.; Silva, D.d.M.e.; Alencastre-Santos, A.B.; Vieira, T.B. The first record of ingestion and inhalation of micro- and mesoplastics by neotropical bats from the Brazilian amazon. Acta Chiropterologica 2024, 25, 371–383. [Google Scholar] [CrossRef]
- Schnitzler, H.U.; Kalko, E.K.V. Echolocation by insect-eating bats. Bioscience 2001, 51, 557–569. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh county, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, Y.; Liang, C.; Song, J.; Yu, S.; Liao, G.; Zou, P.; Tang, K.H.D.; Wu, C. Airborne microplastics: Occurrence, sources, fate, risks and mitigation. Sci. Total Environ. 2023, 858, 159943. [Google Scholar] [CrossRef]
- Xie, M.; Lin, L.; Xu, P.; Zhou, W.; Zhao, C.; Ding, D.; Suo, A. Effects of microplastic fibers on lates calcarifer juveniles: Accumulation, oxidative stress, intestine microbiome dysbiosis and histological damage. Ecol. Indic. 2021, 133, 108370. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Xie, J.; Ji, J.; Sun, Y.; Ma, Y.; Wu, D.; Zhang, Z. Blood-brain barrier damage accelerates the accumulation of micro- and nanoplastics in the human central nervous system. J. Hazard. Mater. 2024, 480, 136028. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Bianchi, S.; Giacomelli, G.; Manariti, A.; Vinciguerra, V. Microplastics in fish meal: Contamination level analyzed by polymer type, including polyester (PET), polyolefins, and polystyrene. Environ. Pollut. 2021, 273, 115792. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2017; Volume 111, ISBN 9782831718279. [Google Scholar]
- Wickramasinghe, L.P.; Harris, S.; Jones, G.; Jennings, N.V. Abundance and species richness of nocturnal insects on organic and conventional farms: Effects of agricultural intensification on bat foraging. Conserv. Biol. 2004, 18, 1283–1292. [Google Scholar] [CrossRef]
- Frank, E.G. The economic impacts of ecosystem disruptions: Costs from substituting biological pest control. Science 2024, 385, eadg0344. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.; Zeta-Flores, M.; Ramos-Baldárrago, S.; Tume-Ruiz, J.; Rangel-Vega, A.; Reyes, E.; Quinde, E.; De-la-Torre, G.E.; Lajo-Salazar, L.; Cárdenas-Alayza, S. Terrestrial mammals of the Americas and their interactions with plastic waste. Environ. Sci. Pollut. Res. 2023, 30, 57759–57770. [Google Scholar] [CrossRef]
- Wang, D.; Su, L.; Ruan, H.D.; Chen, J.; Lu, J.; Lee, C.-H.; Jiang, S.Y. Quantitative and qualitative determination of microplastics in oyster, seawater and sediment from the coastal areas in Zhuhai, China. Mar. Pollut. Bull. 2021, 164, 112000. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Valente, A.J.M.; Romano, A.; Antunes, F.E.; Medronho, B. Dissolution of kraft lignin in alkaline solutions. Int. J. Biol. Macromol. 2020, 148, 688–695. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Fischer, E.K. Various digestion protocols within microplastic sample processing—Evaluating the Resistance of different synthetic polymers and the efficiency of biogenic organic matter destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, Isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Ghosal, S.; Chen, M.; Wagner, J.; Wang, Z.M.; Wall, S. Molecular Identification of polymers and anthropogenic particles extracted from oceanic water and fish stomach—A raman micro-spectroscopy study. Environ. Pollut. 2018, 233, 1113–1124. [Google Scholar] [CrossRef]
- Lavoy, M.; Crossman, J. A novel method for organic matter removal from samples containing microplastics. Environ. Pollut. 2021, 286, 117357. [Google Scholar] [CrossRef]
- Ribeiro-Brasil, D.R.G.; Torres, N.R.; Picanço, A.B.; Sousa, D.S.; Ribeiro, V.S.; Brasil, L.S.; Montag, L.F.d.A. Contamination of stream fish by plastic waste in the Brazilian amazon. Environ. Pollut. 2020, 266, 115241. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.M.; Al Najjar, T.; Taupp, T.; Koop, J.H.E. PVC and PET Microplastics in Caddisfly (Lepidostoma Basale) Cases Reduce Case Stability. Environ. Sci. Pollut. Res. 2020, 27, 22380–22389. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Alencastre-Santos, A.; Silva, D.; Ribeiro-Brasil, D.; Correia, L.; Garcia, M.; Vieira, T. Microplastic contamination in Amazon Vampire Bats (Desmodontinae: Phyllostomidae). Diversity 2024, 17, 31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, L.L.; Brasil, A.d.S.; Vieira, T.B.; Garcia, M.G.; Silva, D.d.M.e.; Alencastre-Santos, A.B.; Ribeiro-Brasil, D.R.G. A Simple Way to Quantify Plastic in Bats (Mammalia: Chiroptera) Using an Ultraviolet Flashlight. Methods Protoc. 2025, 8, 80. https://doi.org/10.3390/mps8040080
Correia LL, Brasil AdS, Vieira TB, Garcia MG, Silva DdMe, Alencastre-Santos AB, Ribeiro-Brasil DRG. A Simple Way to Quantify Plastic in Bats (Mammalia: Chiroptera) Using an Ultraviolet Flashlight. Methods and Protocols. 2025; 8(4):80. https://doi.org/10.3390/mps8040080
Chicago/Turabian StyleCorreia, Letícia Lima, Ariane de Sousa Brasil, Thiago Bernardi Vieira, Magali Gonçalves Garcia, Daniela de Melo e Silva, Ana Beatriz Alencastre-Santos, and Danielle Regina Gomes Ribeiro-Brasil. 2025. "A Simple Way to Quantify Plastic in Bats (Mammalia: Chiroptera) Using an Ultraviolet Flashlight" Methods and Protocols 8, no. 4: 80. https://doi.org/10.3390/mps8040080
APA StyleCorreia, L. L., Brasil, A. d. S., Vieira, T. B., Garcia, M. G., Silva, D. d. M. e., Alencastre-Santos, A. B., & Ribeiro-Brasil, D. R. G. (2025). A Simple Way to Quantify Plastic in Bats (Mammalia: Chiroptera) Using an Ultraviolet Flashlight. Methods and Protocols, 8(4), 80. https://doi.org/10.3390/mps8040080







