Generation of a Recombinant scFv against Deoxycholic Acid and Its Conversion to a Quenchbody for One-Step Immunoassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
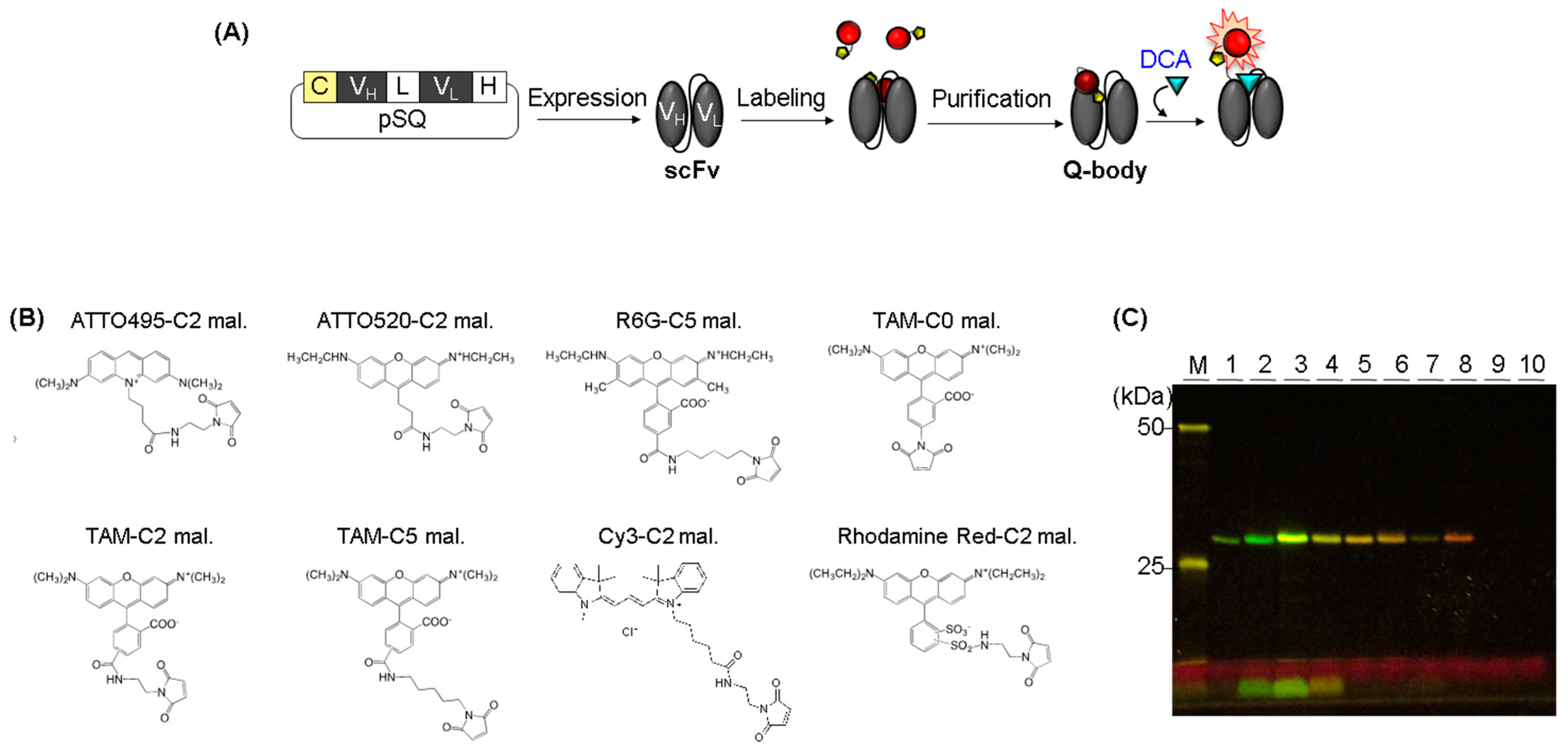
2.2. Gene Cloning
2.3. scFv Expression
2.4. Q-Body Generation
2.5. Fluorescence Measurement
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, Y.; Zhang, T.; Zhang, J.; Wang, Y.; Ding, S. Deoxycholic Acid Could Induce Apoptosis and Trigger Gastric Carcinogenesis on Gastric Epithelial Cells by Quantitative Proteomic Analysis. Gastroenterol. Res. Pract. 2016, 2016, 9638963. [Google Scholar] [CrossRef]
- Quilty, F.; Freeley, M.; Gargan, S.; Gilmer, J.; Long, A. Deoxycholic acid induces proinflammatory cytokine production by model oesophageal cells via lipid rafts. J. Steroid Biochem. Mol. Biol. 2021, 214, 105987. [Google Scholar] [CrossRef]
- Xu, M.; Cen, M.; Shen, Y.; Zhu, Y.; Cheng, F.; Tang, L.; Hu, W.; Dai, N. Deoxycholic Acid-Induced Gut Dysbiosis Disrupts Bile Acid Enterohepatic Circulation and Promotes Intestinal Inflammation. Dig. Dis. Sci. 2021, 66, 568–576. [Google Scholar] [CrossRef]
- Liu, Y.; Rong, Z.; Xiang, D.; Zhang, C.; Liu, D. Detection technologies and metabolic profiling of bile acids: A comprehensive review. Lipids Health Dis. 2018, 17, 121. [Google Scholar] [CrossRef]
- Maekawa, M.; Shimada, M.; Iida, T.; Goto, J.; Mano, N. Tandem mass spectrometric characterization of bile acids and steroid conjugates based on low-energy collision-induced dissociation. Steroids 2014, 80, 80–91. [Google Scholar] [CrossRef]
- Kakiyama, G.; Muto, A.; Takei, H.; Nittono, H.; Murai, T.; Kurosawa, T.; Hofmann, A.F.; Pandak, W.M.; Bajaj, J.S. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: Validation by GC-MS and LC-MS[S]. J. Lipid Res. 2014, 55, 978–990. [Google Scholar] [CrossRef]
- Mingtao, F.; Jiang, H. Recent Progress in Noncompetitive Hapten Immunoassays: A Review. In Trends in Immunolabelled and Related Techniques; Eltayb, A., Ed.; IntechOpen: Rijeka, Yugoslavia, 2012; Chapter 5; pp. 53–66. [Google Scholar]
- Bai, Y.; Fei, J.; Wu, W.; Dou, L.; Liu, M.; Shao, S.; Yu, W.; Wen, K.; Shen, J.; Wang, Z. Minimum Distance Between Two Epitopes in Sandwich Immunoassays for Small Molecules. Anal. Chem. 2022, 94, 17843–17852. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, Y.; Li, P.; Li, Q.; Yu, W.; Wen, K.; Eremin, S.A.; Shen, J.; Yu, X.; Wang, Z. Dual-Wavelength Fluorescence Polarization Immunoassay for Simultaneous Detection of Sulfonamides and Antibacterial Synergists in Milk. Biosensors 2022, 12, 1053. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xie, X.X.; Zhong, N.; Sun, L.C.; Lin, D.; Zhang, L.J.; Weng, L.; Jin, T.; Cao, M.J. Research Progresses and Applications of Fluorescent Protein Antibodies: A Review Focusing on Nanobodies. Int. J. Mol. Sci. 2023, 24, 4307. [Google Scholar] [CrossRef]
- Renard, M.; Bedouelle, H. Improving the sensitivity and dynamic range of reagentless fluorescent immunosensors by knowledge-based design. Biochemistry 2004, 43, 15453–15462. [Google Scholar] [CrossRef]
- Rani, A.Q.; Zhu, B.; Ueda, H.; Kitaguchi, T. Recent progress in homogeneous immunosensors based on fluorescence or bioluminescence using antibody engineering. Analyst 2023, 148, 1422–1429. [Google Scholar] [CrossRef]
- Renard, M.; Belkadi, L.; Hugo, N.; England, P.; Altschuh, D.; Bedouelle, H. Knowledge-based Design of Reagentless Fluorescent Biosensors from Recombinant Antibodies. J. Mol. Biol. 2002, 318, 429–442. [Google Scholar] [CrossRef]
- Ueda, H.; Dong, J. From fluorescence polarization to Quenchbody: Recent progress in fluorescent reagentless biosensors based on antibody and other binding proteins. Biochim. Biophys. Acta 2014, 1844, 1951–1959. [Google Scholar] [CrossRef]
- Dong, J.; Ueda, H. Recent Advances in Quenchbody, a Fluorescent Immunosensor. Sensors 2021, 21, 1223. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Kawamura, T.; Dong, J.; Ueda, H. Q-Bodies from Recombinant Single-Chain Fv Fragment with Better Yield and Expanded Palette of Fluorophores. ACS Sens. 2016, 1, 88–94. [Google Scholar] [CrossRef]
- Abe, R.; Jeong, H.-J.; Arakawa, D.; Dong, J.; Ohashi, H.; Kaigome, R.; Saiki, F.; Yamane, K.; Takagi, H.; Ueda, H. Ultra Q-bodies: Quench-based antibody probes that utilize dye-dye interactions with enhanced antigen-dependent fluorescence. Sci. Rep. 2014, 4, 4640. [Google Scholar] [CrossRef]
- Abe, R.; Ohashi, H.; Iijima, I.; Ihara, M.; Takagi, H.; Hohsaka, T.; Ueda, H. “Quenchbodies”: Quench-Based Antibody Probes That Show Antigen-Dependent Fluorescence. J. Am. Chem. Soc. 2011, 133, 17386–17394. [Google Scholar] [CrossRef]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence Quenching by Photoinduced Electron Transfer: A Reporter for Conformational Dynamics of Macromolecules. ChemPhysChem 2009, 10, 1389–1398. [Google Scholar] [CrossRef]
- Heinlein, T.; Knemeyer, J.-P.; Piestert, O.; Sauer, M. Photoinduced Electron Transfer between Fluorescent Dyes and Guanosine Residues in DNA-Hairpins. J. Phys. Chem. B 2003, 107, 7957–7964. [Google Scholar] [CrossRef]
- Marmé, N.; Knemeyer, J.-P.; Sauer, M.; Wolfrum, J. Inter- and Intramolecular Fluorescence Quenching of Organic Dyes by Tryptophan. Bioconjug. Chem. 2003, 14, 1133–1139. [Google Scholar] [CrossRef]
- Ohashi, H.; Matsumoto, T.; Jeong, H.J.; Dong, J.; Abe, R.; Ueda, H. Insight into the Working Mechanism of Quenchbody: Transition of the Dye around Antibody Variable Region That Fluoresces upon Antigen Binding. Bioconjug. Chem. 2016, 27, 2248–2253. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Ueda, H. Strategy for Making a Superior Quenchbody to Proteins: Effect of the Fluorophore Position. Sensors 2014, 14, 13285–13297. [Google Scholar] [CrossRef]
- Liang, J.; Dong, H.; Xu, F.; Li, B.; Li, H.; Chen, L.; Li, M.; Liu, Y.; Jiang, G.; Dong, J. Isolation of a Monoclonal Antibody and its Derived Immunosensor for Rapid and Sensitive Detection of 17β-Estradiol. Front. Bioeng. Biotechnol. 2022, 10, 818983. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, R.; Gao, Y.; Cheng, Y.; Zhao, S.; Li, M.; Li, H.; Dong, J. Immunosensor for Rapid and Sensitive Detection of Digoxin. ACS Omega 2023, 8, 15341–15349. [Google Scholar] [CrossRef]
- Liang, J.; Dong, H.; Wang, H.; Yi, Z.; Jiang, G.; Inagaki, T.; Gomez-Sanchez, C.E.; Dong, J.; Ueda, H. Creation of a quick and sensitive fluorescent immunosensor for detecting the mineralocorticoid steroid hormone aldosterone. J. Steroid. Biochem. Mol. Biol. 2022, 221, 106118. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ohmuro-Matsuyama, Y.; Ohashi, H.; Ohsawa, F.; Tatsu, Y.; Inagaki, M.; Ueda, H. Detection of vimentin serine phosphorylation by multicolor Quenchbodies. Biosens. Bioelectron. 2013, 40, 17–23. [Google Scholar] [CrossRef]
- Inoue, A.; Ohmuro-Matsuyama, Y.; Kitaguchi, T.; Ueda, H. Creation of a Nanobody-Based Fluorescent Immunosensor Mini Q-body for Rapid Signal-On Detection of Small Hapten Methotrexate. ACS Sens. 2020, 5, 3457–3464. [Google Scholar] [CrossRef]
- Jeong, H.J.; Dong, J.; Yeom, C.H.; Ueda, H. Synthesis of Quenchbodies for One-Pot Detection of Stimulant Drug Methamphetamine. Methods Protoc. 2020, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.L. Antibody fragments: Hope and hype. MAbs 2010, 2, 77–83. [Google Scholar] [CrossRef]
- Kang, T.H.; Seong, B.L. Solubility, Stability, and Avidity of Recombinant Antibody Fragments Expressed in Microorganisms. Front. Microbiol. 2020, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Sivaccumar, J.; Sandomenico, A.; Vitagliano, L.; Ruvo, M. Monoclonal Antibodies: A Prospective and Retrospective View. Curr. Med. Chem. 2021, 28, 435–471. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.S.; Anupa, A.; Gupta, J.A.; Rathore, A.S. Challenges in Expression and Purification of Functional Fab Fragments in E. coli: Current Strategies and Perspectives. Fermentation 2022, 8, 175. [Google Scholar] [CrossRef]
- Kobayashi, N.; Ohtoyo, M.; Wada, E.; Kato, Y.; Mano, N.; Goto, J. Generation of a single-chain Fv fragment for the monitoring of deoxycholic acid residues anchored on endogenous proteins. Steroids 2005, 70, 285–294. [Google Scholar] [CrossRef]
- Kobayashi, N.; Katayama, H.; Nagata, M.; Goto, J. Production of a Monoclonal Antibody for Sensitive Monitoring of Deoxycholic Acid Residues Anchored on Endogenous Proteins. Anal. Sci. 2000, 16, 1133–1138. [Google Scholar] [CrossRef][Green Version]
- Northrop, B.H.; Frayne, S.H.; Choudhary, U. Thiol–maleimide “click” chemistry: Evaluating the influence of solvent, initiator, and thiol on the reaction mechanism, kinetics, and selectivity. Polym. Chem. 2015, 6, 3415–3430. [Google Scholar] [CrossRef]
- Zanetti-Domingues, L.C.; Tynan, C.J.; Rolfe, D.J.; Clarke, D.T.; Martin-Fernandez, M. Hydrophobic fluorescent probes introduce artifacts into single molecule tracking experiments due to non-specific binding. PLoS ONE 2013, 8, e74200. [Google Scholar] [CrossRef]
- Zanetti-Domingues, L.C.; Tynan, C.J.; Daniel; Rolfe, J.; Clarke, D.T.; Martin-Fernandez, M.L. Artefacts Caused by Non-Specific Binding of Fluorescent Dyes in Single Molecule Experiments. 2015; Corpus ID: 207912259. [Google Scholar]
- Arbeloa, F.L.; Gonzalez, I.L.; Ojeda, P.R.; Arbeloa, I.L. Aggregate formation of rhodamine 6G in aqueous solution. J. Chem. Soc. Faraday Trans. 1982, 78, 989–994. [Google Scholar] [CrossRef]
- Jing, C.; Cornish, V.W. A fluorogenic TMP-tag for high signal-to-background intracellular live cell imaging. ACS Chem. Biol. 2013, 8, 1704–1712. [Google Scholar] [CrossRef]
- Zhao, S.; Dong, J.; Jeong, H.J.; Okumura, K.; Ueda, H. Rapid detection of the neonicotinoid insecticide imidacloprid using a quenchbody assay. Anal. Bioanal. Chem. 2018, 410, 4219–4226. [Google Scholar] [CrossRef]
- Dong, J.; Banwait, B.; Ueda, H.; Kristensen, P. V(H)-Based Mini Q-Body: A Novel Quench-Based Immunosensor. Sensors 2023, 23, 2251. [Google Scholar] [CrossRef] [PubMed]

| Dye | Exmax (nm) | Emmax (nm) | LogD at pH 7.4 | Net Charge at pH 7.4 | Fluorescence Intensity | |||
|---|---|---|---|---|---|---|---|---|
| Anti-DCA scFv (This Study) | Anti-BGP 1 scFv [17] | Anti-ICP 2 Fab [42] | Anti-Lysozyme VH [43] | |||||
| ATTO495-C2-mal. | 495 | 525 | −2.63 | −0.81 | 1.05 | 1.1 | ≈0.9 | ND |
| ATTO520-C2-mal. | 520 | 546 | −1.31 | −0.98 | 1.12 | 2.7 | ≈1.0 | ≈3.3 |
| R6G-C5-mal. | 530 | 558 | +5.59 | −1.70 | 1.19 | 5.0 | 2.6 | ≈4.5 |
| TAMRA-C0-mal. | 546 | 578 | +4.59 | −1.04 | 1.07 | 2.9 | ≈1.5 | ≈1.6 |
| TAMRA-C2-mal. | 546 | 583 | +3.81 | −1.31 | 1.31 | 2.0 | ≈1.9 | ≈1.4 |
| TAMRA-C5-mal. | 546 | 588 | +4.83 | −1.12 | 2.29 | 4.0 | ≈1.1 | ≈3.2 |
| Cy3-C2-mal. | 535 | 566 | +2.76 | −0.86 | 1.02 | ND | ND | ND |
| Rho. Red-C2-mal. | 565 | 592 | +1.87 | −1.34 | 1.20 | 1.7 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, H.; Jeong, H.-J. Generation of a Recombinant scFv against Deoxycholic Acid and Its Conversion to a Quenchbody for One-Step Immunoassay. Methods Protoc. 2023, 6, 90. https://doi.org/10.3390/mps6050090
Ueda H, Jeong H-J. Generation of a Recombinant scFv against Deoxycholic Acid and Its Conversion to a Quenchbody for One-Step Immunoassay. Methods and Protocols. 2023; 6(5):90. https://doi.org/10.3390/mps6050090
Chicago/Turabian StyleUeda, Hiroshi, and Hee-Jin Jeong. 2023. "Generation of a Recombinant scFv against Deoxycholic Acid and Its Conversion to a Quenchbody for One-Step Immunoassay" Methods and Protocols 6, no. 5: 90. https://doi.org/10.3390/mps6050090
APA StyleUeda, H., & Jeong, H.-J. (2023). Generation of a Recombinant scFv against Deoxycholic Acid and Its Conversion to a Quenchbody for One-Step Immunoassay. Methods and Protocols, 6(5), 90. https://doi.org/10.3390/mps6050090







