Optimization of Nile Tilapia Artificial Breeding Using Human Chorionic Gonadotropin (hCG) Hormone
Abstract
1. Introduction
2. Experimental Design
Materials
- Mature male and mature female Nile tilapia (Oreochromis niloticus);
- Human chorionic gonadotropin (Merck, Darmstadt, Germany);
- NaCl (sodium chloride 98.80%) (Maldon, Essex, UK);
- Nitrate-Nitrite (0–40 mg/L NO3−-N, 0.015–0.60 mg/L NO2−-N) and ammonia test kit (0–2.4 mg/L NH3-N) (product numbers: 1408100 and 2428700, Hach, Loveland, CO, USA);
- 100 L plastic buckets;
- Heater (EHEIM thermocontrol 150 and 300 Watt, cat-4011708011713 and 4011708011744, Eheim GmbH & Co., Deizisau, Germany);
- Aerator (TetraTec APS 120-400, 400 L/h 4.5 watts, Tetra GmbH, Melle, Germany);
- Syringe (0.3 mL, Beckton-Dickinson, Oslo, Norway);
- Needle (Gauge 22, WVR, Radnor, PA, USA);
- Borosilicate glass beaker (500 mL);
- Aquarium tanks (500 L);
- Fine mesh net (7.5 cm)
- Lamp (Unilite SLR-3000, Worcestershire, UK);
- Timer (Bachmann, Bremen, Germany);
- Egg rocker (5 L capacity) (item number 1407, FIAP GmbH, Ursensollen, Germany);
- Air pump (Eheim Compacton 600 Pump, Deizisau, Germany);
- EBI temperature probe (item no. 620-1860P, WVR, Radnor, PA, USA);
- pH meter (item no. 665-0499P, WVR, Radnor, PA, USA);
- Oxygen meter (H05: Polaris C, Oxyguard, Farum, Denmark);
- Leica MZ16 FA dissecting stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany).
3. Procedure
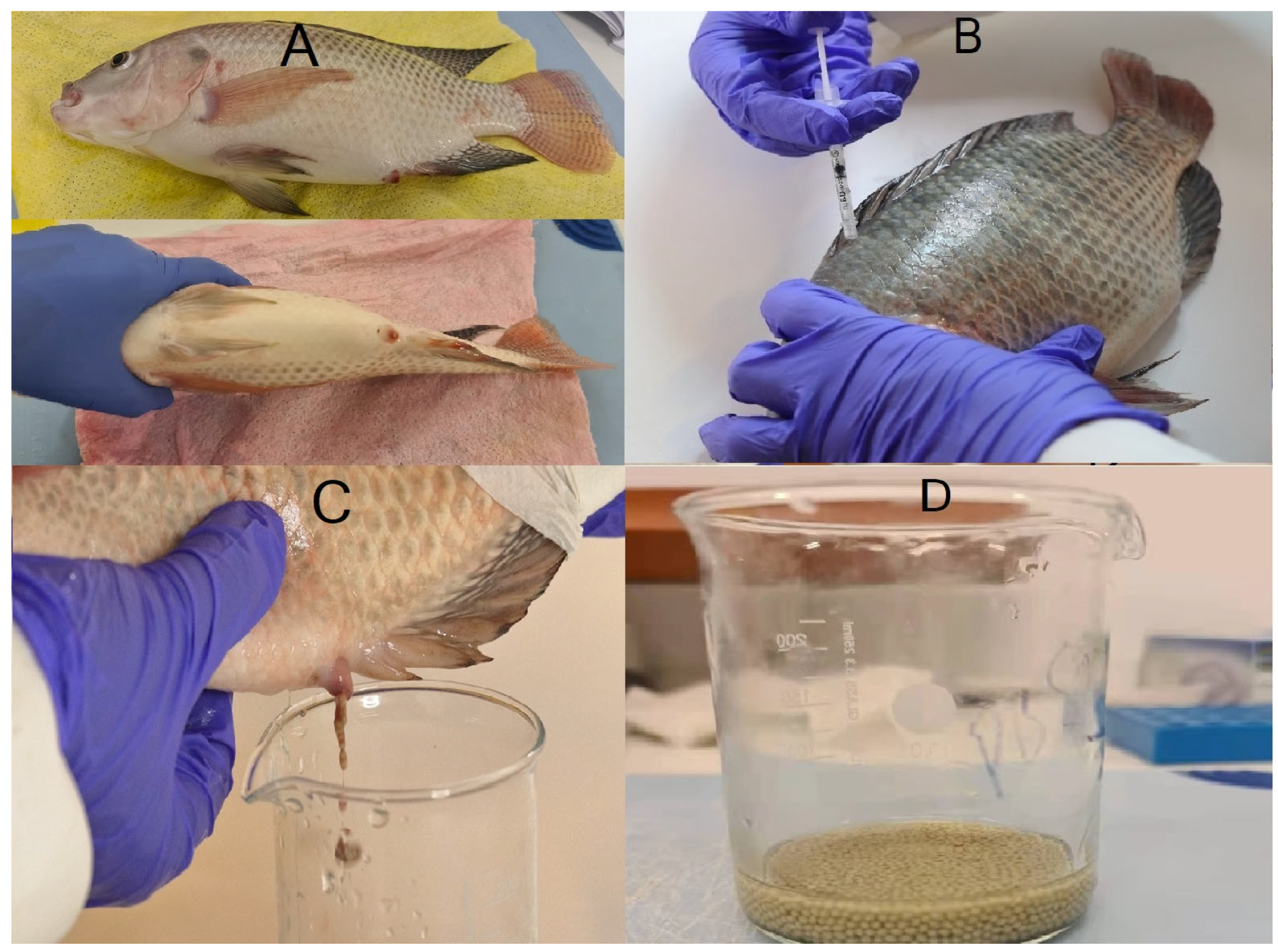
3.1. Selection of Female Tilapia
3.2. Hormone Induction
3.3. Post-Injection Care
3.4. Selection of Male Tilapia
3.5. Egg Collection
3.6. Sperm Collection and Fertilization
3.7. Washing Fertilized Eggs
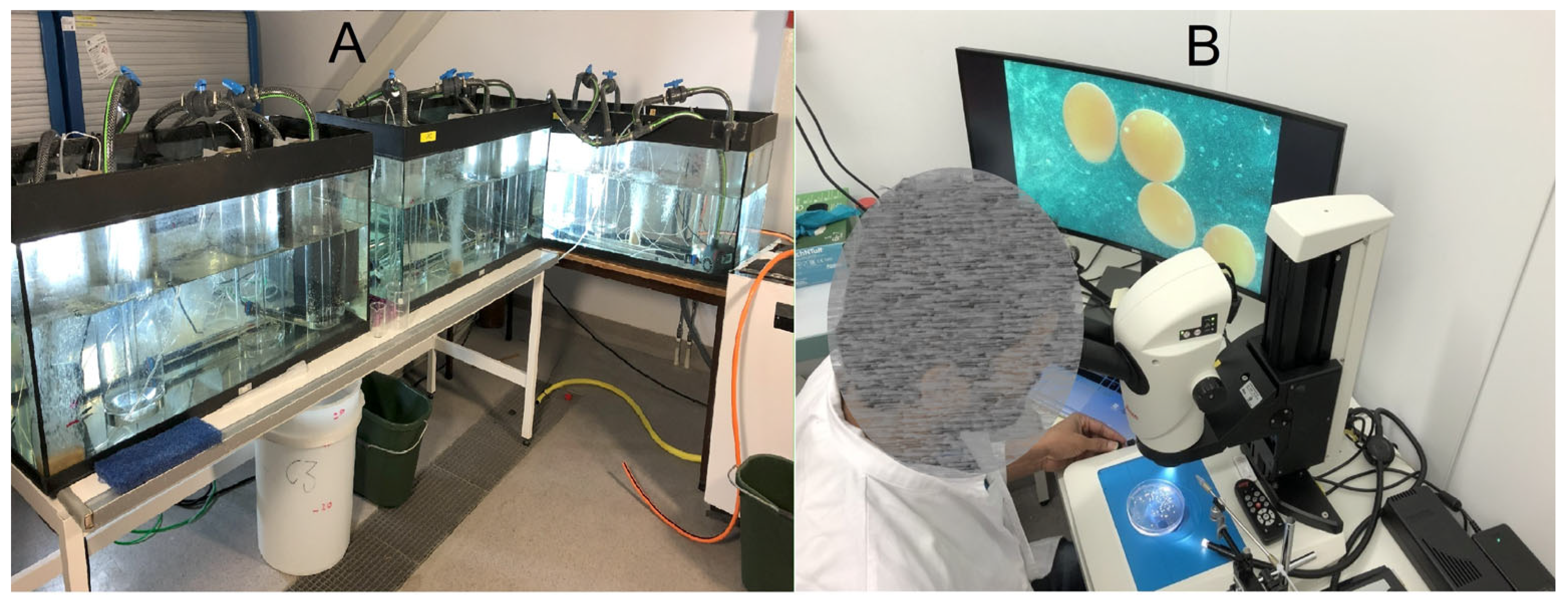
3.8. Incubation
3.9. Water Quality Parameters
3.10. Photoperiod
3.11. Monitoring the Fertilized Eggs
3.12. Data Collection
4. Results and Discussion
- Breeders should be maintained in a controlled wet lab environment for proper evaluation, ensuring the selection of superior females. Housing selected males and females in separate aquarium systems facilitates female maturation for spawning and allows more effective monitoring and management.
- Throughout the incubation process, we carefully monitored the eggs, removing any dead embryos to prevent deterioration of water quality.
- Sperm was stripped from the male and directly added to the eggs to ensure its viability. This immediate mixing helps maximize the chances of successful fertilization.
- It is essential to keep the eggs in motion at the bottom of the egg rockers to ensure successful development.
- The water in the tanks was changed once daily and replaced (75%) with freshwater manually using a bucket.
- When calculating the concentration of hormones used during breeding, particular attention should be given, as even slight variations in hormone dosage can have a significant impact on fertilization rates and larval survival. Proper dosing is crucial to ensuring successful reproduction and optimal outcomes in the breeding process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Li, M.; Sun, L.; Zhou, L.; Wang, D. Tilapia, a good model for studying reproductive endocrinology. Gen. Comp. Endocrinol. 2024, 345, 114395. [Google Scholar] [CrossRef] [PubMed]
- Rbbani, G.; Murshed, R.; Siriyappagouder, P.; Sharko, F.; Nedoluzhko, A.; Joshi, R.; Galindo-Villegas, J.; Raeymaekers, J.A.; Fernandes, J.M. Embryonic temperature has long-term effects on muscle circRNA expression and somatic growth in Nile tilapia. Front. Cell Dev. Biol. 2024, 12, 1369758. [Google Scholar] [CrossRef]
- Rbbani, G.; Nedoluzhko, A.; Siriyappagouder, P.; Sharko, F.; Galindo-Villegas, J.; Raeymaekers, J.A.M.; Joshi, R.; Fernandes, J.M.O. The novel circular RNA CircMef2c is positively associated with muscle growth in Nile tilapia. Genomics 2023, 115, 110598. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.F.M.; Fitzsimmons, K. From Africa to the world—The journey of Nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Abaho, I.; Masembe, C.; Akoll, P.; Jones, C.L. The use of plant extracts to control tilapia reproduction: Current status and future perspectives. J. World Aquac. Soc. 2022, 53, 593–619. [Google Scholar] [CrossRef]
- Biswas, A.K.; Morita, T.; Yoshizaki, G.; Maita, M.; Takeuchi, T. Control of reproduction in Nile tilapia Oreochromis niloticus (L.) by photoperiod manipulation. Aquaculture 2005, 243, 229–239. [Google Scholar] [CrossRef]
- Arumugam, M.; Jayaraman, S.; Sridhar, A.; Venkatasamy, V.; Brown, P.B.; Abdul Kari, Z.; Tellez-Isaias, G.; Ramasamy, T. Recent advances in tilapia production for sustainable developments in Indian aquaculture and its economic benefits. Fishes 2023, 8, 176. [Google Scholar] [CrossRef]
- Akian, D.D.; Quenum, C.L.; Koua, D.N.Z.; Houra, J.A.K.; Clota, F.; Bégout, M.L.; Yao, K. Comparative study of larvae production by the Nile tilapia (Oreochromis niloticus, Linné, 1758) Bouaké strain between earthen ponds and hapas. Aquac. Res. 2022, 53, 6049–6055. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef]
- Azevedo, R.O.; de Alvarenga, É.R.; Fernandes, A.F.A.; da Silva, M.A.; Alves, G.F.d.O.; Menezes, W.F.; Turra, E.M. Use of hCG hormone in the natural and artificial reproduction of Nile tilapia (Oreochromis niloticus). Aquac. Res. 2021, 52, 6380–6388. [Google Scholar] [CrossRef]
- Fernandes, A.; Alvarenga, É.; Oliveira, D.; Aleixo, C.; Prado, S.; Luz, R.; Sarmento, N.; Teixeira, E.; Luz, M.; Turra, E. Production of oocytes of Nile tilapia (Oreochromis niloticus) for in vitro fertilization via hormonal treatments. Reprod. Domest. Anim. 2013, 48, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.; Lin, H.; Kraak, G.v.d.; Little, M. Releasing Hormones, Dopamine Antagonists and Induced Spawning. In Recent Advances in Aquaculture; Blackwell Scientific: Oxford, UK, 1993. [Google Scholar]
- Acharjee, A.; Chaube, R.; Joy, K. Ovaprim, a commercial spawning inducer, stimulates gonadotropin subunit gene transcriptional activity: A study correlated with plasma steroid profile, ovulation and fertilization in the catfish Heteropneustes fossilis. Gen. Comp. Endocrinol. 2017, 251, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Reynalte-Tataje, D.; Lopes, C.; Ávila-Simas, S.; Garcia, J.; Zaniboni-Filho, E. Artificial reproduction of neotropical fish: Extrusion or natural spawning? Nat. Sci. 2013, 5, 1–6. [Google Scholar] [CrossRef]
- Yasui, G.S.; Ferreira do Nascimento, N.; Pereira-Santos, M.; Santos Silva, A.P.d.; Coelho, G.C.Z.; Visintin, J.A.; Porto-Foresti, F.; Okada Nakaghi, L.S.; Vianna, N.C.; Carvalho, G.B. Establishing a model fish for the Neotropical region: The case of the yellowtail tetra Astyanax altiparanae in advanced biotechnology. Front. Genet. 2022, 13, 903990. [Google Scholar] [CrossRef]
- Tuzine, T.A.R.; Freitas, R.T.d.F.d.; Gaya, L.d.G.; Paula, D.A.d.J.; Tuzine, M.S.; Felizardo, V.d.O. Hormonal induction and synchronization in the reproduction of nile tilapia (Oreochromis Niloticus). Bol. De Indústria Anim. 2022, 79, 1–14. [Google Scholar] [CrossRef]
- El-Gamal, A.; El-Greisy, Z.A. Effect of photoperiod, temperature and HCG on ovarian recrudescence and ability of spawning in Nile tilapia Oreochromis niloticus (Teleostei, Cichlidae). Egypt. J. Aquat. Res. 2005, 31, 419–431. [Google Scholar]
- Campos-Mendoza, A.; McAndrew, B.; Coward, K.; Bromage, N. Reproductive response of Nile tilapia (Oreochromis niloticus) to photoperiodic manipulation; effects on spawning periodicity, fecundity and egg size. Aquaculture 2004, 231, 299–314. [Google Scholar] [CrossRef]
- Piamsomboon, P.; Mehl, N.S.; Sirivaidyapong, S.; Wongtavatchai, J. Assisted reproduction in Nile tilapia Oreochromis niloticus: Milt preservation, spawning induction and artificial fertilization. Aquaculture 2019, 507, 139–143. [Google Scholar] [CrossRef]
- Yoshida, G.M.; Oliveira, C.A.L.d.; Kunita, N.M.; Rizzato, G.S.; Ribeiro, R.P. Reproduction performance of female Nile tilapia under different environments and age classes. Acta Scientiarum. Anim. Sci. 2015, 37, 221–226. [Google Scholar] [CrossRef]
- Bezault, E.; Clota, F.; Derivaz, M.; Chevassus, B.; Baroiller, J.-F. Sex determination and temperature-induced sex differentiation in three natural populations of Nile tilapia (Oreochromis niloticus) adapted to extreme temperature conditions. Aquaculture 2007, 272, S3–S16. [Google Scholar] [CrossRef]
- Nivelle, R.; Gennotte, V.; Kalala, E.J.K.; Ngoc, N.B.; Muller, M.; Mélard, C.; Rougeot, C. Temperature preference of Nile tilapia (Oreochromis niloticus) juveniles induces spontaneous sex reversal. PLoS ONE 2019, 14, e0214689. [Google Scholar]
- Li, M.; Yang, H.; Zhao, J.; Fang, L.; Shi, H.; Li, M.; Sun, Y.; Zhang, X.; Jiang, D.; Zhou, L. Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetics 2014, 197, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Dai, S.; Xiao, H.; Wang, D. High efficiency targeting of non-coding sequences using CRISPR/Cas9 system in Tilapia. G3 Genes Genomes Genet. 2019, 9, 287–295. [Google Scholar] [CrossRef]
- Segev-Hadar, A.; Slosman, T.; Rozen, A.; Sherman, A.; Cnaani, A.; Biran, J. Genome editing using the CRISPR-Cas9 system to generate a solid-red germline of Nile tilapia (Oreochromis niloticus). CRISPR J. 2021, 4, 583–594. [Google Scholar] [CrossRef]
| Group | Replicate | Total Eggs | Dead Eggs | Fertilized Eggs | Fertilization Rate (%) | Hatch-Out Larvae | Hatching Rate (%) | Total Surviving Larvae | Survival Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| 32 °C | Rep 1 | 230 | 26 | 204 | 88.7 | 135 | 66.2 | 113 | 83.7 |
| Rep 2 | 230 | 24 | 206 | 89.6 | 129 | 62.6 | 122 | 94.6 | |
| Rep 3 | 230 | 20 | 210 | 91.3 | 127 | 60.5 | 116 | 91.3 | |
| 28 °C | Rep 1 | 230 | 22 | 208 | 90.4 | 140 | 67.3 | 128 | 91.4 |
| Rep 2 | 230 | 32 | 198 | 86.1 | 145 | 73.2 | 131 | 90.3 | |
| Rep 3 | 230 | 25 | 205 | 89.1 | 148 | 72.2 | 133 | 89.9 | |
| 24 °C | Rep 1 | 230 | 22 | 208 | 90.4 | 147 | 70.7 | 137 | 93.2 |
| Rep 2 | 230 | 31 | 199 | 86.5 | 151 | 75.9 | 138 | 91.4 | |
| Rep 3 | 230 | 40 | 190 | 82.6 | 153 | 80.5 | 136 | 88.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rbbani, G.; Siriyappagouder, P.; Murshed, R.; Joshi, R.; Nedoluzhko, A.; Galindo-Villegas, J.; Fernandes, J.M.O. Optimization of Nile Tilapia Artificial Breeding Using Human Chorionic Gonadotropin (hCG) Hormone. Methods Protoc. 2025, 8, 57. https://doi.org/10.3390/mps8030057
Rbbani G, Siriyappagouder P, Murshed R, Joshi R, Nedoluzhko A, Galindo-Villegas J, Fernandes JMO. Optimization of Nile Tilapia Artificial Breeding Using Human Chorionic Gonadotropin (hCG) Hormone. Methods and Protocols. 2025; 8(3):57. https://doi.org/10.3390/mps8030057
Chicago/Turabian StyleRbbani, Golam, Prabhugouda Siriyappagouder, Riaz Murshed, Rajesh Joshi, Artem Nedoluzhko, Jorge Galindo-Villegas, and Jorge M. O. Fernandes. 2025. "Optimization of Nile Tilapia Artificial Breeding Using Human Chorionic Gonadotropin (hCG) Hormone" Methods and Protocols 8, no. 3: 57. https://doi.org/10.3390/mps8030057
APA StyleRbbani, G., Siriyappagouder, P., Murshed, R., Joshi, R., Nedoluzhko, A., Galindo-Villegas, J., & Fernandes, J. M. O. (2025). Optimization of Nile Tilapia Artificial Breeding Using Human Chorionic Gonadotropin (hCG) Hormone. Methods and Protocols, 8(3), 57. https://doi.org/10.3390/mps8030057










