Solid-Phase Microextraction—Gas Chromatography Analytical Strategies for Pesticide Analysis
Abstract
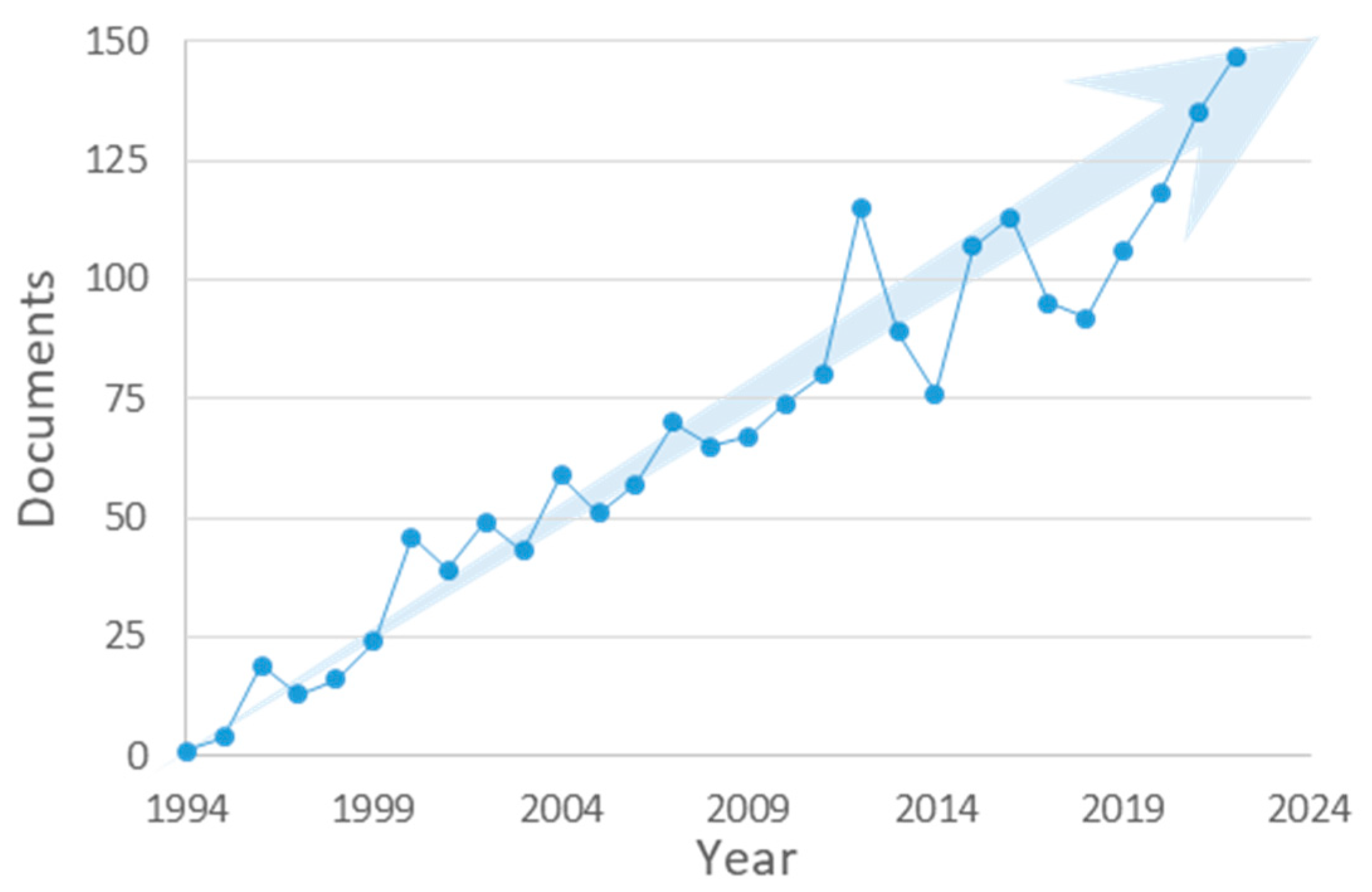
:1. Introduction
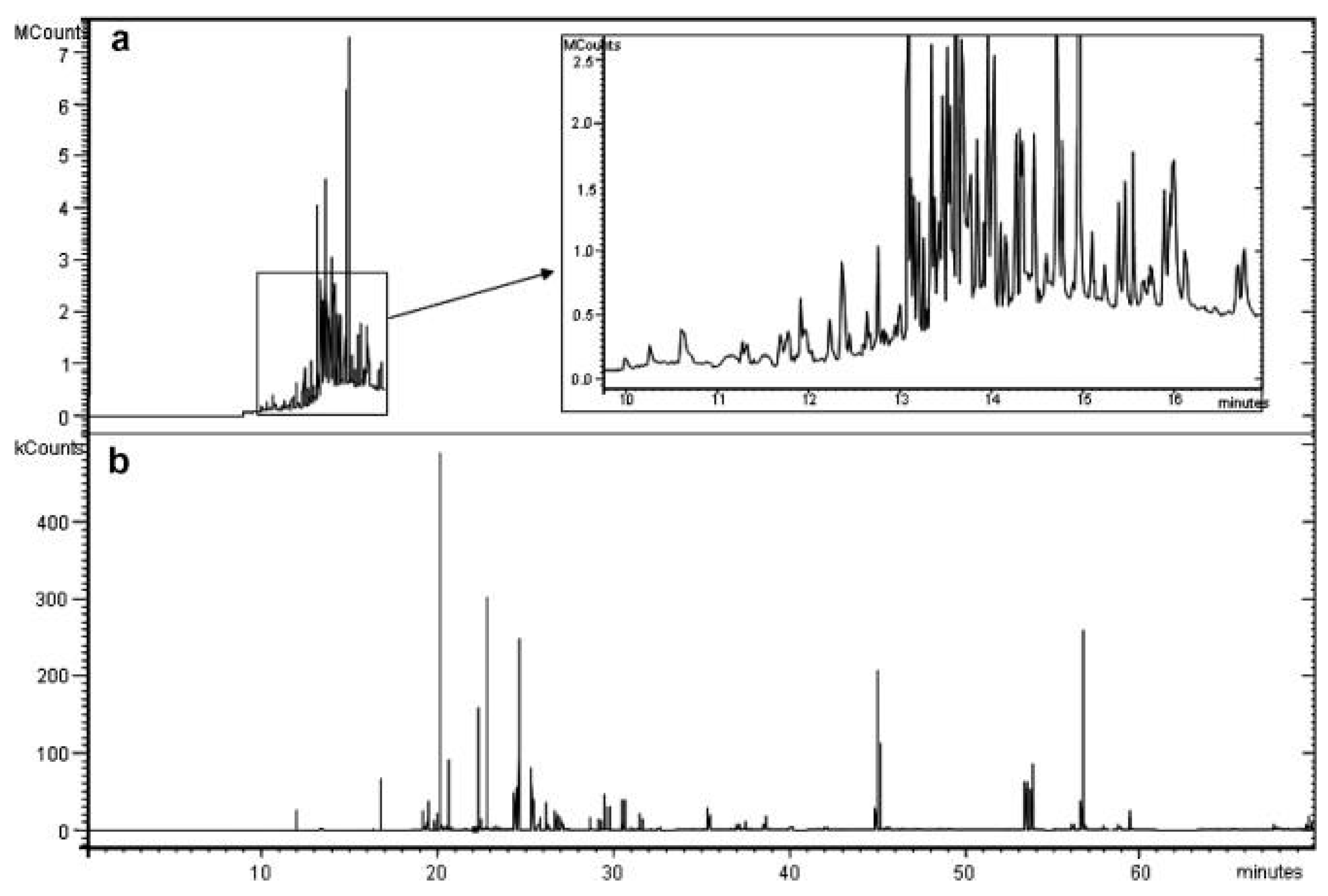
2. General Considerations
| Matrix | SPME Coating Tested 1 | SPME Mode | GC Detector | LOD | Ref. | |
|---|---|---|---|---|---|---|
| Food Samples | ||||||
| Fruits and Vegetables | PA PDMS PDMS/DVB PDMS/CAR DVB/CAR/PDMS | DI | MS (SIM) | 1.00–10.00 | ppb | [36] |
| C-(C3N4@MOF) | DI | MS (SIM) | 0.23–7.5 | ppb | [44] | |
| PDMS/DVB | DI | MS (TIC)2 | 0.013–0.110 (for 2D) | ppt | [42] | |
| PA | DI | MS (identification) FPD (quantification) | 0.01–0.14 | ppb | [45] | |
| PDMS (modified) | DI | TOFMS | 1–50 | ppb | [46] | |
| COF | DI | ECD | 0.04–0.25 | ppb | [47] | |
| PDMS/DVB/PDMS | DI | MS (SIM) | 1.0–33.0 only LOQ reported | ppb | [48] | |
| IL on silica | HS | FID | 0.01–0.93 | ppb | [49] | |
| COF | HS | ECD | 0.0003–0.0023 | ppt | [50] | |
| PDMS | HS | ECD | 0.01–1.0 | ppb | [51] | |
| PDMS PDMS/DVB | HS | MS (TIC) MS (SIM) | 0.11–3.48 | ppb | [35] | |
| Wine and Juice | PA | DI | MS (SIM) NPD FID | 0.01–15 10–6000 200–19000 | ppt | [52] |
| PA | DI | MS (SIM) | 2–90 | ppb | [53] | |
| PDMS PDMS/DVB | DI | MS/MS | 0.8–19.6 | ppb | [39] | |
| PDMS/DVB | HS | MS (TIC) 2 | 0.062–33.515 (for 2D) | ppb | [41] | |
| Milk | PDMS/DVB | DI | µECD | 0.003–0.56 | ppb | [54] |
| PDMS PDMS/DVB | DI HS | MS/MS | 0.01–0.30 | ppb | [40] | |
| PDMS/DVB | HS | MS (SIM) | 2.2–10.9 | ppb | [37] | |
| Honey | PA PDMS | DI | MS/MS | 0.12–50.42 | ppb | [55] |
| PDMS PA | DI | AED | 0.02–10.0 | ppb | [56] | |
| Electrospun nanostructured PS | HS | MS (SIM) | 0.1–2 | ppb | [57] | |
| Environmental samples | ||||||
| Soil and sediment | PDMS | DI | MS (identification) ECD (quantification) | 0.6–30 | ppb | [58] |
| PA | DI | MS (TIC) | 0.1–60 | ppb | [59] | |
| PA | DI | MS (SIM) NPD FID | 0.01–15 10–6000 200–19000 | ppt | [52] | |
| Water (including drinking water) | PDMS/DVB | DI | MS (TIC) ECD NPD | 4–32 | ppt | [60] |
| PA | DI | MS (SIM) | 0.05–19 | ppb | [61] | |
| PA | DI | MS (SIM) | 3–200 | ppt | [62] | |
| PDMS/DVB | DI | MS (SIM) | 0.003–0.322 | ppb | [63] | |
| PDMS/DVB | DI | MS/MS | 0.0002–0.04 | ppb | [64] | |
| NU-1000 (MOF) | DI | MS (SIM) | 0.011–0.058 | ppt | [65] | |
| DVB/CAR/PDMS | DI | ECD | 0.001–0.45 | ppt | [66] | |
| DVB/CAR/PDMS | DI | ECD | 0.002–0.070 | ppb | [67] | |
| PDMS/DVB | DI | ECD | 2.6–5.7 | ppt | [68] | |
| PDMS | DI | MS 3 | 0.001–0.025 | ppb | [43] | |
| Nafion on SBA-15 | HS | MS (TIC) | 0.01–0.09 | ppb | [69] | |
| PDMS PDMS/DVB | HS | MS/MS | 0.9–26.3 | ppt | [70] | |
| PA | HS | HRMS (magnetic sector) | 0.01–350 only LOQ reported | ppt | [71] | |
| PDMS | HS | ECD | 0.034–0.301 | ppb | [72] | |
3. Sample Matrices
3.1. Food Samples
3.2. Environmental Samples
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Panighel, A.; Flamini, R. Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riboni, N.; Fornari, F.; Bianchi, F.; Careri, M. Recent Advances in in Vivo Spme Sampling. Separations 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Moreira, N.; Lopes, P.; Cabral, M.; Guedes de Pinho, P. HS-SPME/GC-MS Methodologies for the Analysis of Volatile Compounds in Cork Material. Eur. Food Res. Technol. 2016, 242, 457–466. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, K. Recent Advances in SPME Techniques in Biomedical Analysis. J. Pharm. Biomed. Anal. 2011, 54, 926–950. [Google Scholar] [CrossRef]
- Pati, S.; Tufariello, M.; Crupi, P.; Coletta, A.; Grieco, F.; Losito, I. Quantification of Volatile Compounds in Wines by HS-SPME-GC/MS: Critical Issues and Use of Multivariate Statistics in Method Optimization. Processes 2021, 9, 662. [Google Scholar] [CrossRef]
- Ouyang, G.; Pawliszyn, J. SPME in Environmental Analysis. Anal. Bioanal. Chem. 2006, 386, 1059–1073. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Jiang, R.; Rodríguez-Lafuente, A.; Gionfriddo, E.; Pawliszyn, J. A Critical Review of the State of the Art of Solid-Phase Microextraction of Complex Matrices I. Environmental Analysis. TrAC Trends Anal. Chem. 2015, 71, 224–235. [Google Scholar] [CrossRef]
- de Fátima Alpendurada, M. Solid-Phase Microextraction: A Promising Technique for Sample Preparation in Environmental Analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef]
- Environmental Protection Agency What Is a Pesticide? Available online: https://www.epa.gov/minimum-risk-pesticides/what-pesticide (accessed on 28 August 2022).
- Hassaan, M.A.; El Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and Determination of Pesticides in Fruits and Vegetables. TrAC-Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Li, Z.; Jennings, A. Worldwide Regulations of Standard Values of Pesticides for Human Health Risk Control: A Review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef] [Green Version]
- Tobiszewski, M.; Mechlinska, A.; Namie, J. Green Analytical Chemistry—Theory and Practice. Chem. Soc. Rev. 2010, 39, 2869–2878. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green Extraction Techniques in Green Analytical Chemistry. TrAC - Trends Anal. Chem. 2019, 116, 248–253. [Google Scholar] [CrossRef]
- Elsevier Scopus. Available online: https://www.scopus.com/results/results.uri?sort=plf-f&src=s&st1=pesticides&st2=gas+chromatography&searchTerms=SPME%3f%21%22*%24&sid=b6292b113259b87b3290315e5d5d5364&sot=b&sdt=b&sl=89&s=%28TITLE-ABS-KEY%28pesticides%29+AND+TITLE-ABS-KEY%28gas+chromatography%29+AND+TITLE-ABS-KEY%28SPME%29%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Present (accessed on 28 August 2022).
- Zhang, Q.H.; Zhou, L.D.; Chen, H.; Wang, C.Z.; Xia, Z.N.; Yuan, C.S. Solid-Phase Microextraction Technology for in Vitro and in Vivo Metabolite Analysis. TrAC-Trends Anal. Chem. 2016, 80, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacl, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of Solid-Phase Microextraction. In Handbook of Solid Phase Microextraction; Elsevier: Amsterdam, The Netherlands, 2012; pp. 13–59. ISBN 9780124160170. [Google Scholar]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Garcés, N.R.; Tascon, M. Smart Materials in Solid Phase Microextraction (SPME). In Handbook of Smart Materials in Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 581–620. [Google Scholar]
- Patel, D.I.; Roychowdhury, T.; Shah, D.; Jacobsen, C.; Herrington, J.S.; Hoisington, J.; Myers, C.; Salazar, B.G.; Walker, A.V.; Bell, D.S.; et al. 6-Phenylhexyl Silane Derivatized, Sputtered Silicon Solid Phase Microextraction Fiber for the Parts-per-trillion Detection of Polyaromatic Hydrocarbons in Water and Baby Formula. J. Sep. Sci. 2021, 44, 2824–2836. [Google Scholar] [CrossRef]
- Dugheri, S.; Mucci, N.; Cappelli, G.; Trevisani, L.; Bonari, A.; Bucaletti, E.; Squillaci, D.; Arcangeli, G. Advanced Solid-Phase Microextraction Techniques and Related Automation: A Review of Commercially Available Technologies. J. Anal. Methods Chem. 2022, 2022. [Google Scholar] [CrossRef]
- David, F.; Ochiai, N.; Sandra, P. Two Decades of Stir Bar Sorptive Extraction: A Retrospective and Future Outlook. TrAC Trends Anal. Chem. 2019, 112, 102–111. [Google Scholar] [CrossRef]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting Molecules in Complex Matrices with Spme Arrows: A Review. Separations 2020, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Pawliszyn, J. Thin-Film Microextraction Offers Another Geometry for Solid-Phase Microextraction. TrAC Trends Anal. Chem. 2012, 39, 245–253. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Gionfriddo, E.; Grandy, J.J.; Alam, M.N.; Pawliszyn, J. Development and Validation of Eco-Friendly Strategies Based on Thin Film Microextraction for Water Analysis. J. Chromatogr. A 2018, 1579, 20–30. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef] [Green Version]
- Raeppel, C.; Fabritius, M.; Nief, M.; Appenzeller, B.M.R.; Millet, M. Coupling ASE, Sylilation and SPME–GC/MS for the Analysis of Current-Used Pesticides in Atmosphere. Talanta 2014, 121, 24–29. [Google Scholar] [CrossRef]
- Henriksen, T.; Svensmark, B.; Lindhardt, B.; Juhler, R.K. Analysis of Acidic Pesticides Using in Situ Derivatization with Alkylchloroformate and Solid-Phase Microextraction (SPME) for GC–MS. Chemosphere 2001, 44, 1531–1539. [Google Scholar] [CrossRef]
- Pena-Pereira, F. Miniaturization in Sample Preparation; De Gruyter Open: Berlin, Germany, 2014; ISBN 978-3-11-041017-4. [Google Scholar]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC-Trends Anal. Chem. 2022, 148. [Google Scholar] [CrossRef]
- Abdulra’uf, L.B.; Tan, G.H. Chemometric Approach to the Optimization of HS-SPME/GC–MS for the Determination of Multiclass Pesticide Residues in Fruits and Vegetables. Food Chem. 2015, 177, 267–273. [Google Scholar] [CrossRef]
- Menezes Filho, A.; dos Santos, F.N.; de Paula Pereira, P.A. Development, Validation and Application of a Methodology Based on Solid-Phase Micro Extraction Followed by Gas Chromatography Coupled to Mass Spectrometry (SPME/GC–MS) for the Determination of Pesticide Residues in Mangoes. Talanta 2010, 81, 346–354. [Google Scholar] [CrossRef]
- Rodrigues, F.d.M.; Mesquita, P.R.R.; de Oliveira, L.S.; de Oliveira, F.S.; Menezes Filho, A.; de P. Pereira, P.A.; de Andrade, J.B. Development of a Headspace Solid-Phase Microextraction/Gas Chromatography–Mass Spectrometry Method for Determination of Organophosphorus Pesticide Residues in Cow Milk. Microchem. J. 2011, 98, 56–61. [Google Scholar] [CrossRef]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. A Multiresidue Method for the Analysis of 90 Pesticides, 16 PAHs, and 22 PCBs in Honey Using QuEChERS–SPME. Anal. Bioanal. Chem. 2017, 409, 5157–5169. [Google Scholar] [CrossRef]
- Cortés-Aguado, S.; Sánchez-Morito, N.; Arrebola, F.J.; Frenich, A.G.; Vidal, J.L.M. Fast Screening of Pesticide Residues in Fruit Juice by Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Food Chem. 2008, 107, 1314–1325. [Google Scholar] [CrossRef]
- González-Rodríguez, M.J.; Arrebola Liébanas, F.J.; Garrido Frenich, A.; Martínez Vidal, J.L.; Sánchez López, F.J. Determination of Pesticides and Some Metabolites in Different Kinds of Milk by Solid-Phase Microextraction and Low-Pressure Gas Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2005, 382, 164–172. [Google Scholar] [CrossRef]
- Del Castillo, M.L.R.; Rodriguez-Valenciano, M.; De La Peña Moreno, F.; Blanch, G.P. Evaluation of Pesticide Residue Contents in Fruit Juice by Solid-Phase Microextraction and Multidimensional Gas Chromatography Coupled with Mass Spectrometry. Talanta 2012, 89, 77–83. [Google Scholar] [CrossRef]
- Ruiz del Castillo, M.L.; Rodríguez-Valenciano, M.; Flores, G.; Blanch, G.P. New Method Based on Solid Phase Microextraction and Multidimensional Gas Chromatography-Mass Spectrometry to Determine Pesticides in Strawberry Jam. LWT-Food Sci. Technol. 2019, 99, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Purcaro, G.; Quinto Tranchida, P.; Conte, L.; Obiedzińska, A.; Dugo, P.; Dugo, G.; Mondello, L. Performance Evaluation of a Rapid-Scanning Quadrupole Mass Spectrometer in the Comprehensive Two-Dimensional Gas Chromatography Analysis of Pesticides in Water. J. Sep. Sci. 2011, 34, 2411–2417. [Google Scholar] [CrossRef]
- Pang, Y.; Zang, X.; Li, H.; Liu, J.; Chang, Q.; Zhang, S.; Wang, C.; Wang, Z. Solid-Phase Microextraction of Organophosphorous Pesticides from Food Samples with a Nitrogen-Doped Porous Carbon Derived from g-C3N4 Templated MOF as the Fiber Coating. J. Hazard. Mater. 2020, 384, 121430. [Google Scholar] [CrossRef]
- Sapahin, H.A.; Makahleh, A.; Saad, B. Determination of Organophosphorus Pesticide Residues in Vegetables Using Solid Phase Micro-Extraction Coupled with Gas Chromatography–Flame Photometric Detector. Arab. J. Chem. 2015, 12, 1934–1944. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Pawliszyn, J. Direct Immersion Solid-Phase Microextraction with Matrix-Compatible Fiber Coating for Multiresidue Pesticide Analysis of Grapes by Gas Chromatography–Time-of-Flight Mass Spectrometry (DI-SPME-GC-ToFMS). J. Agric. Food Chem. 2015, 63, 4464–4477. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, X.; Zang, X.; Pang, Y.; Chang, Q.; Wang, C.; Wang, Z. Determination of Pesticides Residues in Vegetable and Fruit Samples by Solid-Phase Microextraction with a Covalent Organic Framework as the Fiber Coating Coupled with Gas Chromatography and Electron Capture Detection. J. Sep. Sci. 2018, 41, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gionfriddo, E.; Acquaro, V.; Pawliszyn, J. Direct Immersion Solid-Phase Microextraction Analysis of Multi-Class Contaminants in Edible Seaweeds by Gas Chromatography-Mass Spectrometry. Anal. Chim. Acta 2018, 1031, 83–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delinska, K.; Yavir, K.; Kloskowski, A. Head-Space SPME for the Analysis of Organophosphorus Insecticides by Novel Silica IL-Based Fibers in Real Samples. Molecules 2022, 27, 4688. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Ma, J.; Liu, P.; Jia, Q. Fabrication of Cross-Linked Hydrazone Covalent Organic Frameworks by Click Chemistry and Application to Solid Phase Microextraction. Talanta 2016, 161, 350–358. [Google Scholar] [CrossRef]
- Mee Kin, C.; Guan Huat, T. Headspace Solid-Phase Microextraction for the Evaluation of Pesticide Residue Contents in Cucumber and Strawberry after Washing Treatment. Food Chem. 2010, 123, 760–764. [Google Scholar] [CrossRef]
- Boyd-boland, A.A.; Pawliszyn, J.B. Solid-Phase Microextraction of Nitrogen-Containing Herbicides. J. Chromatogr. A 1995, 704, 163–172. [Google Scholar] [CrossRef]
- Zambonin, C.G.; Quinto, M.; De Vietro, N.; Palmisano, F. Solid-Phase Microextraction–Gas Chromatography Mass Spectrometry: A Fast and Simple Screening Method for the Assessment of Organophosphorus Pesticides Residues in Wine and Fruit Juices. Food Chem. 2004, 86, 269–274. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, M.; Llompart, M.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Cela, R.; Dagnac, T. Development of a Solid-Phase Microextraction Gas Chromatography with Microelectron-Capture Detection Method for a Multiresidue Analysis of Pesticides in Bovine Milk. Anal. Chim. Acta 2008, 617, 37–50. [Google Scholar] [CrossRef]
- Akbarzade, S.; Chamsaz, M.; Rounaghi, G.H.; Ghorbani, M. Zero Valent Fe-Reduced Graphene Oxide Quantum Dots as a Novel Magnetic Dispersive Solid Phase Microextraction Sorbent for Extraction of Organophosphorus Pesticides in Real Water and Fruit Juice Samples Prior to Analysis by Gas Chromatography-Mass Spectrom. Anal. Bioanal. Chem. 2018, 410, 429–439. [Google Scholar] [CrossRef]
- Campillo, N.; Peñalver, R.; Aguinaga, N.; Hernández-Córdoba, M. Solid-Phase Microextraction and Gas Chromatography with Atomic Emission Detection for Multiresidue Determination of Pesticides in Honey. Anal. Chim. Acta 2006, 562, 9–15. [Google Scholar] [CrossRef]
- Zali, S.; Jalali, F.; Es-haghi, A.; Shamsipur, M. Electrospun Nanostructured Polystyrene as a New Coating Material for Solid-Phase Microextraction: Application to Separation of Multipesticides from Honey Samples. J. Chromatogr. B 2015, 1002, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bouaid, A.; Ramos, L.; Gonzalez, M.; Fernández, P.; Cámara, C. Solid-Phase Microextraction Method for the Determination of Atrazine and Four Organophosphorus Pesticides in Soil Samples by Gas Chromatography. J. Chromatogr. A 2001, 939, 13–21. [Google Scholar] [CrossRef]
- Boyd-boland, A.A.; Magdic, S.; Pawliszyn, J.B. Simultaneous Determination of 60 Pesticides in Water Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Analyst 1996, 121, 929–938. [Google Scholar] [CrossRef]
- Beceiro-González, E.; Concha-Graña, E.; Guimaraes, A.; Gonçalves, C.; Muniategui-Lorenzo, S.; Alpendurada, M.F. Optimisation and Validation of a Solid-Phase Microextraction Method for Simultaneous Determination of Different Types of Pesticides in Water by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2007, 1141, 165–173. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; García-Hermida, C.; Rodríguez-Gonzalo, E.; Ruano-Miguel, L. Behaviour of Carbamate Pesticides in Gas Chromatography and Their Determination with Solid-Phase Extraction and Solid-Phase Microextraction as Preconcentration Steps. J. Sep. Sci. 2005, 28, 2130–2138. [Google Scholar] [CrossRef]
- Eisert, R.; Levsen, K. Determination of Pesticides in Aqueous Samples by Solid-Phase Microextraction in-Line Coupled to Gas Chromatography—Mass Spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Elizarragaz-de la Rosa, D.; Guzmán-Mar, J.L.; Salas-Espinosa, E.A.; Heras-Ramírez, M.E.; Hinojosa-Reyes, L.; Gaspar-Ramírez, O.; Ruiz-Ruiz, E.J. Multi-Residual Determination of Multi-Class Pesticides in Groundwater by Direct Immersion Solid-Phase Microextraction with Gas Chromatography-Selected Ion Monitoring Mass Spectrometry (GC–MS/SIM) Detection. Water Air Soil Pollut. 2022, 233, 76. [Google Scholar] [CrossRef]
- Gonçalves, C.; Alpendurada, M.F. Solid-Phase Micro-Extraction-Gas Chromatography-(Tandem) Mass Spectrometry as a Tool for Pesticide Residue Analysis in Water Samples at High Sensitivity and Selectivity with Confirmation Capabilities. J. Chromatogr. A 2004, 1026, 239–250. [Google Scholar] [CrossRef]
- Gong, X.; Xu, L.; Huang, S.; Kou, X.; Lin, S.; Chen, G.; Ouyang, G. Application of the NU-1000 Coated SPME Fiber on Analysis of Trace Organochlorine Pesticides in Water. Anal. Chim. Acta 2022, 1218, 339982. [Google Scholar] [CrossRef]
- Junior, J.; Repoppi, N. Determination of Organochlorine Pesticides in Ground Water Samples Using Solid-Phase Microextraction by Gas Chromatography-Electron Capture Detection. Talanta 2007, 72, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-P.; Li, G.-C.; Jen, J.-F. Determination of Organochlorine Pesticides in Water Using Microwave Assisted Headspace Solid-Phase Microextraction and Gas Chromatography. J. Chromatogr. A 2003, 1012, 129–137. [Google Scholar] [CrossRef]
- Perez-Trujillo, J.P.; Frías, S.; Sáchez, M.J.; Conde, J.E.; Rodríguez-Delgado, M.A. Determination of Organochlorine Pesticides by Gas Chromatography with Solid-Phase Microextraction. Chromatographia 2002, 56, 191–197. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Hassani, S.; Bamorowat, M. Efficient Solid-Phase Microextraction of Triazole Pesticides from Natural Water Samples Using a Nafion-Loaded Trimethylsilane-Modified Mesoporous Silica Coating of Type SBA-15. Microchim. Acta 2016, 183, 889–895. [Google Scholar] [CrossRef]
- Derouiche, A.; Driss, M.R.; Morizur, J.P.; Taphanel, M.H. Simultaneous Analysis of Polychlorinated Biphenyls and Organochlorine Pesticides in Water by Headspace Solid-Phase Microextraction with Gas Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2007, 1138, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, I.; Arrebola, F.J.; Gavara, R.; Martínez Vidal, J.L.; Frenich, A.G. Automated and Simultaneous Determination of Priority Substances and Polychlorinated Biphenyls in Wastewater Using Headspace Solid Phase Microextraction and High Resolution Mass Spectrometry. Anal. Chim. Acta 2017, 1002, 39–49. [Google Scholar] [CrossRef]
- Chang, S.M.; Doong, R.A. Concentration and Fate of Persistent Organochlorine Pesticides in Estuarine Sediments Using Headspace Solid-Phase Microextraction. Chemosphere 2006, 62, 1869–1878. [Google Scholar] [CrossRef]
- López, F.J.; Pitarch, E.; Egea, S.; Beltran, J.; Hernández, F. Gas Chromatographic Determination of Organochlorine and Organophosphorus Pesticides in Human Fluids Using Solid Phase Microextraction. Anal. Chim. Acta 2001, 433, 217–226. [Google Scholar] [CrossRef]
- Tsoukali, H.; Theodoridis, G.; Raikos, N.; Grigoratou, I. Solid Phase Microextraction Gas Chromatographic Analysis of Organophosphorus Pesticides in Biological Samples. J. Chromatogr. B 2005, 822, 194–200. [Google Scholar] [CrossRef]
- Hernandez, F.; Pitarch, E.; Beltran, J.; Lopez, F.J. Headspace Solid-Phase Microextraction in Combination with Gas Chromatography and Tandem Mass Spectrometry for the Determination of Organochlorine and Organophosphorus Pesticides in Whole Human Blood Q. J. Chromatogr. B 2002, 769, 65–77. [Google Scholar] [CrossRef]
- Zhu, F.; Ruan, W.; He, M.; Zeng, F.; Luan, T.; Tong, Y.; Lu, T.; Ouyang, G. Application of Solid-Phase Microextraction for the Determination of Organophosphorus Pesticides in Textiles by Gas Chromatography with Mass Spectrometry. Anal. Chim. Acta 2009, 650, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.-H.; Teh, S.-Y.; Hossain, M.M.; Nadarajaw, T.; Zabidi-Hussin, Z.; Chin, S.-Y.; Lai, K.-S.; Lim, S.-H.E. Application, Monitoring and Adverse Effects in Pesticide Use: The Importance of Reinforcement of Good Agricultural Practices (GAPs). J. Environ. Manag. 2020, 260, 109987. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 396/2005, Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin; Publications Office of the European Union: Copenhagen, Denmark, 2005.
- Page, B.D.; Lacroix, G. Application of Solid-Phase Microextraction to the Headspace Gas Chromatographic Analysis of Halogenated Volatiles in Selected Foods. J. Chromatogr. A 1993, 648, 199–211. [Google Scholar] [CrossRef]
- Mascrez, S.; Psillakis, E.; Purcaro, G. A Multifaceted Investigation on the Effect of Vacuum on the Headspace Solid-Phase Microextraction of Extra-Virgin Olive Oil. Anal. Chim. Acta 2020, 1103, 106–114. [Google Scholar] [CrossRef]
- Möder, M.; Popp, P.; Eisert, R.; Pawliszyn, J. Determination of Polar Pesticides in Soil by Solid Phase Microextraction Coupled to High-Performance Liquid Chromatography-Electrospray/Mass Spectrometry. Fresenius. J. Anal. Chem. 1999, 363, 680–685. [Google Scholar] [CrossRef]
- Hernandez, F.; Beltran, J.; Lopez, F.J.; Gaspar, J.V. Use of Solid-Phase Microextraction for the Quantitative Determination of Herbicides in Soil and Water Samples. Anal. Chem. 2000, 72, 2313–2322. [Google Scholar] [CrossRef]
- European Commission. 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified under Document Number C(2002) 3044); Publications Office of the European Union: Copenhagen, Denmark, 2002.
- Nollet, L.M.L.; De Gelder, L.S.P. (Eds.) Handbook of Water Analysis; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780849384868. [Google Scholar]
- Barceló, D. Environmental Protection Agency and Other Methods for the Determination of Priority Pesticides and Their Transformation Products in Water. J. Chromatogr. 1993, 643, 117–143. [Google Scholar] [CrossRef]
- Ghiasvand, A.; Yazdankhah, F.; Paull, B. Heating-, Cooling- and Vacuum-Assisted Solid-Phase Microextraction (HCV-SPME) for Efficient Sampling of Environmental Pollutants in Complex Matrices. Chromatographia 2020, 83, 531–540. [Google Scholar] [CrossRef]
- Psillakis, E. Vacuum-Assisted Headspace Solid-Phase Microextraction: A Tutorial Review. Anal. Chim. Acta 2017, 986, 12–24. [Google Scholar] [CrossRef]
- Lv, F.; Gan, N.; Huang, J.; Hu, F.; Cao, Y.; Zhou, Y.; Dong, Y.; Zhang, L.; Jiang, S. A Poly-Dopamine Based Metal-Organic Framework Coating of the Type PDA-MIL-53(Fe) for Ultrasound-Assisted Solid-Phase Microextraction of Polychlorinated Biphenyls Prior to Their Determination by GC-MS. Microchim. Acta 2017, 184, 2561–2568. [Google Scholar] [CrossRef]
- Wei, M.C.; Jen, J.F. Determination of Polycyclic Aromatic Hydrocarbons in Aqueous Samples by Microwave Assisted Headspace Solid-Phase Microextraction and Gas Chromatography/Flame Ionization Detection. Talanta 2007, 72, 1269–1274. [Google Scholar] [CrossRef]
- Mascrez, S.; Purcaro, G. Exploring Multiple-cumulative Trapping Solid-phase Microextraction for Olive Oil Aroma Profiling. J. Sep. Sci. 2020, 43, 1934–1941. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Mascrez, S.; McGregor, L.; Purcaro, G. Exploring Multiple-Cumulative Trapping Solid-Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry for Quality and Authenticity Assessment of Olive Oil. Food Chem. 2022, 383, 132438. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aspromonte, J.; Lancioni, C.; Purcaro, G. Solid-Phase Microextraction—Gas Chromatography Analytical Strategies for Pesticide Analysis. Methods Protoc. 2022, 5, 82. https://doi.org/10.3390/mps5050082
Aspromonte J, Lancioni C, Purcaro G. Solid-Phase Microextraction—Gas Chromatography Analytical Strategies for Pesticide Analysis. Methods and Protocols. 2022; 5(5):82. https://doi.org/10.3390/mps5050082
Chicago/Turabian StyleAspromonte, Juan, Carlina Lancioni, and Giorgia Purcaro. 2022. "Solid-Phase Microextraction—Gas Chromatography Analytical Strategies for Pesticide Analysis" Methods and Protocols 5, no. 5: 82. https://doi.org/10.3390/mps5050082
APA StyleAspromonte, J., Lancioni, C., & Purcaro, G. (2022). Solid-Phase Microextraction—Gas Chromatography Analytical Strategies for Pesticide Analysis. Methods and Protocols, 5(5), 82. https://doi.org/10.3390/mps5050082









