Investigating the Impact of Pressure Relief Performance on the Occurrence of Pressure Injuries and Shoulder Pain in Wheelchair Users with Spinal Cord Injury (PRperf Study): Study Protocol for a Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedures and Outcomes
2.3. Quality Assurance and Safety Provisions
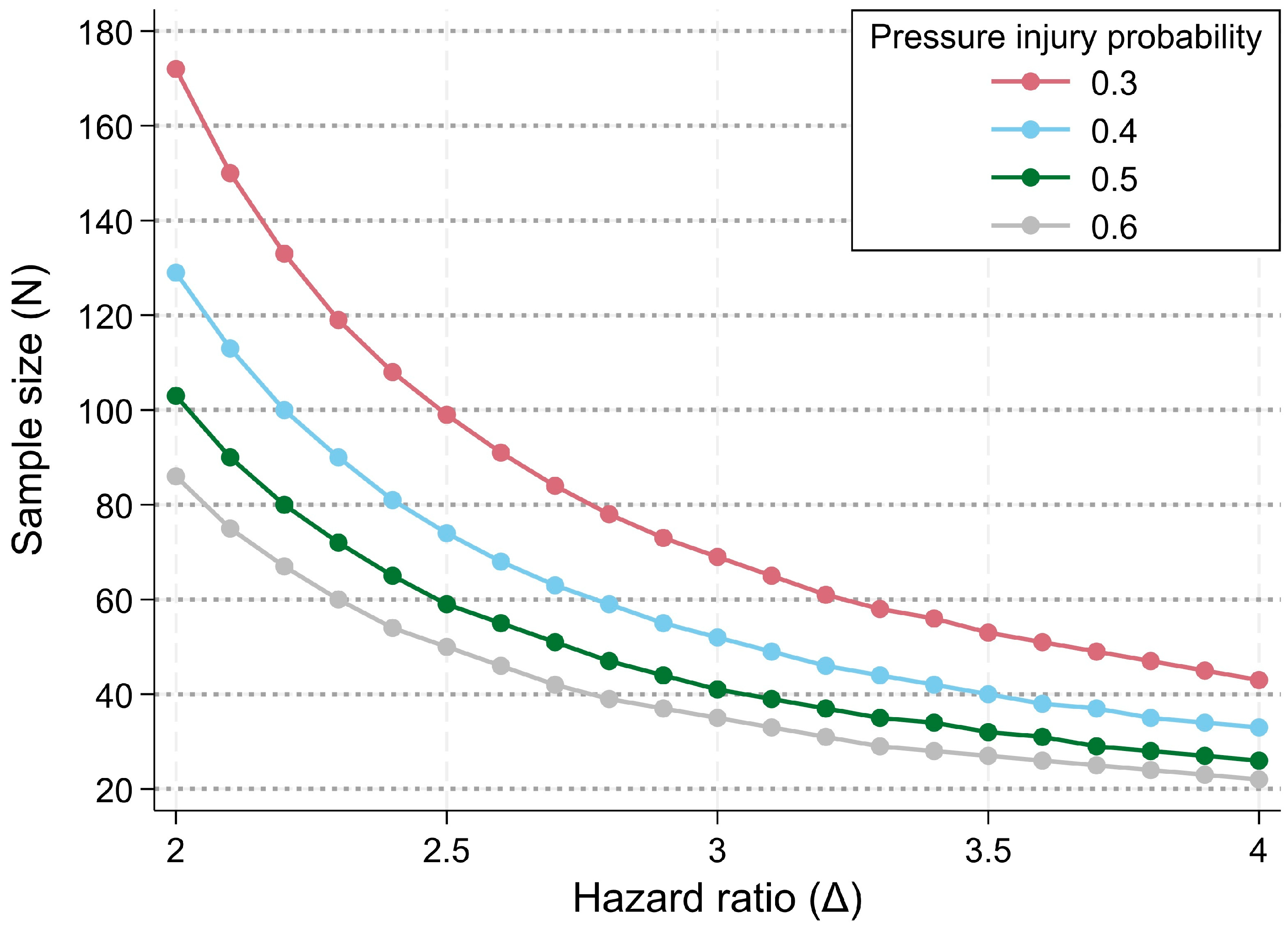
2.4. Data Analysis Plan
2.5. Dissemination Policy
3. Strengths and Limitations
4. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | American Spinal Injury Association Impairment Scale |
| EKNZ | Swiss Ethics Committee for Northwest/Central Switzerland |
| IMU | Inertial measurement unit |
| PI | Pressure injury |
| PR | Pressure relief |
| PRperf | Pressure relief performance |
| SCI | Spinal cord injury |
| SCIM-SR | Spinal Cord Independence Measure Self-Report |
| SP | Shoulder pain |
| SwiSCI | Swiss Spinal Cord Injury cohort study |
| WUSPI | Wheelchair User’s Shoulder Pain Index |
Appendix A
| Exposure and Main Outcomes | Assessment Method | Parameter/Unit | Assessment Timepoints | Literature | |
|---|---|---|---|---|---|
| PR performance | Measured using a textile sensor mat (Sensomative wheelchair, Sensomative GmbH, Rothenburg, Switzerland) | PR technique (weight relief lift, forward lean, side lean, or other) PR frequency (times/h) PR duration (s) | T1+, T2+, T3+, T4+ | [30] | |
| PI occurrence | Questioning | Location and grade (following National Pressure Injury Advisory Panel (NPIAP) pressure injury stages 1 to 4, including unstageable pressure injuries) | Screening, T1, T2, T3, T4, T5/Tocc | [7,46] | |
| SP | Wheelchair User’s Shoulder Pain Index (WUSPI) | Score of 0 to 150 points | T1+, T2+, T3+, T4+ | [40] | |
| Confounder | Assessment Method | Parameter/Unit | Assessment Timepoints | Confounder for | Literature |
| Age | Questioning | Years | Screening | PI, SP | [11,12,36,47,48,49,50] |
| Alcohol consumption | Questioning | Days/week and units/day | T1, T2, T3, T4, T5/Tocc | PI | [51] |
| Anxiety and depression | Hospital Anxiety and Depression Scale (HADS) | Score of 0 to 21 points for anxiety and depression, respectively | T1, T2, T3, T4, T5/Tocc | PI, SP | [48,52] |
| Autonomic dysreflexia | ISAFSCI questionnaire | Binary (yes/no) | T1, T2, T3, T4, T5/Tocc | PI | [10,53] |
| Bed rest during daytime/day | Questioning | Hours | T1+, T2+, T3+, T4+ | PI | |
| Diabetes mellitus | Questioning | Binary (yes/no) | T1, (T2), (T3), (T4), (T5/Tocc) | PI | [50,54,55] |
| Feverish infection | Questioning | Binary (yes/no) | T1, T2, T3, T4, T5/Tocc | PI | [10,54] |
| Height | Questioning | cm | T1 | PI, SP | |
| Incontinence | ISAFSCI questionnaire | Score of 0 to 2 points | T1, T2, T3, T4, T5/Tocc | PI | [11,48,53,54] |
| Independence in activities of daily life | SCIM-SR questionnaire | Score of 0 to 100 points; divisible into three subscales | T1, (T2), (T3), (T4), (T5/Tocc) | PI, SP | [10,54,55,56] |
| Lesion AIS | Questioning, Medical record | Score of A to E | T1, (T2), (T3), (T4), (T5/Tocc) | PI, SP | [2,10,12,36,49,55] |
| Lesion duration | Questioning, Medical record | Years since injury | Screening | PI, SP | [12,22,36,47,49,57] |
| Lesion level | Questioning, Medical record | C1 to S5 | T1, (T2), (T3), (T4), (T5/Tocc) | PI, SP | [2,12,22,49,57] |
| Medication intake | Questioning | Type of medication | T1, T2, T3, T4, T5/Tocc | PI, SP | [47,57] |
| Menopausal status | Questioning | Binary (menopause yes/no) | T1, T2, T3, T4, T5/Tocc | PI | |
| Mobility independence | SCIM-SR questionnaire—Mobility during short distances (10–100 m) | Score of 0 to 8 | Screening, T1, T2, T3, T4, T5/Tocc | PI, SP | [11,56] |
| MVPA duration/week | Questioning | Hours/week | T1, T2, T3, T4, T5/Tocc | PI, SP | |
| PI location | Questioning | Location | T1, T2, T3, T4, T5/Tocc | PI | [11,48,55] |
| PI stage | Questioning | Stage 1 to 4 | T1, T2, T3, T4, T5/Tocc | PI | [7,11,46,48,54] |
| PI treatment | Questioning | Conservative/surgical | T1, T2, T3, T4, T5/Tocc | PI | |
| Pressure relief quality | Pressure mat (ForeSite SS, Xsensor, Calgary, Canada) | % of pressure compared to upright sitting | T1, (T2), (T3), (T4), (T5/Tocc) | PI | |
| RoM upper extremity | Goniometer | Degrees | T1, (T2), (T3), (T4), (T5/Tocc) | SP | [22] |
| Rotator cuff function | Belly-Press Test (also Napoleon-Test) | Binary (positive/negative) | T1, (T2), (T3), (T4), (T5/Tocc) | SP | [58,59] |
| Self-efficacy | General self-efficacy scale | Score from 0 to 40 | T1, T2, T3, T4, T5/Tocc | PI, SP | [60,61,62] |
| Sex | Questioning | Binary (male/female) | Screening | PI, SP | [2,37,49] |
| Shoulder injuries | Questioning | Type of injury | T1, (T2), (T3), (T4), (T5/Tocc) | SP | [12] |
| Shoulder intervention | Questioning | Type of intervention | T1, (T2), (T3), (T4), (T5/Tocc) | SP | |
| Shoulder joint disease/pathology | Questioning | Type of disease/pathology | T1, (T2), (T3), (T4), (T5/Tocc) | SP | [12,22,58] |
| Sitting time/day | Questioning during screening, afterwards using measurement mat (Sensomative) | Hours | Screening, T1+, T2+, T3+, T4+ | PI, SP | |
| Skin check performance | Questioning | Times/week | T1, T2, T3, T4, T5/Tocc | PI | [11] |
| Smoking | Questioning | Cigarettes/day | T1, T2, T3, T4, T5/Tocc | PI | [47,51,55,57] |
| Spasticity lower extremities | Modified Ashworth Scale | Score from 0 to 4 | T1, (T2), (T3), (T4), (T5/Tocc) | PI | [2,48,63] |
| Spasticity upper extremities | Modified Ashworth Scale | Score from 0 to 4 | T1, (T2), (T3), (T4), (T5/Tocc) | SP | [2,48,63] |
| SR-PR performance | Questioning | Times/hour and seconds/PR | T1+, T2+, T3+, T4+ | PI | |
| Transfer number/day | Measurement mat (Sensomative) | Times/day | T1+, T2+, T3+, T4+ | PI, SP | [11,64] |
| Transfer quality | Transfer Assessment Instrument | Score from 0 to 10 | T1, (T2), (T3), (T4), (T5/Tocc) | PI, SP | [11,64,65,66] |
| Weight | Measurement on a wheelchair-accessible scale, measurement of wheelchair separately from participant | kg | T1, (T2), (T3), (T4), (T5/Tocc) | PI, SP | [11,12,22,48,50,55] |
| Wheelchair hip angle | Goniometer | Degrees | T1, (T2), (T3), (T4), (T5/Tocc) | PI | [12] |
| Wheelchair shoulder position | Assessment | In front of hip/above hip/behind hip | T1, (T2), (T3), (T4), (T5/Tocc) | SP | [12] |
| Wheelchair cushion type | Questioning | Cushion brand and model | T1, (T2), (T3), (T4), (T5/Tocc) | PI | [11,67] |
| Wheelchair mobility | Wearable IMU sensor attached to wheelchair wheel (Axivity AX6, Axivity Ltd., Newcastle upon Tyne, UK) | Distance covered (km/day), linear velocity of the wheelchair (km/h), number of pushes (pushes/day), duration of pushes (s/push), number of turns (turns/day), magnitude of turns (degrees/turn) | T1+, T2+, T3+, T4+ | PI, SP | [22,39] |
References
- Anthony, D.; Alosaimi, D.; Shiferaw, W.S.; Korsah, K.; Safari, R. Prevalence of pressure ulcers in africa: A systematic review and meta-analysis. J. Tissue Viability 2021, 30, 137–145. [Google Scholar] [CrossRef]
- Bossuyt, F.M.; Arnet, U.; Brinkhof, M.W.G.; Eriks-Hoogland, I.; Lay, V.; Muller, R.; Sunnaker, M.; Hinrichs, T. Shoulder pain in the Swiss spinal cord injury community: Prevalence and associated factors. Disabil. Rehabil. 2018, 40, 798–805. [Google Scholar] [CrossRef]
- Liampas, A.; Neophytou, P.; Sokratous, M.; Varrassi, G.; Ioannou, C.; Hadjigeorgiou, G.M.; Zis, P. Musculoskeletal pain due to wheelchair use: A systematic review and meta-analysis. Pain Ther. 2021, 10, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, W.S.; Akalu, T.Y.; Mulugeta, H.; Aynalem, Y.A. The global burden of pressure ulcers among patients with spinal cord injury: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 334. [Google Scholar] [CrossRef]
- Richardson, A.; Samaranayaka, A.; Sullivan, M.; Derrett, S. Secondary health conditions and disability among people with spinal cord injury: A prospective cohort study. J. Spinal Cord Med. 2021, 44, 19–28. [Google Scholar] [CrossRef]
- Chen, H.L.; Cai, J.Y.; Du, L.; Shen, H.W.; Yu, H.R.; Song, Y.P.; Zha, M.L. Incidence of pressure injury in individuals with spinal cord injury: A systematic review and meta-analysis. J. Wound Ostomy Cont. Nurs. 2020, 47, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Edsberg, L.E.; Black, J.M.; Goldberg, M.; McNichol, L.; Moore, L.; Sieggreen, M. Revised National Pressure Ulcer Advisory Panel pressure injury staging system: Revised pressure injury staging system. J. Wound Ostomy Cont. Nurs. 2016, 43, 585–597. [Google Scholar] [CrossRef]
- Vecin, N.M.; Gater, D.R. Pressure injuries and management after spinal cord injury. J. Pers. Med. 2022, 12, 1130. [Google Scholar] [CrossRef]
- Sprigle, S.; Sonenblum, S. Assessing evidence supporting redistribution of pressure for pressure ulcer prevention: A review. J. Rehabil. Res. Dev. 2011, 48, 203–213. [Google Scholar] [CrossRef]
- Najmanova, K.; Neuhauser, C.; Krebs, J.; Baumberger, M.; Schaefer, D.J.; Sailer, C.O.; Wettstein, R.; Scheel-Sailer, A. Risk factors for hospital acquired pressure injury in patients with spinal cord injury during first rehabilitation: Prospective cohort study. Spinal Cord 2022, 60, 45–52. [Google Scholar] [CrossRef]
- Benbow, M. Pressure ulcer prevention and pressure-relieving surfaces. Br. J. Nurs. 2008, 17, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Dyson-Hudson, T.A.; Kirshblum, S.C. Shoulder pain in chronic spinal cord injury, Part I: Epidemiology, etiology, and pathomechanics. J. Spinal Cord Med. 2004, 27, 4–17. [Google Scholar] [CrossRef]
- Cao, Y.; Krause, J.S. The association between secondary health conditions and indirect costs after spinal cord injury. Spinal Cord 2021, 59, 306–310. [Google Scholar] [CrossRef]
- Dryden, D.M.; Saunders, L.D.; Rowe, B.H.; May, L.A.; Yiannakoulias, N.; Svenson, L.W.; Schopflocher, D.P.; Voaklander, D.C. Utilization of health services following spinal cord injury: A 6-year follow-up study. Spinal Cord 2004, 42, 513–525. [Google Scholar] [CrossRef]
- Gould, L.J.; Alderden, J.; Aslam, R.; Barbul, A.; Bogie, K.M.; El Masry, M.; Graves, L.Y.; White-Chu, E.F.; Ahmed, A.; Boanca, K.; et al. WHS guidelines for the treatment of pressure ulcers—2023 update. Wound Repair. Regen. 2024, 32, 6–33. [Google Scholar] [CrossRef] [PubMed]
- Young, D.L.; Shen, J.J.; Estocado, N.; Landers, M.R. Financial impact of improved pressure ulcer staging in the acute hospital with use of a new tool, the NE1 Wound Assessment Tool. Adv. Skin Wound Care 2012, 25, 158–166. [Google Scholar] [CrossRef] [PubMed]
- White, B.A.B.; Dea, N.; Street, J.T.; Cheng, C.L.; Rivers, C.S.; Attabib, N.; Kwon, B.K.; Fisher, C.G.; Dvorak, M.F. The economic burden of urinary tract infection and pressure ulceration in acute traumatic spinal cord injury admissions: Evidence for comparative economics and decision analytics from a matched case-control study. J. Neurotrauma 2017, 34, 2892–2900. [Google Scholar] [CrossRef]
- Smit, C.A.; Zwinkels, M.; van Dijk, T.; de Groot, S.; Stolwijk-Swuste, J.M.; Janssen, T.W. Gluteal blood flow and oxygenation during electrical stimulation-induced muscle activation versus pressure relief movements in wheelchair users with a spinal cord injury. Spinal Cord 2013, 51, 694–699. [Google Scholar] [CrossRef]
- Sonenblum, S.E.; Sprigle, S.H.; Martin, J.S. Everyday sitting behavior of full-time wheelchair users. J. Rehabil. Res. Dev. 2016, 53, 585–598. [Google Scholar] [CrossRef]
- European Pressure Ulcer Advisory Panel; National Pressure Ulcer Advisory Panel; Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide, 2nd ed.; Cambridge Media: Osborne Park, WA, Australia, 2014. [Google Scholar]
- Sonenblum, S.E.; Vonk, T.E.; Janssen, T.W.; Sprigle, S.H. Effects of wheelchair cushions and pressure relief maneuvers on ischial interface pressure and blood flow in people with spinal cord injury. Arch. Phys. Med. Rehabil. 2014, 95, 1350–1357. [Google Scholar] [CrossRef]
- Arnet, U.; Boninger, M.L.; Cools, A.; Bossuyt, F.M. Effect of fatiguing wheelchair propulsion and weight relief lifts on subacromial space in wheelchair users. Front. Rehabil. Sci. 2022, 3, 849629. [Google Scholar] [CrossRef] [PubMed]
- van Drongelen, S.; van der Woude, L.H.; Janssen, T.W.; Angenot, E.L.; Chadwick, E.K.; Veeger, D.H. Glenohumeral contact forces and muscle forces evaluated in wheelchair-related activities of daily living in able-bodied subjects versus subjects with paraplegia and tetraplegia. Arch. Phys. Med. Rehabil. 2005, 86, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Nawoczenski, D.A.; Clobes, S.M.; Gore, S.L.; Neu, J.L.; Olsen, J.E.; Borstad, J.D.; Ludewig, P.M. Three-dimensional shoulder kinematics during a pressure relief technique and wheelchair transfer. Arch. Phys. Med. Rehabil. 2003, 84, 1293–1300. [Google Scholar] [CrossRef]
- State Spinal Cord Injury Service. Spinal Seating Modules: Module 8—Pressure Management. Available online: https://aci.health.nsw.gov.au/networks/spinal-cord-injury/spinal-seating/module-8/recognizing-key-relationships-between-the-client-and-wheelchair#_ftn1 (accessed on 10 April 2025).
- Northwest Regional Spinal Cord Injury System. Spinal Cord Injury Model Systems Consumer Information: Skin Care & Pressure Sores. Available online: http://sci.washington.edu/info/pamphlets/msktc-pressure_relief.asp (accessed on 10 April 2025).
- Coggrave, M.J.; Rose, L.S. A specialist seating assessment clinic: Changing pressure relief practice. Spinal Cord 2003, 41, 692–695. [Google Scholar] [CrossRef]
- Schofield, R.; Porter-Armstrong, A.; Stinson, M. Reviewing the literature on the effectiveness of pressure relieving movements. Nurs. Res. Pract. 2013, 2013, 124095. [Google Scholar] [CrossRef]
- Sprigle, S.; Sonenblum, S.E.; Feng, C. Pressure redistributing in-seat movement activities by persons with spinal cord injury over multiple epochs. PLoS ONE 2019, 14, e0210978. [Google Scholar] [CrossRef]
- Hubli, M.; Zemp, R.; Albisser, U.; Camenzind, F.; Leonova, O.; Curt, A.; Taylor, W.R. Feedback improves compliance of pressure relief activities in wheelchair users with spinal cord injury. Spinal Cord 2021, 59, 175–184. [Google Scholar] [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gotzsche, P.C.; Krleza-Jeric, K.; Hrobjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Post, M.W.; Brinkhof, M.W.; von Elm, E.; Boldt, C.; Brach, M.; Fekete, C.; Eriks-Hoogland, I.; Curt, A.; Stucki, G.; SwiSCI Study Group. Design of the Swiss Spinal Cord Injury Cohort Study. Am. J. Phys. Med. Rehabil. 2011, 90, S5–S16. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane Collaboration: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 10 April 2025).
- Gross-Hemmi, M.H.; Gemperli, A.; Fekete, C.; Brach, M.; Schwegler, U.; Stucki, G. Methodology and study population of the second Swiss national community survey of functioning after spinal cord injury. Spinal Cord 2021, 59, 363–372. [Google Scholar] [CrossRef]
- Raghavan, P.; Raza, W.A.; Ahmed, Y.S.; Chamberlain, M.A. Prevalence of pressure sores in a community sample of spinal injury patients. Clin. Rehabil. 2003, 17, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Scheel-Sailer, A.; Wyss, A.; Boldt, C.; Post, M.W.; Lay, V. Prevalence, location, grade of pressure ulcers and association with specific patient characteristics in adult spinal cord injury patients during the hospital stay: A prospective cohort study. Spinal Cord 2013, 51, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Sprigle, S.; McNair, D.; Sonenblum, S. Pressure ulcer risk factors in persons with mobility-related disabilities. Adv. Skin Wound Care 2020, 33, 146–154. [Google Scholar] [CrossRef]
- Verschueren, J.H.; Post, M.W.; de Groot, S.; van der Woude, L.H.; van Asbeck, F.W.; Rol, M. Occurrence and predictors of pressure ulcers during primary in-patient spinal cord injury rehabilitation. Spinal Cord 2011, 49, 106–112. [Google Scholar] [CrossRef]
- de Vries, W.H.K.; van der Slikke, R.M.A.; van Dijk, M.P.; Arnet, U. Real-life wheelchair mobility metrics from IMUs. Sensors 2023, 23, 7174. [Google Scholar] [CrossRef]
- Curtis, K.A.; Roach, K.E.; Applegate, E.B.; Amar, T.; Benbow, C.S.; Genecco, T.D.; Gualano, J. Reliability and validity of the Wheelchair User’s Shoulder Pain Index (WUSPI). Paraplegia 1995, 33, 595–601. [Google Scholar] [CrossRef]
- Brosteanu, O.; Houben, P.; Ihrig, K.; Ohmann, C.; Paulus, U.; Pfistner, B.; Schwarz, G.; Strenge-Hesse, A.; Zettelmeyer, U. Risk analysis and risk adapted on-site monitoring in noncommercial clinical trials. Clin. Trials 2009, 6, 585–596. [Google Scholar] [CrossRef]
- Zemp, R.; Fliesser, M.; Wippert, P.M.; Taylor, W.R.; Lorenzetti, S. Occupational sitting behaviour and its relationship with back pain—A pilot study. Appl. Ergon. 2016, 56, 84–91. [Google Scholar] [CrossRef]
- Zemp, R.; Tanadini, M.; Pluss, S.; Schnuriger, K.; Singh, N.B.; Taylor, W.R.; Lorenzetti, S. Application of Machine Learning Approaches for Classifying Sitting Posture Based on Force and Acceleration Sensors. Biomed. Res. Int. 2016, 2016, 5978489. [Google Scholar] [CrossRef]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Carpenter, J.R.; Smuk, M. Missing data: A statistical framework for practice. Biom. J. 2021, 63, 915–947. [Google Scholar] [CrossRef] [PubMed]
- National Pressure Injury Advisory Panel. NPUAP Pressure Injury Stages. Available online: https://cdn.ymaws.com/npiap.com/resource/resmgr/online_store/npiap_pressure_injury_stages.pdf (accessed on 10 April 2025).
- Gould, L.J.; Bohn, G.; Bryant, R.; Paine, T.; Couch, K.; Cowan, L.; McFarland, F.; Simman, R. Pressure ulcer summit 2018: An interdisciplinary approach to improve our understanding of the risk of pressure-induced tissue damage. Wound Repair Regen. 2019, 27, 497–508. [Google Scholar] [CrossRef]
- Hajhosseini, B.; Longaker, M.T.; Gurtner, G.C. Pressure Injury. Ann. Surg. 2020, 271, 671–679. [Google Scholar] [CrossRef]
- Kentar, Y.; Zastrow, R.; Bradley, H.; Brunner, M.; Pepke, W.; Bruckner, T.; Raiss, P.; Hug, A.; Almansour, H.; Akbar, M. Prevalence of upper extremity pain in a population of people with paraplegia. Spinal Cord 2018, 56, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Bien, M.; Dumond, K.; Brown, K.; Myers, A. Etiology and incidence of pressure ulcers in surgical patients. AORN J. 1999, 70, 434–449. [Google Scholar] [CrossRef]
- Li, C.; DiPiro, N.D.; Cao, Y.; Szlachcic, Y.; Krause, J. The association between metabolic syndrome and pressure ulcers among individuals living with spinal cord injury. Spinal Cord 2016, 54, 967–972. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Wecht, J.M.; Krassioukov, A.V.; Alexander, M.; Handrakis, J.P.; McKenna, S.L.; Kennelly, M.; Trbovich, M.; Biering-Sorensen, F.; Burns, S.; Elliott, S.L.; et al. International Standards to document Autonomic Function following SCI (ISAFSCI): Second Edition. Top. Spinal Cord Inj. Rehabil. 2021, 27, 23–49. [Google Scholar] [CrossRef]
- Coleman, S.; Gorecki, C.; Nelson, E.A.; Closs, S.J.; Defloor, T.; Halfens, R.; Farrin, A.; Brown, J.; Schoonhoven, L.; Nixon, J. Patient risk factors for pressure ulcer development: Systematic review. Int. J. Nurs. Stud. 2013, 50, 974–1003. [Google Scholar] [CrossRef]
- Gould, L.J.; Olney, C.M.; Nichols, J.S.; Block, A.R.; Simon, R.M.; Guihan, M. Spinal cord injury survey to determine pressure ulcer vulnerability in the outpatient population. Med. Hypotheses 2014, 83, 552–558. [Google Scholar] [CrossRef]
- Fekete, C.; Eriks-Hoogland, I.; Baumberger, M.; Catz, A.; Itzkovich, M.; Luthi, H.; Post, M.W.; von Elm, E.; Wyss, A.; Brinkhof, M.W. Development and validation of a self-report version of the Spinal Cord Independence Measure (SCIM III). Spinal Cord 2013, 51, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; DiPiro, N.D.; Krause, J. A latent structural equation model of risk behaviors and pressure ulcer outcomes among people with spinal cord injury. Spinal Cord 2017, 55, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S. Rotator cuff tendinopathy: A model for the continuum of pathology and related management. Br. J. Sports Med. 2010, 44, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Tokish, J.M.; Decker, M.J.; Ellis, H.B.; Torry, M.R.; Hawkins, R.J. The belly-press test for the physical examination of the subscapularis muscle: Electromyographic validation and comparison to the lift-off test. J. Shoulder Elbow Surg. 2003, 12, 427–430. [Google Scholar] [CrossRef]
- Hug, K.; Stumm, C.; Debecker, I.; Fellinghauer, C.S.; Peter, C.; Hund-Georgiadis, M. Self-efficacy and pressure ulcer prevention after spinal cord injury-results from a nationwide community survey in Switzerland (SwiSCI). PM&R 2018, 10, 573–586. [Google Scholar] [CrossRef]
- King, R.B.; Champion, V.L.; Chen, D.; Gittler, M.S.; Heinemann, A.W.; Bode, R.K.; Semik, P. Development of a measure of skin care belief scales for persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2012, 93, 1814–1821. [Google Scholar] [CrossRef]
- Lazić, M.; Jovanović, V.; Gavrilov-Jerković, V. The general self-efficacy scale: New evidence of structural validity, measurement invariance, and predictive properties in relationship to subjective well-being in Serbian samples. Curr. Psychol. 2021, 40, 699–710. [Google Scholar] [CrossRef]
- Meseguer-Henarejos, A.B.; Sanchez-Meca, J.; Lopez-Pina, J.A.; Carles-Hernandez, R. Inter- and intra-rater reliability of the Modified Ashworth Scale: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 576–590. [Google Scholar] [CrossRef]
- Gagnon, D.; Nadeau, S.; Noreau, L.; Dehail, P.; Piotte, F. Comparison of peak shoulder and elbow mechanical loads during weight-relief lifts and sitting pivot transfers among manual wheelchair users with spinal cord injury. J. Rehabil. Res. Dev. 2008, 45, 863–873. [Google Scholar] [CrossRef]
- Hogaboom, N.S.; Worobey, L.A.; Boninger, M.L. Transfer technique is associated with shoulder pain and pathology in people with spinal cord injury: A cross-sectional investigation. Arch. Phys. Med. Rehabil. 2016, 97, 1770–1776. [Google Scholar] [CrossRef]
- Worobey, L.A.; Zigler, C.K.; Huzinec, R.; Rigot, S.K.; Sung, J.; Rice, L.A. Reliability and validity of the revised Transfer Assessment Instrument. Top. Spinal Cord Inj. Rehabil. 2018, 24, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ong, B.; Wilson, J.R.; Henzel, M.K. Management of the patient with chronic spinal cord injury. Med. Clin. N. Am. 2020, 104, 263–278. [Google Scholar] [CrossRef] [PubMed]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schürch, Y.; Hertig-Godeschalk, A.; Eriks-Hoogland, I.; Scheel-Sailer, A.; Brinkhof, M.W.G.; Arnet, U. Investigating the Impact of Pressure Relief Performance on the Occurrence of Pressure Injuries and Shoulder Pain in Wheelchair Users with Spinal Cord Injury (PRperf Study): Study Protocol for a Prospective Observational Study. Methods Protoc. 2025, 8, 62. https://doi.org/10.3390/mps8030062
Schürch Y, Hertig-Godeschalk A, Eriks-Hoogland I, Scheel-Sailer A, Brinkhof MWG, Arnet U. Investigating the Impact of Pressure Relief Performance on the Occurrence of Pressure Injuries and Shoulder Pain in Wheelchair Users with Spinal Cord Injury (PRperf Study): Study Protocol for a Prospective Observational Study. Methods and Protocols. 2025; 8(3):62. https://doi.org/10.3390/mps8030062
Chicago/Turabian StyleSchürch, Yannik, Anneke Hertig-Godeschalk, Inge Eriks-Hoogland, Anke Scheel-Sailer, Martin W. G. Brinkhof, and Ursina Arnet. 2025. "Investigating the Impact of Pressure Relief Performance on the Occurrence of Pressure Injuries and Shoulder Pain in Wheelchair Users with Spinal Cord Injury (PRperf Study): Study Protocol for a Prospective Observational Study" Methods and Protocols 8, no. 3: 62. https://doi.org/10.3390/mps8030062
APA StyleSchürch, Y., Hertig-Godeschalk, A., Eriks-Hoogland, I., Scheel-Sailer, A., Brinkhof, M. W. G., & Arnet, U. (2025). Investigating the Impact of Pressure Relief Performance on the Occurrence of Pressure Injuries and Shoulder Pain in Wheelchair Users with Spinal Cord Injury (PRperf Study): Study Protocol for a Prospective Observational Study. Methods and Protocols, 8(3), 62. https://doi.org/10.3390/mps8030062









