3.1. Characterisation of Gold and Silver Nanoparticles
The synthesis of AuNPs and AgNPs was achieved using trisodium citrate as the reducing agent for AuNPs and silver nitrate for AgNPs. The reduction reactions were conducted under carefully optimised conditions including controlled temperature and reaction time to facilitate the nucleation and growth of stable nanoparticles. The particle spectra and images are presented in
Figure 1.
Figure 1a,b show the UV-visible spectra of the synthesised silver and gold NPs exhibit distinct absorption peaks in the 300 nm to 800 nm spectral range. Evident within these spectra are well-defined characteristic plasmon resonance peaks observed at 520 nm for AuNPs and 400 nm for AgNPs. These plasmon resonance peaks indicate the collective oscillation of electrons on the nanoparticle surface. This confirms the successful formation of colloidal Ag and Au nanoparticles.
The TEM images revealed that both nanoparticles display a spherical morphology with a relatively narrow size distribution (
Figure 1c,d). AgNPs exhibited diameters ranging from 17.6 nm to 25.8 nm, while AuNPs demonstrated an average diameter spanning from 5.95 nm to 11.9 nm. When conjugating the AuNPs and AgNPs with antibodies, the spherical morphology of the synthesised AuNPs and AgNPs may be advantageous. This is primarily attributed to the potential enhancement in achieving a well-orientated distribution of antibodies across the nanoparticle surface. Consequently, this may potentially enhance both the binding efficiency and sensitivity [
21].
The size of nanoparticles holds the potential to significantly influence the overall performance of the application in lateral flow assay [
22]. Dynamic Light Scattering (DLS) was employed to determine the hydrodynamic size distributions of the nanoparticles. The obtained size distribution by intensity profiles, which indicates that the AuNPs exhibited a size range of 9 nm to 120 nm, while the AgNPs were observed to range from 2.5 nm to 120 nm. These findings denote the presence of a well-dispersed population. The range suggests that the nanoparticles are predominantly polydisperse. AgNPs have a relatively broader range of hydrodynamic sizes compared to AuNPs. The introduction of larger nanoparticles could be advantageous. According to their findings, increasing the nanoparticle size to as high as 115 nm resulted in a significant drop in the limit of detection (LOD) [
23]. The polydispersity index (PDI) and Zeta Potential (ZP) values are shown in
Table 1.
Au and Ag nanoparticles had zeta potentials of −26.3 ± 4.6 mV and −20.07 ± 0.5 mV, respectively, showing strong negative surface charges. They are relatively stable as zeta potential values other than −30 mV to +30 mV are deemed stable [
24]. Furthermore, the zeta potential of the AuNPs was comparatively stronger than that of the AgNPs, indicating better stability.
It was reported that PDI greater than 0.7 is an indication of aggregated nanoparticles in the solution [
25]. The current result shows a PDI value of 0.2 for the AuNPs, indicating a narrow size distribution, meaning that the majority of the particles are similar in size. On the other hand, a PDI value of 0.5 was obtained for the AgNPs, which is typically acceptable and suggests a broader size distribution.
3.2. Analysis of Gold and Silver Nanoparticles Aggregation Behaviour in Differing Alkaline Environments
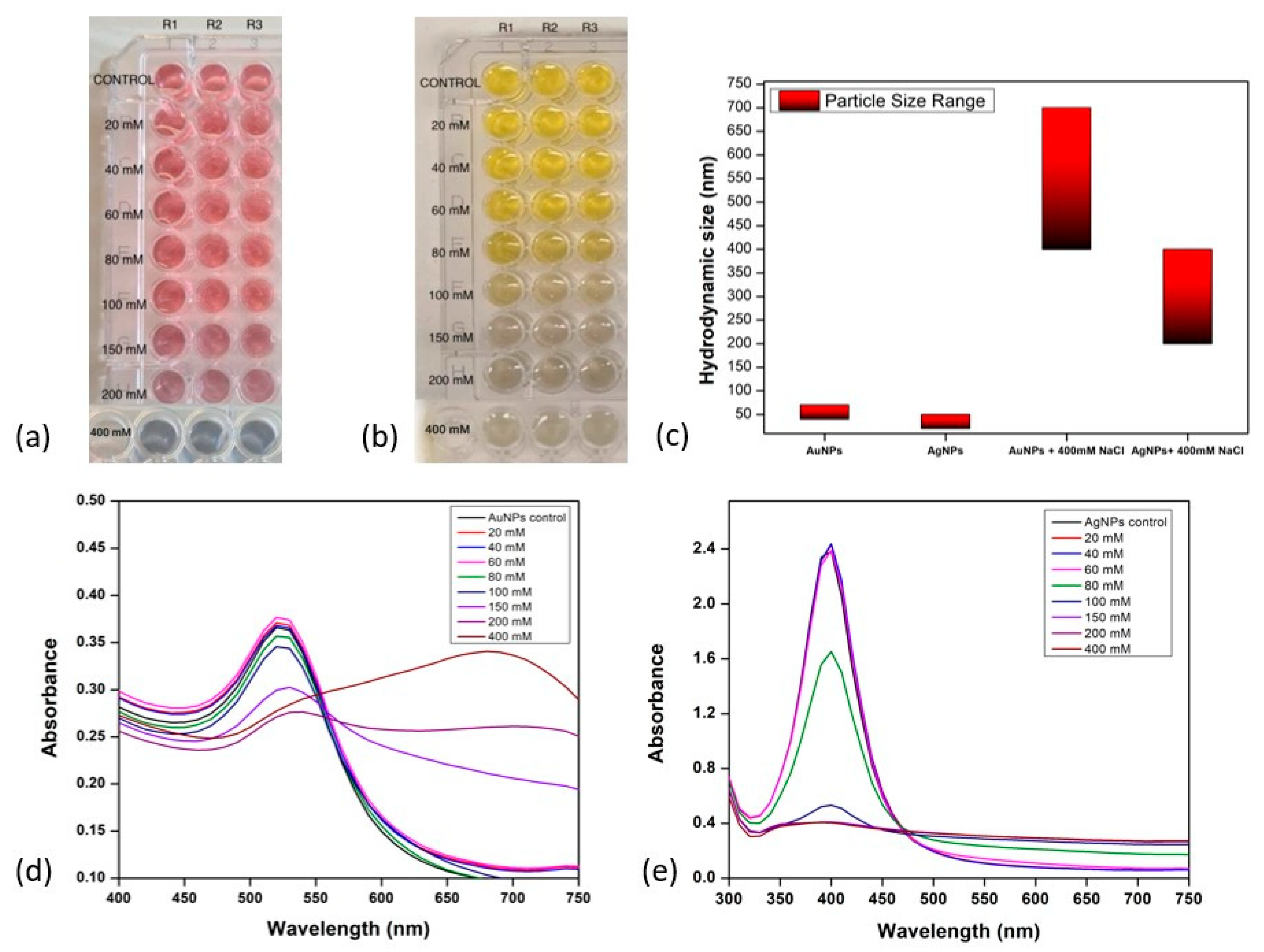
Prior to antibody conjugation, the stability of the synthesised Au and Ag nanoparticles was investigated in NaCl concentrations ranging from 20 mM to 400 mM. The aim was to investigate the effects of different NaCl levels on nanoparticle stability. For accuracy, the experiment used a 96-well plate format with triplicate samples (
Figure 2).
Figure 2 depicts the aggregation behaviour of AuNPs (
Figure 2a,d) across various NaCl concentrations. At NaCl concentrations of 20 mM, 40 mM, 60 mM, 80 mM, and 100 mM, AuNPs displayed no aggregation while retaining their typical brilliant red colour and stability. However, around 150 mM, a shift from red to pink-purple occurred, suggesting that the physical and chemical characteristics had changed in response to unfavourable alkaline conditions. At a NaCl concentration of 150 mM, the stability of AuNPs was disturbed, resulting in aggregation. The observed grey colour indicates that saturation was achieved at 400 mM. Similar trends were observed for AgNPs, with slight variations. The onset aggregation of AgNPs was observed at 80 mM of NaCl, characterised by a transition from brilliant yellow to light grey colouration (
Figure 2b,e). Following that, saturation at 150 mM of NaCl was observed, resulting in the development of dark grey nanoparticles. These varied observations together highlight the complex relationship between nanoparticle stability and NaCl concentration.
For additional insight into the behaviour of the AuNPs in the alkaline environment, the optical density (OD) was measured at 520 nm and 630 nm to monitor changes in their SPR properties as a function of NaCl concentration. The OD measurements obtained at 520 nm and 630 nm were plotted on a graph, which is shown in
Figure 3.
The OD at 520 nm decreased slightly at NaCl concentrations 20 mM to 100 mM, while at NaCl concentrations 150 mM to 400 mM, a more significant decrease was observed (
Figure 3a). This indicated a decrease in the concentration of dispersed nanoparticles as the NaCl concentration increased due to increased aggregation. The SPR of the nanoparticles slightly shifted to a longer wavelength at NaCl concentrations of 20 to 100 mM. These shifts were more significant as the NaCl concentration increased beyond 100 mM; hence, a significant decrease in OD was observed. This is consistent with the red shift in the SPR peak observed in
Figure 2d. These shifts in SPR occurred as a result of the interaction of Na+ and Cl- ions with the surface charge of nanoparticles, with stronger interactions occurring at higher NaCl concentrations. Conversely, at 630 nm, the OD increased with increasing NaCl concentration (
Figure 3b). This was clearly due to the shift in the SPR of nanoparticles to longer wavelengths. The increase in OD became more significant at 150 mM, which is the concentration at which the onset of nanoparticle aggregation occurs, as determined by the corresponding SPR peak on the UV-visible absorption spectra.
The hydrodynamic sizes of both gold and silver nanoparticles (without NaCl influence) and with NaCl were measured using dynamic light scattering analysis (
Figure 2c). The data reveals the size of AuNPs in the control sample was in the range of 40–70 nm and of AgNPs was 20–40 nm. After the addition of NaCl at the concentration of 400 mM, a change in colour was observed, which shows the aggregation of both AuNPs and AgNPs. The aggregation was confirmed by checking the hydrodynamic size, zeta potential, and PDI values of dispersions of AuNPs and AgNPs. The size was found to be in the range of 450–700 nm for AuNPs and 200–400 nm in the case of AgNPs. PDI values were also found to be close to 1, along with zeta potential close to zero, indicating unstable and aggregated dispersions. The colour changed to grey, indicating that high NaCl concentration led to aggregation. Hence, for further experiments, the concentration of NaCl used was 150 mM for AuNPs and 80 mM for AgNPs.
3.3. Influence of Colloidal Solution pH on Antibody Conjugation to Gold and Silver Nanoparticles
The effect of varying pH levels on the interaction between the nanoparticles and antibodies was investigated. K
2CO
3 was employed to adjust the pH, with concentrations ranging from 0.2 mM to 1.2 mM. To verify the conjugation, a suboptimal NaCl concentration of 150 mM was introduced for AuNPs and 80 mM for AgNPs. The study aimed to identify the optimal pH for antibody–nanoparticle (Ab-NP) conjugation. The pH of colloidal Au and Ag nanoparticle solutions was regulated using K
2CO
3, while antibody concentration remained constant at 2 µg/mL. The details of the K
2CO
3 concentrations used to adjust the pH of the colloidal AuNPs and AgNPs solutions are provided in
Figure 4a,c, respectively.
Figure 4 shows the effect of varying pH levels maintained via K
2CO
3 on the interaction between the nanoparticles and antibodies.
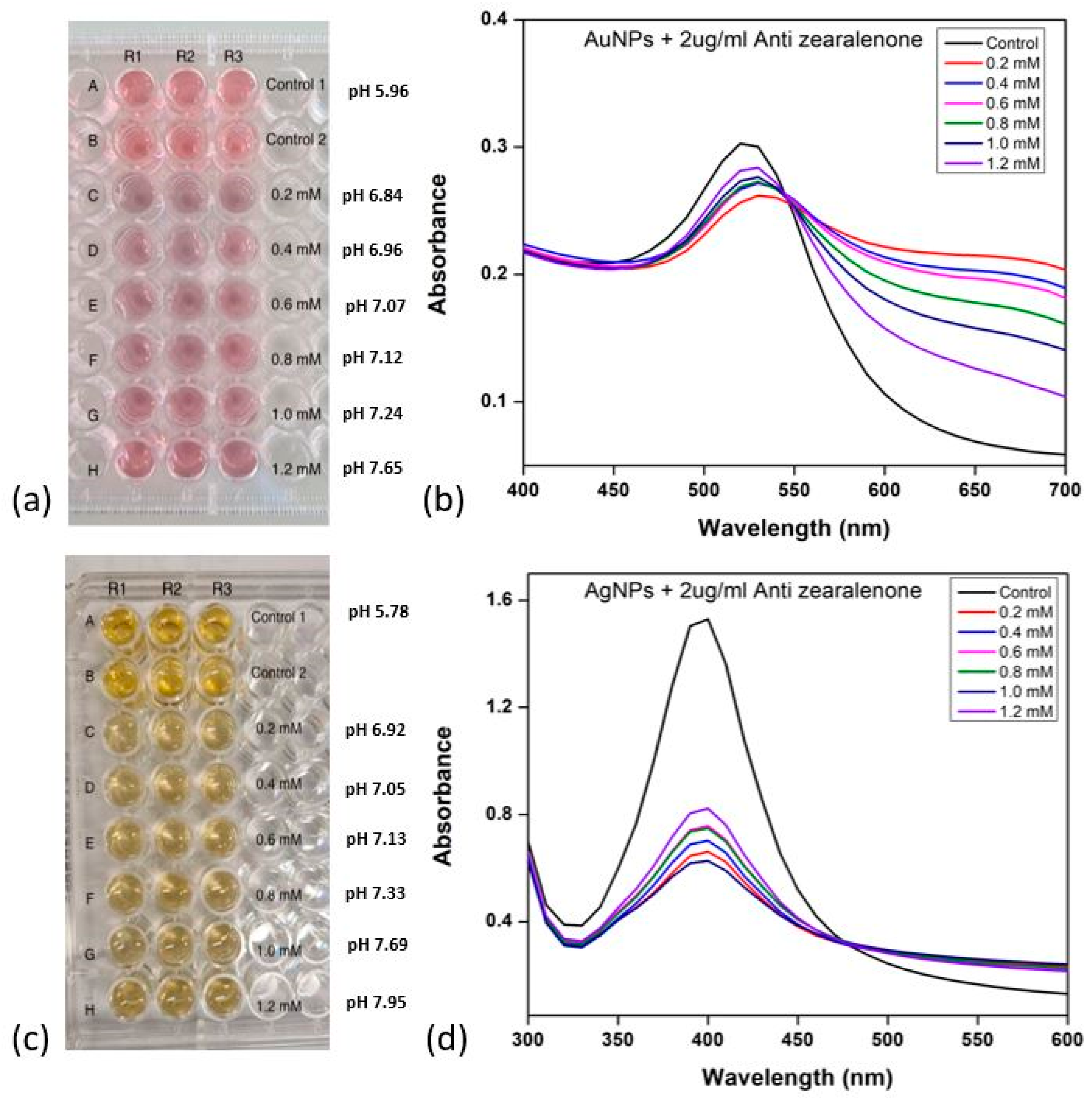
Figure 4a,c illustrates visually monitored solutions for colour changes in the 96-well plate.
The image in
Figure 4a shows the plate used for the colourimetric assay of Ab-AuNP conjugates. Colour changes occurred at concentrations of 0.2, 0.4, 0.6, 0.8, and 1 mM, signifying nanoparticle aggregation after NaCl addition. This indicated unsuccessful antibody–AuNP conjugation due to suboptimal pH. Lower K
2CO
3 concentrations created a low-pH environment, causing electrostatic bridging between antibodies and negatively charged citrate-capped nanoparticles, resulting in aggregation. At 1.2 mM K
2CO
3, corresponding to the pH 7.65, the nanoparticles exhibited minimal colour change compared to the control, indicating successful antibody coating onto nanoparticle surfaces.
To investigate the effect of pH on AuNP optical properties, nanoparticle absorbance was measured using UV-visible spectroscopy (300–750 nm).
Figure 4b depicts the obtained absorbance spectra. SPR peaks at around 520 nm, similar to standard AuNPs, were identified. Control 1 (AuNPs and deionised water) and Control 2 (AuNPs, deionised water, and antibodies) exhibited no changes in SPR, optimal antibody orientation on the nanoparticle surface, and stability. As the reaction pH decreased, slight redshifts appeared, suggesting augmented nano-moiety size. Changes in interparticle distances and dielectric constants of the surrounding medium are two causing factors. Lower pH resulted in larger, less defined SPR peaks, indicating destabilisation and aggregation of nanoparticle conjugates. The SPR peak closely mirrored the control peaks at 1.2 mM K
2CO
3, indicating stable antibody–AuNP conjugation in solution.
Figure 4c is an image of the 96-well plate employed for examining AgNPs. Control 1 contains AgNPs and deionized water, and Control 2 also includes antibodies. AgNPs followed a similar aggregation pattern to AuNPs, aggregating at 0.2, 0.4, 0.6, 0.8, and 1 mM, evident by colour shifts from bright yellow to pale yellow to light grey. Retention of bright yellow colour at 1.2 mM, which corresponds to pH 7.95, suggested optimal K
2CO
3 concentration for AgNPs-Ab conjugation.
The particle size distribution for control 1 (AuNPs and deionised water) was reported between 10 to 140 nm, while the values changed from 8 to 90 nm for control 2 (AuNPs, deionised water, and antibodies). At 1.2 mM K
2CO
3, the particle size distribution increased from 10 to 410 nm following conjugation, which shows an increase in the nanoparticle standard size and indicates the effective coverage of the nanoparticle’s surface with the antibodies.
Figure 4d presents UV-visible absorbance spectra of Ab-AgNP complexes at varied K
2CO
3 concentrations. The SPR peaks are positioned around 400 nm. However, a significant change in peak shape and intensity is observed at low concentrations of K
2CO
3, indicating aggregation stability. Conjugates showed a significant absorbance drop at 400 nm for K
2CO
3 concentrations 0.2 mM to 1.2 mM, with 1.2 mM exhibiting a slightly smaller yet well-defined peak, suggesting stability.
Table 2 illustrates the zeta potential (ZP) and PDI of AuNPs in control 1, control 2, and at 1.2 mM of K
2CO
3 concentration.
Control 1, control 2, and Ab-AuNP at 1.2 mM K
2CO
3 all had significantly negative zeta potential values of −33.4 ± 8.2 mV, −30.9 ± 10.6 mV, and −23.2 ± 1.5 mV, indicating highly stable conjugates in
Table 2. A slight reduction in ZP at 1.2 mM of K
2CO
3 suggested a modest decrease in surface charge, presumably due to pH-driven changes in ionic strength influencing electrostatic interactions between nanoparticles in the surrounding medium. The Polydispersity Index (PDI) values were 0.229, 0.329, and 0.347 (
Table 2).
Notably, the conjugates at optimum pH had a larger PDI, indicating a greater variance in nanoparticle size and enhanced solution heterogeneity [
26,
27]. This corresponds to the increased hydrodynamic diameter range of single AuNP–antibody conjugates. Similar results were obtained for the AgNPs and their antibody conjugates at the 1.2 mM concentration of K
2CO
3 (
Table 3).
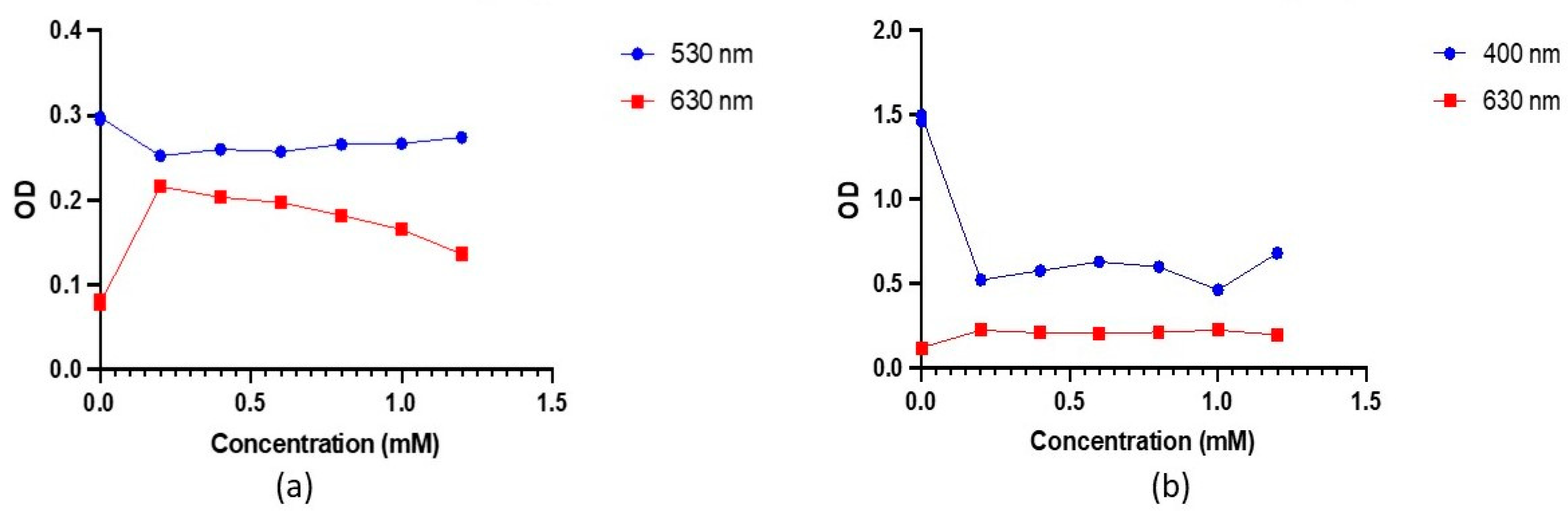
To gain further insights into antibody and nanoparticle behaviour across varying pH, OD measurements were taken at 530 nm and 630 nm on different concentrations of K
2CO
3 (
Figure 5).
At 530 nm, a decrease in OD values was observed, with a notable decrease from control 1 to control 2. As pH increased, OD increased consistently. This pattern is mirrored in the OD measurements at 630 nm—a significant increase from control 1 to control 2—followed by a steady decrease with rising pH. The variations in pH influence the surface charge of the nanoparticles, impacting the adsorption of antibodies. These changes in surface properties can impact the SPR phenomenon, causing shifts towards longer wavelengths as a result of more prevalent repulsive interactions between particles. This is consistent with the redshift in SPR peaks at low K
2CO
3 concentrations observed in the UV-visible spectra. The behaviour observed at both wavelengths suggests that antibody–nanoparticle interactions are closely connected to pH-induced changes in surface charge and nanoparticle stability (
Figure 5a).
OD measurements of AgNPs-Ab conjugates in
Figure 5b reveal a decrease at lower pH and an increase at 1.2 mM at 400 nm. Contrastingly, variations at 630 nm are minimal. For conjugates at 1.2 mM K
2CO
3 concentration, the DLS data demonstrate a broad size distribution of 10 to 400 nm, highly negative ZP of −33.6 ± 1.11 mV, and slightly higher PDI of 0.312 compared to the controls (
Table 3). These findings are similar to that of AuNPs antibody conjugates in this study, providing evidence of successful antibody binding for stable conjugates at 1.2 mM of K
2CO
3 concentration.
Kasoju et al. [
12] found that the onset of nanoparticle aggregation occurred at 40 mM and reached saturation at 80 mM. This differs from the findings of this study, as the nanoparticles began aggregating at 80 mM and reached saturation at 400 mM. This means that the nanoparticles in this study have a higher stability, are more resistant to aggregation, and require higher NaCl concentrations to induce aggregation compared to the AuNPs synthesised by Kasoju et al. [
12]. Additionally, the higher concentration range for saturation suggests that AuNPs have a comparatively higher sensitivity to changes in NaCl concentration. In this study, the AuNPs size ranged between 9 and 120 nm, while the AuNPs used by Kasoju et al. [
12] were approximately 18 to 20 nm. The difference in aggregation behaviour can be attributed to the size distribution of the particles, as particle size plays an important role in their stability and sensitivity to aggregation [
28]. As the nanoparticles in this study were larger, they exhibited higher stability and began aggregating at a relatively higher NaCl concentration. This is advantageous for the use of these conjugates as they would be less likely to aggregate during migration on the sample pad, therefore ensuring better dispersion and enhanced signal generation for the lateral flow test strips. However, it is important to note that if the size of NPs is too large and excessively high NaCl concentrations are required to reach saturation, it could result in reduced assay sensitivity [
29,
30].
3.4. Stability Study of Gold and Silver Nanoparticles at Varying Antibody Concentrations under Controlled pH and NaCl Conditions
This study aimed to establish the optimal antibody concentration for enhancing nanoparticle surface binding and subsequently amplifying signal output. The antibody concentration was increased in 2 µg/mL increments, and pH was controlled using 1.2 mM K
2CO
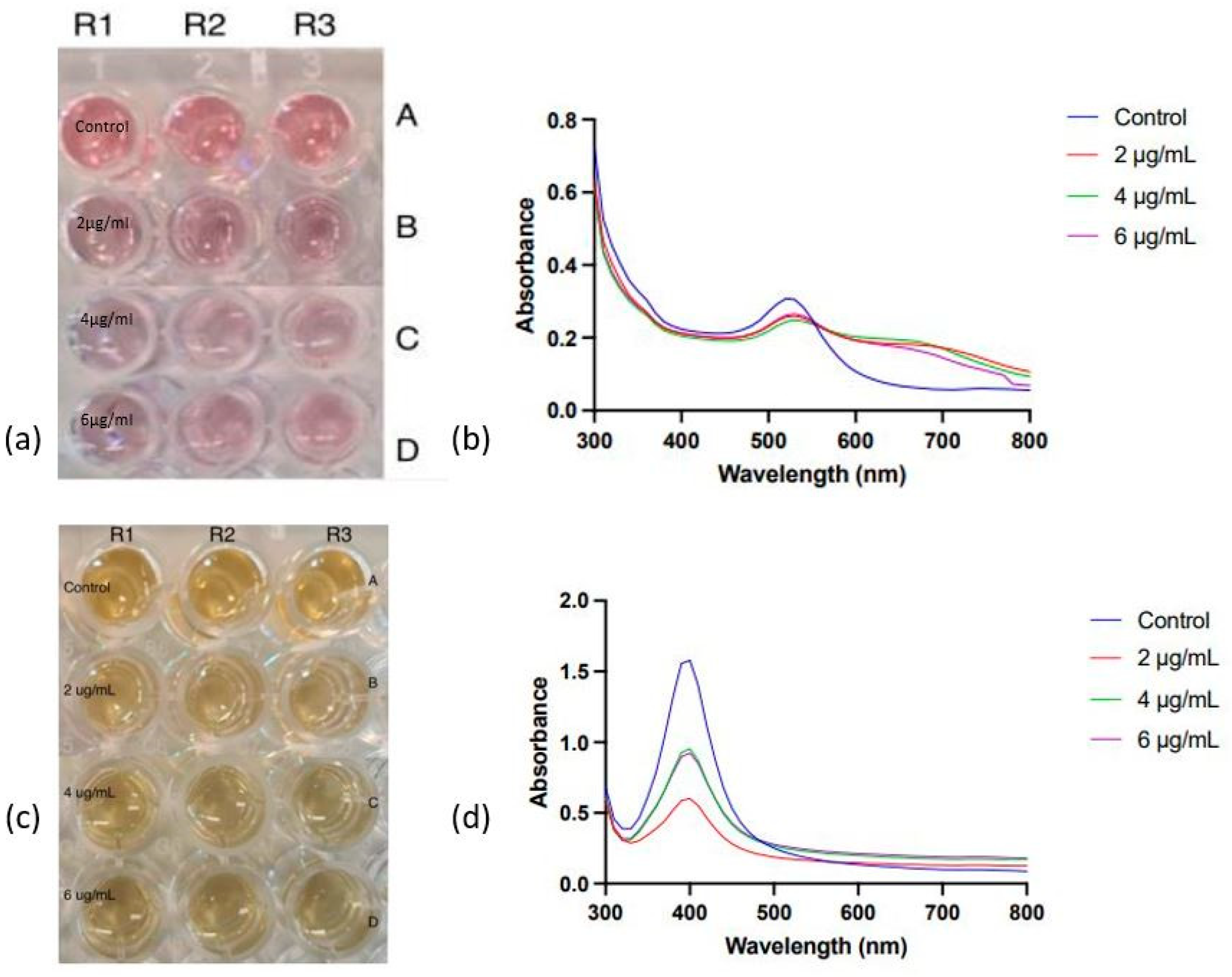
3 concentration. The experimentation was conducted within a 96-well plate setup, with a control comprising nanoparticles, deionised water, and varying antibody concentrations. The arrangement of nanoparticles in the plate is illustrated in
Figure 6.
AuNPs and AgNPs were conjugated with 2,4 and 6 µg/mL of anti-zearalenone antibodies at the optimised pH value of ⪆7.5. After adding the required concentration of antibodies and nanoparticles, NaCl solution was added. Nanoparticle conjugates exhibited minor colouration changes when exposed to suboptimal NaCl concentrations of 150 mM (AuNPs) and 80 mM (AgNPs). For further evaluation, OD measurements were taken at 520 nm, and variations in the OD of AuNP conjugates were evident (
Figure 6b). Specifically, at 150 mM NaCl and 4 µg/mL of antibody concentration, the OD of nanoparticles (0.250) closely resembled the control sample’s OD (0.298). This suggests that the SPR was similar under these conditions, indicating successful antibody attachment. At 630 nm, a similar pattern was observed, suggesting optimal conjugation at 4 µg/mL of antibody concentration at 150 mM of NaCl. Similarly, in the case of AgNPs, the OD measurements were taken at 400 nm. However, the extent of aggregation can be regarded as acceptable, as the Ab-AuNP and Ab-AgNP conjugates retained a substantial degree of the nanoparticles’ original pinkish-red and bright yellow colour, respectively.
The influence of the varying antibody concentrations on the SPR of nanoparticle conjugates was evaluated via absorbance measurements employing UV-visible spectroscopy. In the UV-visible spectra of AuNPs-Ab conjugates (
Figure 6b), the SPR peak of the control conjugates is distinct and well-defined. Conversely, the peaks corresponding to the AuNPs conjugated with 2 and 4 µg/mL of antibody concentrations are relatively broader and moved toward the higher wavelength, suggesting successful conjugation of AuNPs with the antibodies.
In the case of AgNP-Ab conjugates (
Figure 6c), the UV visible absorbance spectra display SPR peaks at 400 nm (
Figure 6d). For conjugates at 2, 4, and 6 µg/mL of antibody concentrations, SPR peak intensities are lower than that of the control. Notably, 2 µg/mL exhibits the lowest intensity, while 4 µg/mL and 6 µg/mL share slightly higher intensities. An optimal antibody concentration for AgNP conjugation may not be 2 µg/mL due to the lowest SPR intensity, while 4 µg/mL and 6 µg/mL show comparable intensities, suggesting either could be optimal. Since the study’s goal is to maximise the nanoparticle-to-antibody conjugation, 6 µg/mL emerges as the optimal antibody concentration.
The OD measurements for AgNP conjugates revealed no distinct trend at 400 nm and 630 nm, likely due to comparable surface plasmon resonances among conjugates at varying antibody concentrations under controlled pH and salt conditions. Notably, control containing only nanoparticles and antibodies exhibited significantly higher OD at 400 nm compared to conjugates with varying antibody concentrations. This divergence implied differing SPR bands, possibly due to non-optimised conditions for these control conjugates.
The hydrodynamic diameter of AuNP-Ab conjugates at 2 µg/mL in 0 mM NaCl, 2 µg/mL in 150 mM NaCl, and 4 µg/mL in 150 mM NaCl were 10–90 nm, 150–450 nm, and 150–400 nm, respectively. These measurements indicated that the conjugates exhibited larger sizes compared to the control. The same pattern was observed for AgNP-Ab conjugates where the size distribution of the control and conjugates at 2 µg/mL, 4 µg/mL, and 6 µg/mL ranged from 1 to 110 nm, 1–500 nm, 150–350 nm, and 195–500 nm, respectively. These observations strongly demonstrate the effective binding of antibodies to nanoparticles within the controlled pH environment. ZP and PDI of AuNPs at varying NaCl and Ab concentrations are detailed in
Table 4 and
Table 5.
ZP measurements indicated comparable surface charges between control and antibody conjugates at 4 µg/mL of antibody concentration in 150 mM of NaCl, with values of −30.9 mV and −30.53 ± 1.80 mV, respectively. These highly negative readings denote substantial stability. In contrast, nanoparticle conjugates at 2 µg/mL of antibody in 150 mM of NaCl exhibited markedly lower negative ZP value of −0.529 ± 0.54 mV. This weak ZP signifies insufficient antibody coverage on nanoparticle surfaces, causing interactions with NaCl ions and the formation of large and destabilised nanoparticle aggregates. The low ZP aligns with DLS data, revealing larger particle sizes for the sample with 2 µg/mL of antibody concentration in 150 mM of NaCl. Additionally, the highest PDI appeared at 4 µg/mL of antibody in 150 mM of NaCl, indicative of increased heterogeneity and broad size distribution in these conjugates.
These findings effectively underscore that a more efficient conjugation process can be achieved at 4 µg/mL of antibody concentration in 150 mM NaCl with 1.2 mM of K
2CO
3 concentration. A similar study was conducted by Kasoju et al. [
12], where AuNPs stability was examined using varying NaCl concentrations ranging from 20 mM to 200 mM. The aggregation pattern observed by the researcher was identical to the pattern observed in this study, where the nanoparticles at low NaCl concentration are stable and exhibit no colour change. Contrarily, at high concentrations of NaCl, the physical stability of the nanoparticles is affected, resulting in the formation of aggregates. The ZP of AgNP-Ab conjugates at 2 and 4 µg/mL of antibody concentrations were 0.329 ± 0.16 mV and 0.403 ± 0.639 mV, respectively. These lowly positive values typically suggest the potential destabilisation of conjugates in the reaction mixture. However, this contradicts the well-defined SPR peaks and comparable OD values, both indicative of conjugate stability. The discrepancy may stem from potential contamination during reaction mixture preparation or the conjugates’ quality compromised due to prolonged room temperature exposure during analysis. The conjugates might have lost stability, possibly due to antibody sensitivity to temperature fluctuations in the environment and their requirement for low temperatures to preserve stability.
Conversely, in AgNPs at 6 µg/mL of concentration, the conjugates displayed a notably negative ZP value of −36 ± 2.11 mV, underscoring robust stability under optimised pH and salt conditions. This strongly implies enhanced temperature resistance compared to the 2 µg/mL and 4 µg/mL of antibody concentration conjugates, indicative of enhanced shelf life and sensitivity for biosensing. Hence, the optimal antibody concentration for AgNPs-Ab conjugation is 6 µg/mL. Bélteky et al. [
31] performed a comparable study on AgNPs to determine the effect of NaCl on the colloidal stability of AgNP suspension. The results of the study also concluded that the SPR of the AgNPs declined with increasing NaCl concentration. The reasoning behind this is that the relative permittivity of the surrounding medium is increased with increasing NaCl concentration, thereby affecting the SPR [
32]. Moreover, Bélteky et al. [
31] observed changes in SPR at 10 mM and 50 mM of NaCl for AgNPs that were 10 nm and 20 to 50 nm, respectively, while in this study, this pattern was observed at 80 mM NaCl for AgNPs that are 2.5 to 120 nm. Therefore, this indicated that higher NaCl concentrations may be required to induce mild aggregation of larger nanoparticles.
Lou et al. [
19] conducted a comparable investigation to determine an appropriate K
2CO
3 and antibody concentration for developing stable antibody-labelled AuNPs. The results demonstrated that the stability of the conjugates improved as the antibody concentration increased. This was because the nanoparticles were saturated by the antibodies, and hence the conjugates were less sensitive to NaCl and K
2CO
3 in the reaction mixture, preventing aggregation. A similar pattern was also observed in this study, where AuNP and AgNP conjugates were stable when the antibody concentration increased from 2 µg/mL to 4 µg/mL and 6 µg/mL, respectively. It was also found that a higher pH value is required for conjugating smaller-sized nanoparticles with antibodies compared to larger ones. This contrasted with the results of this study, as a higher pH value was required for developing stable AuNP and AgNP conjugates using Au and Ag NPs with a large hydrodynamic diameter of up to 120 nm.