A Simple and Fast Method for the Formation and Downstream Processing of Cancer-Cell-Derived 3D Spheroids: An Example Using Nicotine-Treated A549 Lung Cancer 3D Spheres
Abstract
:1. Introduction
2. Experimental Design
3. Materials and Methods
3.1. Equipment and Instruments
- Echo Revolve microscope for imaging (Discover Echo, San Diego, CA, USA);
- Centrifuge provided with a microliter plate rotor (optional);
- Cell culture incubator with a humidified environment at 5% CO2 and 37 °C;
- Tecan Spark (Tecan, AG, Männedorf, Switzerland);
- 96-well BIOFLOAT ULA ultra-low-attachment plates (FaCellitate, Mannheim, Germany);
- Opaque-walled 96-well plates;
- NanoPhotometer (AxonLab, Baden, Switzerland);
- ViiA 7 Real-Time PCR System (Applied Biosystems/Thermo Fisher Scientific, Schwerte, Germany).
3.2. Reagents
- PBS (Gibco, Waltham, MA, USA);
- FBS (Gibco, Waltham, MA, USA);
- Trypsin (Gibco, Waltham, MA, USA);
- Medium: MEM (Gibco, Waltham, MA, USA);
- Medium: DMEM (Gibco, Waltham, MA, USA);
- CellTiter-Glo 3D assay (Promega, Madison, WI, USA);
- RNeasy Plus Micro Kit (Qiagen, Hilden, Germany);
- Nicotine (Tocris, Bristol, UK);
- Mecamylamine (MEC) (Tocris, Bristol, UK);
- High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Dreieich, Germany);
- TaqMan Gene Expression Assay and Master Mix (Thermo Fisher Scientific, Dreieich, Germany);
- L-glutamine (Gibco, Waltham, MA, USA);
- MEM Non-Essential Amino Acids Solution (Gibco, Waltham, MA, USA).
3.3. Cell Culture
- MCF7 (breast cancer);
- A549 (lung cancer);
- DU145 (prostate cancer);
- HCT116 (colon cancer);
- Lovo (colon cancer).
4. Detailed Procedure
4.1. Stepwise Generation of Spheres
- Culture the cells aseptically, and once the flask is at 70–80% confluence, proceed to dissociation. Note: cells with a passage number of below 20 are recommended for spheroid generation.
- Aspirate corresponding cell medium.
- Wash the cells with sterile PBS to remove any traces of serum.
- Add appropriate amounts of pre-warmed trypsin–EDTA solution to cover the cells.
- Place the flask in a humidified incubator at 5% CO2 and 37 °C for 5 min. Inspect the cells using a microscope to ensure complete cell detachment.
- To stop the action of trypsin, add the corresponding medium with 10% serum.
- Gently resuspend the cell suspension until no cell clumps are visible.
- Count the number of cells, e.g., using an automated cell counter.
- Initially titrate the optimal number of cells needed to form spheres depending on cell type. We recommend starting with seeding at 1000–2500–5000 and 10,000 cells per well. Note: the reader can also refer to [12], in which seeding numbers of 60 diverse cell lines for successful sphere formation using ULA plates are provided.
- Dilute cells to the desired cell concentration/number per ml. Note: take into consideration that the final volume per well is 200 µL.
- Next, add 100 µL of pre-warmed medium using a multichannel pipette to each well of a ULA 96-well plate.
- Then, seed the selected number of cells, adding the corresponding 100 µL of cell suspension into the wells of the ULA plate. Note: these ULA plates can also be used to form individual patient-derived spheroids from tumor tissue [20].
- In case of long incubation times, we recommend filling the non-used wells of the plate with 1X PBS or sterile water to prevent evaporation of medium during incubation.
- Centrifuge the plate at 300 rpm for 5 min. Note: This step assists cell aggregation, leading to uniform spheroid formation. Change centrifuge parameters as needed for each cell line.
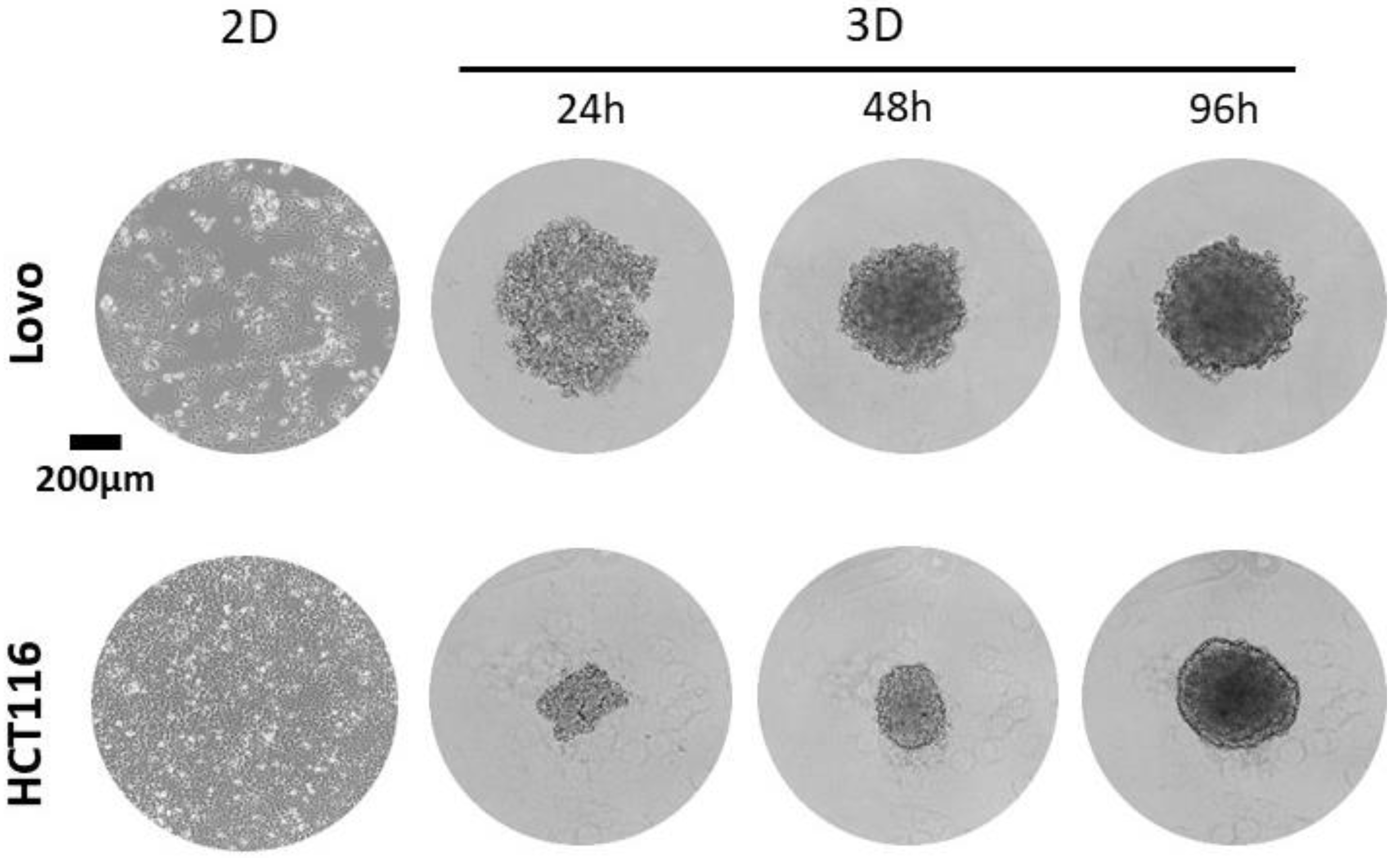
- Place the multiwell plate in the incubator at 37 °C and 5% CO2. Note: Spheres must be monitored by using a microscope every day. For most cell lines using this protocol, spheres are formed after 48–72 h and are stable for up to two weeks.
- Change medium twice per week. To disturb the spheres as little as possible, carefully remove 80 µL using a multichannel pipette (optional) and then add 100 µL of fresh full medium. Afterwards, centrifuge the plate at 300 rpm for 5 min and return it to the cell culture incubator to help maintain sphere integrity.
- Note: If spheres are going to be used for a drug screening assay, molecules can be co-incubated with the spheres from day 1 of seeding (this study, see below). Alternatively, spheres can be grown for 3–7 days to allow final organoid formation before drug testing, as described in [12,20]. Here, one has the possibility to use several assays, including sphere growth, which can be helpful to assess potential anti-cancer compounds [14].
- Afterwards, spheres can be subjected to viability assays and qRT-PCR analyses, as detailed below. Furthermore, they can be used for Western blotting; for the protocol, please refer to [16].
- Note: Images of the spheroids should be collected every day using brightfield settings and a 10× magnification on an Echo Revolve microscope, using annotation tools to determine length and area. Alternatively, using an inverted transmission light microscope, one can calculate the corresponding volume of the sphere by using the mathematical formula as in [14].
4.2. Downstream Application I: Viability Assay of Spheres
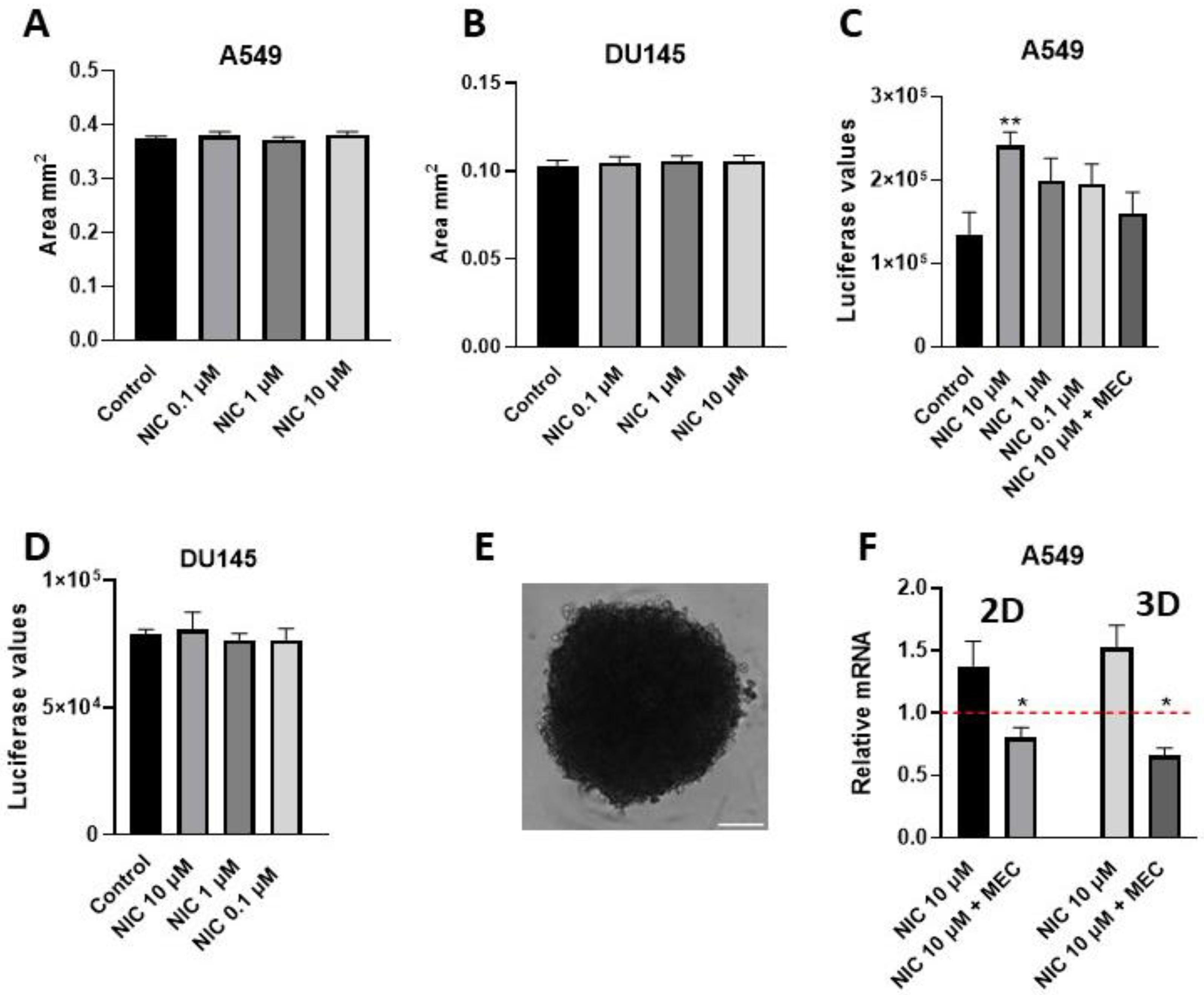
- Prepare spheres as described above. Note: spheroid size and the number of days in culture must be determined experimentally for each cell line.
- Thaw the CellTiter-Glo 3D reagent at 4 °C overnight.
- Place the reagent at room temperature (RT) 30 min prior to use. Mix before using.
- Pick/transfer each individual spheroid together with 100 µL of medium from the 96-well ULA plate to an individual well of an opaque-walled 96-well plate. Note: as the spheres must not be disturbed in this process, use 1 mL pipette tips (cut off the front part of each tip to create a larger opening) for handling.
- Add 100 µL of CellTiter-Glo 3D reagent.
- Mix by vigorous shaking for 5 min to induce sphere/cell lysis. Note: This step ensures the optimal readout from all cells composing the sphere structure. As this assay relies on the lysis of the sphere, it is considered as an endpoint measurement [4].
- Incubate the plate at RT for 25 min.
- Record luminescence according to the manufacturer’s instructions.
- Note: A minimum of three technical replicates per condition must be used. In addition, a blank control for each respective culture medium of readout must be included.
- Note: plate shaking, incubation, and luminescence recording may be performed using a Tecan Spark.
- Note: Optionally, the concentration of ATP from the spheres may be determined. In this case, an ATP standard curve must be created.
4.3. Downstream Application II: Obtaining RNA from Spheres
- Place 15 spheres per condition/treatment into an individual Eppendorf tube. Note: do not disturb the spheres in this process, use 1 mL pipette tips with cut tips for handling.
- Centrifuge the tube at 800 rpm for 10 min.
- Carefully remove the supernatant without disturbing the pellet.
- Wash the pellet with 1× PBS.
- Carefully remove the supernatant without disturbing the pellet. Note: at this point, the sphere pellet obtained can be stored at −80 °C for later use if necessary.
- Continue with sphere RNA extraction using the RNeasy Plus Micro Kit as per the manufacturer’s instructions. In brief:
- Into each tube, add 350 µL of RLT lysis buffer provided with the kit.
- Homogenize by pipetting, transfer to the gDNA Eliminator spin column, and centrifuge for 30 s at top speed.
- Add an equal volume of 70% ethanol to the above obtained flow-through, place in a new RNeasy column, and centrifuge for 30 s at top speed.
- To the spin column, add 700 µL RW1 buffer, and centrifuge for 30 s at top speed.
- Next, add 500 µL RPE buffer, and centrifuge for 30 s at top speed.
- Add 500 µL 80% ethanol to the column, then centrifuge for 2 min at top speed.
- Place the column in a new tube and elute the purified RNA using 10 µL RNAse-free water.
- Measure concentration and purity using a nanophotometer. Note: the size of the spheres may affect the yield of extracted RNA.
- Store the samples at −80 °C until later use. RNA obtained can be used for the analysis of the expression of diverse genes using qPCR.
- Note: the number of spheres to be harvested per condition must be optimized for each cell line.
5. Expected Results
5.1. Generation of Spheres
5.2. Viability Assay of Spheres: Example from Nicotine-Treated A549-Derived Spheres
5.3. Obtaining RNA from Spheres: Example Determination of CD47 Expression from Nicotine-Treated Spheres
- It is essential to use a low-passage cell line when making cell-derived spheres, and always limit the number of passaging of cell lines.
- As mentioned above, e.g., Corning ultra-low-attachment round-bottom plates can be used as an alternative to FaCellitate ULA 96-well plates.
- Although we recommend using centrifugation steps, it is also possible to change parameters of time and rpm. If no spheroid growth is observed, it may be necessary to use less intense centrifugation conditions.
- Spheres can be stained using propidium iodine PI and annexin labeling; this can give a good readout of tumorsphere health. Additionally, it can be used to monitor the results of drug/compound screening.
- Our protocol has a limitation, as one sphere is formed per well. To obtain a high number of spheres for large-scale experiments, such as protein/RNA extraction, it requires the multiple seeding of numerous wells in parallel. The number of such wells must be determined experimentally.
- In Table 1, optimal numbers of cells were determined experimentally in our laboratory, where a lower or higher number yielded in 3D structures with reduced viability (the structure disintegrated after 2–7 days of culture) was determined visually using an appropriate microscope.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sereti, E.; Papapostolou, I.; Dimas, K. Pancreatic Cancer Organoids: An Emerging Platform for Precision Medicine? Biomedicines 2023, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Sherman, H.; Gitschier, H.J.; Rossi, A.E. A Novel Three-Dimensional Immune Oncology Model for High-Throughput Testing of Tumoricidal Activity. Front. Immunol. 2018, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, E.; Veghini, L.; Corbo, V. Modeling Cell Communication in Cancer with Organoids: Making the Complex Simple. Front. Cell Dev. Biol. 2020, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.; Harrington, D.A.; Farach-Carson, M.C.; Yu, H. Heralding a new paradigm in 3D tumor modeling. Biomaterials 2016, 108, 197–213. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110264. [Google Scholar] [CrossRef]
- Tosca, E.M.; Ronchi, D.; Facciolo, D.; Magni, P. Replacement, Reduction, and Refinement of Animal Experiments in Anticancer Drug Development: The Contribution of 3D In Vitro Cancer Models in the Drug Efficacy Assessment. Biomedicines 2023, 11, 1058. [Google Scholar] [CrossRef]
- Perche, F.; Torchilin, V.P. Cancer cell spheroids as a model to evaluate chemotherapy protocols. Cancer Biol. Ther. 2012, 13, 1205–1213. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Prim. 2022, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Selby, M.; Delosh, R.; Laudeman, J.; Ogle, C.; Reinhart, R.; Silvers, T.; Lawrence, S.; Kinders, R.; Parchment, R.; Teicher, B.A.; et al. 3D Models of the NCI60 Cell Lines for Screening Oncology Compounds. SLAS Discov. 2017, 22, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Bär, S.I.; Biersack, B.; Schobert, R. 3D cell cultures, as a surrogate for animal models, enhance the diagnostic value of preclinical in vitro investigations by adding information on the tumour microenvironment: A comparative study of new dual-mode HDAC inhibitors. Investig. New Drugs 2022, 40, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Zhao, Y.; Wei, J.; Han, J.; Chen, L.; Xiao, Z.; Chen, B.; Dai, J. The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials 2013, 34, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Edsbäcker, E.; Serviss, J.T.; Kolosenko, I.; Palm-Apergi, C.; De Milito, A.; Tamm, K.P. STAT3 is activated in multicellular spheroids of colon carcinoma cells and mediates expression of IRF9 and interferon stimulated genes. Sci. Rep. 2019, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Cheaito, K.; Chalhoub, R.M.; Hadadeh, O.; Monzer, A.; Ballout, F.; El-Hajj, A.; Mukherji, D.; Liu, Y.N.; Daoud, G.; et al. Sphere-Formation Assay: Three-Dimensional in vitro Culturing of Prostate Cancer Stem/Progenitor Sphere-Forming Cells. Front. Oncol. 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Tsvirkun, D.; Revilloud, J.; Giannetti, A.; Verdier, C. The intriguing role of collagen on the rheology of cancer cell spheroids. J. Biomech. 2022, 141, 111229. [Google Scholar] [CrossRef]
- Preti, B.; Rougier, J.-S.; Papapostolou, I.; Bochen, F.; Gerber, C.E.; Abriel, H.; Lochner, M.; Peinelt, C. Targeting Ion Channel TRPM4. Chimia 2022, 76, 1039. [Google Scholar] [CrossRef]
- Karkampouna, S.; La Manna, F.; Benjak, A.; Kiener, M.; De Menna, M.; Zoni, E.; Grosjean, J.; Klima, I.; Garofoli, A.; Bolis, M.; et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat. Commun. 2021, 12, 1117. [Google Scholar] [CrossRef]
- Dasgupta, P.; Chellappan, S.P. Nicotine-mediated cell proliferation and angiogenesis: New twists to an old story. Cell Cycle 2006, 5, 2324–2328. [Google Scholar] [CrossRef]
- Dasgupta, P.; Kinkade, R.; Joshi, B.; Decook, C.; Haura, E.; Chellappan, S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc. Natl. Acad. Sci. USA 2006, 103, 6332–6337. [Google Scholar] [CrossRef]
- Wu, S.Y.; Xing, F.; Sharma, S.; Wu, K.; Tyagi, A.; Liu, Y.; Zhao, D.; Deshpande, R.P.; Shiozawa, Y.; Ahmed, T.; et al. Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J. Exp. Med. 2020, 217, e20191131. [Google Scholar] [CrossRef]

| Cell Line | Origin | Number of Cells Seeded Per Well a |
|---|---|---|
| MCF7 | Human, breast cancer | 5000 |
| A549 | Human, lung cancer | 10,000 |
| DU145 | Human, prostate cancer | 10,000 |
| HCT116 | Human, colon cancer | 1000 |
| Lovo | Human, colon cancer | 4000 |
| Protein | Gene Name | Probe ID |
|---|---|---|
| CD47 | CD47 | Hs00179953_m1 |
| TATA-binding protein | TBP | Hs00427621_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapostolou, I.; Bochen, F.; Peinelt, C.; Maldifassi, M.C. A Simple and Fast Method for the Formation and Downstream Processing of Cancer-Cell-Derived 3D Spheroids: An Example Using Nicotine-Treated A549 Lung Cancer 3D Spheres. Methods Protoc. 2023, 6, 94. https://doi.org/10.3390/mps6050094
Papapostolou I, Bochen F, Peinelt C, Maldifassi MC. A Simple and Fast Method for the Formation and Downstream Processing of Cancer-Cell-Derived 3D Spheroids: An Example Using Nicotine-Treated A549 Lung Cancer 3D Spheres. Methods and Protocols. 2023; 6(5):94. https://doi.org/10.3390/mps6050094
Chicago/Turabian StylePapapostolou, Irida, Florian Bochen, Christine Peinelt, and Maria Constanza Maldifassi. 2023. "A Simple and Fast Method for the Formation and Downstream Processing of Cancer-Cell-Derived 3D Spheroids: An Example Using Nicotine-Treated A549 Lung Cancer 3D Spheres" Methods and Protocols 6, no. 5: 94. https://doi.org/10.3390/mps6050094
APA StylePapapostolou, I., Bochen, F., Peinelt, C., & Maldifassi, M. C. (2023). A Simple and Fast Method for the Formation and Downstream Processing of Cancer-Cell-Derived 3D Spheroids: An Example Using Nicotine-Treated A549 Lung Cancer 3D Spheres. Methods and Protocols, 6(5), 94. https://doi.org/10.3390/mps6050094






