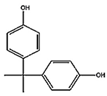
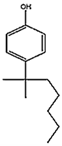
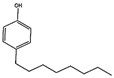
Abstract
Aquaculture, a mass supplier of seafood, relies on plastic materials that may contain the endocrine disruptors bisphenol-A (BPA) and tert-octylphenol (t-OCT). These pollutants present toxicity to Artemia, the live aquaculture feed, and are transferred through it to the larval stages of the cultured organisms. The purpose of this work is the development and validation of an analytical method to determine BPA and t-OCT in Artemia and their culture medium, using n-octylphenol as the internal standard. Extraction of the samples was performed with H2O/TFA (0.08%)–methanol (3:1), followed by SPE. Analysis was performed in a Nucleosil column with mobile phases A (95:5, v/v, 0.1% TFA in H2O:CH3CN) and B (5:95, v/v, 0.08% TFA in H2O:CH3CN). Calibration curves were constructed in the range of concentrations expected following a 24 h administration of BPA (10 μg/mL) or t-OCT (0.5 μg/mL), below their respective LC50. At the end of exposure to the pollutants, their total levels appeared reduced by about 32% for BPA and 35% for t-OCT, and this reduction could not be accounted for by photodegradation (9–19%). The developed method was validated in terms of linearity, accuracy, and precision, demonstrating the uptake of BPA and t-OCT in Artemia.
1. Introduction
The worldwide accumulation of plastic pollution in the marine environment poses a great risk towards the wellbeing of the vertebrate and invertebrate organisms that live in it, as well as of humans as the end consumer. The protective gear that was extensively produced during the COVID-19 pandemic, has greatly contributed to the plastic waste, a large amount of which ends up in the marine environment, where leaching occurs of its hydrophobic additives [1]. The highly hydrophobic organic contaminants include endocrine disrupting chemicals (EDCs), such as bisphenol A (BPA), nonylphenol, and tert-octylphenol (t-OCT). EDCs are incorporated into plastics as building blocks or stabilizers [2] and leach out, due to aging and heat [3,4]. Bisphenol-A (4, 4′-Isopropylidenediphenol) is widely used as a component of synthetic plastic [2] while 4-tert-octylphenol (4-(1,1,3,3-tetramethylbutyl) phenol) is used as a component of polyethoxylates, applied in detergents, industrial cleaners, and emulsifiers [3]. The percentage of stabilizers and antioxidants, including BPA and t-OCT in plastics, depends on the chemical structure of the produced plastic polymer, ranging from 0.05 to 3% w/w [5]. Studies on the levels of BPA and t-OCT in microplastics are gaining attention, and marine microplastics sampled in the open Pacific and the Atlantic Ocean, as well as from beaches in Asia and Central America, reported concentrations reaching 1–730 ng/g for BPA, and 0.1–153 ng/g for t-OCT [6]. BPA has been listed as one of the very high concern compounds by the European Chemicals Agency [7]. The European Food Safety Authority (EFSA) reassessed in 2015 the current tolerable daily intake (TDI) for BPA and reduced it from 50 to a temporary value of 4 μg /kg b. w. daily [8]. However, the plastic industries are in a legal dispute against the European Chemicals Agency’s (ECHA) decision to identify bisphenol A (BPA) as a “substance of very high concern” [9]. It appears that, although BPA is banned in some countries such as Canada, the fight for BPA to stay on shelves continues in Europe [10]. On the other hand, t-OCT is under assessment as persistent, bio—accumulative, and toxic, especially to aquatic life [11,12]. Both compounds present endocrine disrupting properties, resulting from their structural resemblance to the human 17β-estradiol [13], with t-OCT presenting higher estrogenicity than the other pollutants [14,15].
Following uptake, BPA is metabolized through its transformation to glucuronide and sulfate derivatives by vertebrates, such as tadpoles and fish [16], as well as mammals [17], while bacteria lead to the biotransformation of BPA to Hydroquinone, 4-Hydroxyacetophenone, 4-Hydroxybenzoic acid, and 4-Isopropenylphenol [18]. Although BPA has been detected in invertebrates, the reports on its metabolism by aquatic invertebrates are available only for bivalves, which transform it to mono- and disulfate [19]. In a similar manner, alkylphenols, including t-OCT, are metabolized by glucuronidation and/or sulfation in the liver of mammals such as rats [20] and humans [21], while bacteria transform it to end products including hydroquinone, 4-Hydroxybenzaldehyde and 4-Hydroxybenzoic acid [22].
Endocrine disruptors, such as BPA, induce oxidative stress and exert toxic effects on freshwater aquatic organisms by altering bacteria composition in their environment [23]. In the marine environment, sorption of EDCs from plastic particles in the surrounding seawater has been documented, indicating that marine plastics, under environmentally induced stress conditions, can act as carriers of organic contaminants and transfer them to marine organisms [3]. Marine organisms ingest the microplastics together with their contaminants. Amounts of BPA have been detected in fish [24], bivalves [25], and seawater [26], while t-OCT was detected in fish [27], mollusks [28], and shrimp [29]. Nowadays, the largest portion of all seafood that are used as food for humans, originate from aquaculture [30]. Aquaculture relies on plastic use in many aspects, such as fish cages, fish feeders, and fish tanks [31,32]. Moreover, aquaculture hatcheries require the live feed Artemia nauplii or metanauplii for feeding of the cultured fish and crustacea larvae [33,34], due to its high nutritional value characterized by high contents of neutral lipids [35]. Although Artemia is a crustacean adaptable to a wide range of environmental conditions, it is absent in common marine ecosystems, with its natural habitat being high salinity environments such as salt lakes, where it is found in the form of dormant cysts [36]. This saltwater planktonic crustacean, being a filter-feeder, consumes small-sized particles such as microalgae or organic manure [37], and even microplastics that are present in the water column [38,39]. The transfer of pollutants from microplastics to Artemia nauplii, and then to the zebrafish that consumed these nauplii as a feed, has been demonstrated for benzopyrene [40]. The genus Artemia spp. is widely used as a toxicity testing model due to its handling advantages, which include the fast hatching of cysts to nauplii, metanauplii, or adults, and hence the ease of access to these developmental stages [41,42]. Moreover, BPA toxicity against the first developmental stages (Instar I-II) of Artemia franciscana nauplii [43,44] as well as of the alkylphenol n-hexylphenol against A. sinica [45] have been reported, without, however, including t-octylphenol or a determination of the compounds in Artemia. The transfer of the endocrine disruption across species from the freshwater planktonic crustacean D. magna to both its consumer organisms, namely fish [46], as well as to its prey that is the algae it feeds on [47], was considered an indication of a broader action of endocrine disruptors that extends beyond the target organisms, further emphasizing the ecological risk.
Bisphenol-A has gained the focus of attention and the determination of BPA in food [48], and environmental samples [49] has been extensively reviewed. The reported methods mostly employ solvent extraction and SPE (polymer, Oasis, or C18) for the isolation of BPA from samples, followed by HPLC analysis (C18 columns). Moreover, the determination of BPA has been reported in biological samples such as rat tissues by HPLC, employing different extraction protocols for the serum and tissues and using a C18 column and an acetonitrile and water mobile phase with gradient elution [50]. In human breast milk, BPA was extracted using Matrix Solid Phase Dispersion (LiChrolut cartridges) and reversed phase HPLC, followed with isocratic elution of a C18 column with acetonitrile-water (70:30, v/v) [51]. The determination of the residual monomers, including BPA, released from resin-based dental restorative materials employed HPLC analysis in a Kromasil 100-C18 column eluted with methanol: acetonitrile: water, 60:15:25%, v/v [52], while the same HPLC analysis in saliva samples was performed using a Perfect Sil Target ODS-3 column eluted with acetonitrile/water, 58/42% v/v [53,54]. Fewer studies are engaged in the determination of multiple endocrine disruptors in complex biological samples, since extensive clean-up due to matrix interferences is required and only trace levels of the compounds are present. To this end, elaborate techniques including microwave-assisted extraction, C18 SPE, derivatization, and GC analysis were employed for the simultaneous determination of steroid EDCs, t-OCT, 4-cumylphenol, 4-nonylphenol, and BPA in fish samples [55]. The determination of BPA either alone or in combination with 4-t-octylphenol and 4-nonylphenol in human media has been thoroughly reviewed, and was performed in blood serum by ELISA or RIA, in plasma and urine by LC equipped with a fluorescence or electrochemical detector and a C18 column, while more complex samples, such as semen and placental tissue, required the use of LC-MS using a Shodex column, or LC -MS/MS using a C18 column [56]. A recent report on the determination of BPA and other xenoestrogens including t-OCT in human urine, serum, and breast milk samples, employed liquid chromatography-tandem mass spectrometry (LC-MS/MS) method on a silica Acquity column [57]. Analysis of several emerging contaminants, including BPA but not t-OCT, was performed in poultry manure using ultrasound-assisted matrix solid-phase dispersion for the extraction of the analytes and analysis following derivatization was performed by GC coupled to tandem mass spectrometry [58]. Simultaneous determination of BPA and alkylphenols has recently been reported for fish and gull [27], water samples [59], mussels [60], and naturally occurring marine zooplankton [61], using ultrasonic extraction followed by SPE (Oasis HLB) and analysis was performed by HPLC -FLD using a HYPERSIL GOLD C18 column and gradient elution with acetonitrile: water. The methods for the simultaneous determination of BPA and certain alkylphenols mentioned above, require specific equipment including ultrasonic extraction, fluorescence, MS or MS/MS detectors, which are not always available. A simple HPLC method, for the determination of BPA and its metabolites in bacterial cultures, was previously developed [18,62] and employed filtering of the medium samples and SPE (C18) extraction of BPA form bacteria, while HPLC analysis was conducted on a C18 Nucleosil column with gradient elution (acetonitrile: water containing TFA). However, this method did not include t-OCT and was validated only regarding the determination of BPA and its metabolites in cultures of bacteria in minimal salt media with BPA as the main carbon source. The live fish feed Artemia nauplii and metanauplii are unique regarding their biochemical composition, habitat, and extensive use as a live larval feed in aquaculture. Artemia is not included in the naturally occurring zooplanktonic communities, since it lives in high salinity habitats [36]. Its developmental stages, the nauplii and metanauplii, contain high levels of protein and lipids and especially unsaturated fatty acids and their composition can be enhanced through their enrichment with the appropriate nutrients, a fact that renders them suitable to serve as a live feed of larval stages in aquaculture [63]. However, this particular composition of Artemia as well as of its culture medium, namely seawater 35 ppt, poses significant analysis problems due to matrix interferences. Although the transfer of organophosphorus pesticides [64] and nonylphenol [65] from the crustacean Artemia to fish has been documented, the risk of the transfer of BPA and t-OCT to the cultured larvae of marine organisms through their live feed has not been evaluated, possibly due to the scarcity of methods on the determination of bisphenol and t-octylphenol in Artemia.
In the present study, Artemia franciscana metanauplii were used to develop and validate an analytical method that would enable the quantification of BPA and t-octylphenol, in the organisms as well as in their culture medium. The application of this method demonstrates that both bisphenol and t-octylphenol are ingested by Artemia and allows their quantification.
2. Materials and Methods
2.1. Chemicals and Reagents
The organic solvents, methanol and absolute ethanol, were supplied by Fisher Scientific (Loughborough, UK), while acetonitrile was supplied by VWR Chemicals (Paris, France). Trifluoroacetic acid (TFA) and sodium hypochlorite solution 10% w/v were purchased from AppliChem GmbH (Darmstadt, Germany). The sea salt used as the Artemia culture medium was purchased from Instant Ocean (Blacksburg, VA, USA). Artemia franciscana cysts were kindly supplied by INVE Aquaculture (INVE HELLAS SA, 93, Kyprou str., 16451 Argyroupoli, Athens, Greece). The C18 sorbent material, columns, and frits for SPE were supplied by Grace Davison Discovery Sciences (Bannockburn, IL, USA).
The internal standard 4-n-Octylphenol and Bisphenol-A were supplied by Alfa Aesar (Karlsruhe, Germany), while 4-tert-Octylphenol was obtained from Sigma-Aldrich (Germany). The investigated compounds are presented in Table 1.

Table 1.
Compounds investigated and characteristic parameters.
2.2. Animals
Experiments were performed on A. franciscana (Kellogg) cysts (e.g., grade, GSL strain) provided by INVE (INVE HELLAS S.A., Athens, Greece). The cysts were stored at 4 °C until use. The axenic culture of Artemia cysts was performed following their hydration and decapsulation in hypochlorite solution, as previously described [72,73]. In all procedures the artificial sweater was autoclaved prior to use and the containers were rinsed with ethanol to ensure bacteria-free cultures. The nauplii (instar I) were cultured for a total of 96h at a density of 15 nauplii per mL and they were fed on processed yeast provided by P. Sorgeloos (Laboratory of Aquaculture and Artemia Reference Center, University of Ghent, Belgium). The sterile culture medium was renewed daily, and feeding was stopped prior to the addition of pollutants at 72 h. In the preliminary experiments, the LC50 was determined for each endocrine disruptor. To this end, two experimental series of nauplii were employed at 72 h since the onset of cyst incubation after being fasted for six hours, and the first series received increasing concentrations of bisphenol (0, 5, 10, 15, 20, 25 or 30 μg/mL) while the second series received increasing concentration of 4-tert-octylphenol (0, 0.25, 0.5, 0.75, 2, 4 or 6 μg/mL). Each series employed samples containing 0 μg/mL of endocrine disruptor to serve as controls. Survival and mortality were recorded following a 24 h exposure to the pollutant and LC50 was estimated using linear regression and Probit analysis.
2.3. Preparation of Stock and Standard Solutions
The stock solution of BPA (500 μg/mL) and of t-OCT (200 μg/mL) and of the internal standard n -octylphenol (500 μg/mL) were prepared in methanol. All stock solutions were kept in 4 °C, in glass volumetric flasks protected from light to avoid photodegradation of the analytes.
A set of six working standard solutions were prepared for BPA in two sets containing 2.5, 5, 7.5, 10, 12.5 and 15 μg/ mL or 1, 25, 50, 75, 100 and 125 μg/ mL. For t-OCT a set of six working standard solutions were prepared containing 1, 2.5, 5, 7.5 10 and 12.5 μg/mL. Each spiked sample contained the internal standard n-octylphenol at a concentration of 75 μg/mL. The standard solutions were transferred in amber glass vials (SU860083, Supelco) and stored at 4 °C. Calibration curves were prepared at six points, with concentrations ranging from 2.5 to 12.5 μg/mL for BPA in tissue, from 1 to 125 μg/mL for BPA in medium and from 1 to 12.5 μg/mL for t-OCT.
2.4. Sample Preparation
The protocol previously developed for the extraction of Bisphenol-A in bacterial cultures [61] was modified appropriately and validated to facilitate purification of both BPA and t-OCT in Artemia nauplii, as well as in their culture medium.
Regrading extraction of BPA and t-OCT from biological samples, 0.2 g wet weight of A. franciscana nauplii were homogenized (4 °C) in 2 mL methanol and 10.5 mL of double distilled water containing 0.08% (v/v) TFA, following the addition of the internal standard n-octylphenol (final concentration of 75 μg/mL). A Kinematica Polytron PCU homogenizer was employed for 5 min and the sample was kept in ice. The homogenate was centrifuged for 10 min at 10,000 rpm. The supernatant was submitted to SPE (0.5 g of C18 sorbent) conditioned with 10 mL methanol, 5 mL ddH2O, and 5 mL ddH2O containing 0.08% TFA. Loading of the samples (manual flow 1 mL/min) followed and columns were washed with 5 mL ddH2O. Elution of the compounds was performed with 4 mL methanol and the eluates were evaporated to dryness (rotary evaporator, 40 °C). Acetonitrile (1 mL) was added to the dried samples, and they were stored at −20 °C, protected from light. During the analyses all tubes were protected from light to avoid photodegradation of the analytes BPA and 4-tOP.
For the extraction of the analytes from the culture medium of Artemia nauplii, a 10 mL sample of the culture medium was used, its pH value was adjusted to 3.0 ± 0.1 using a 10% water solution of TFA (v/v) and the internal standard was added (75 μg/mL). Following the addition of 2 mL methanol, the sample was centrifuged for 10 min at 10,000 rpm and the supernatant was submitted to SPE, as described for biological samples.
2.5. Pollutant Administration
For the feeding assays, nauplii cultures (1000 mL cultures in filtered and autoclaved artificial seawater, salinity 35 ppt) [72,73] were fasted for 6 h and then Bisphenol-A or 4-tert-octylphenol was administered at 72 h, at a final concentration of each substance in each culture of 10 μg/mL and 0.5 μg/mL, respectively. The cultures were then incubated for 24 h under natural light. At the end of the incubation the nauplii and their culture medium were separated by filtration and stored at −20 °C.
2.6. Photodegradation Assay
Experiments on the potential photodegradation of the analytes were performed in glass tubes containing sterile seawater and BPA or t-OCT at a concentration of 50 μg/mL and 10 μg/mL, respectively. The tubes were incubated under intense sunlight for a total of 48 h. The concentration of the analytes BPA and t-OCT was determined at the onset (t = 0), at t = 24 h and at the end of the incubation period (t = 48 h), following extraction of the analytes.
2.7. Chromatography
The HPLC system comprised of an LC20AD pump and an SPD-20A photodiode array detector (DAD) (Shimadzu, Kyoto, Japan), a Rheodyne 7125 injection valve, with a loop of 80 μL volume (Rheodyne, Cotati, CA, USA) and a Nucleosil 100 C18 column (250 × 4.6 mm, 5 μm), (Macherey-Nagel GmbH & Co., Duren, Germany) equipped with a 10 × 4.6 mm I.D., Nucleosil C18 precolumn. Separation of BPA, t-OCT, and n-OCT was performed using a binary mobile phase system of mobile Phase A (95:5, v/v, 0.1 % TFA in H2O: Acetonitrile) and mobile Phase B (5:95, v/v, 0.08 % TFA in H2O: Acetonitrile) at room temperature, as previously reported [62] but with adjustments to the gradient, starting at 100% mobile Phase A and proceeding to an increasing mobile Phase B concentration to 20% over 2 min, 70% over 5 min, 90% at 7 min, and until the end of the analysis. Flow rate was 0.5 mL/min, and the analytes were detected at 220 nm.
3. Results and Discussion
3.1. Analytes Extraction Efficiency
Artemia metanauplii are a solid sample that require homogenization to achieve cell lysis, and this was performed by homogenization of the sample in methanol-H2O containing 0.08% (v/v) TFA and centrifugation. The previously described protocol for the extraction of BPA from bacterial cultures [62] was modified regarding the methanol aqueous phase ratio from 5:1 to about 1:3, since this amount of methanol produced better extraction of analytes. Since alkylphenols are characterized by low solubility in water compared to Bisphenol-A (Table 1), methanol contributed to the increased solubility of t-octylphenol and BPA. The use of ethyl acetate or water, instead of methanol-TFA, was also employed in trial experiments but compound recovery was deemed inadequate as it resulted in the coextraction of Artemia lipids and matrix interferences. In the present study, 0.2 g wet weight tissue, corresponding to 0.01 ± 0.003 g dry weight, were processed since they resulted in satisfactory chromatograms with no interfering peaks in the blank samples (Figure S1). Although microwave-assisted solvent extraction [74], as well as ultrasonic bath extraction [75], were reported for the extraction of bisphenol and alkylphenols, homogenization is common in biological samples [75,76].
Following the first methanol-water-TFA extraction step, the samples were subjected to SPE, an acknowledged technique for the selective sample preparation prior to analysis of endocrine disruptors in liquid or pre-extracted solid samples [75]. SPE has been previously used for the extraction of bisphenol from complex matrices, such as rat tissues [50], human serum [77], or bacterial cultures [18]. The commonly used sorbent is C18 [18,50,77] although the use of polymeric sorbents [59] and silica gel [78] have been reported. However, C18 cartridges provided the cleanest extracts of fish samples compared to polymeric sorbents, employed in the analysis of bisphenol and alkylphenols [55]. A flow rate of 1 mL/min during the elution of SPE, and a water bath temperature lower than 40 °C during the evaporation of methanol, after SPE, were employed as previously recommended for bacteria samples [62] to facilitate the effective recovery of both analytes and the internal standard. However, it should be noted that although simple centrifuging and filtering was sufficient for medium samples originating from bacterial cultures in basal salt medium [62], this was not the case with the Artemia medium samples, since it resulted in clogging of the injection loop, due to the higher salt concentration of the sample. Hence, the SPE step was critical for efficient analysis.
The extraction in water-methanol and the following SPE step resulted in excellent recovery of BPA (100.4 ± 3.1) and of t-OCT (100.6 ± 3.9) in the case of the Artemia samples. The mean recovery in culture medium samples amounted to 99.9 ± 0.8 for BPA, while for t-OCT it amounted to 101.7 ± 1.9 (Table 2). The recovery values reported in the present work are higher to the previously reported values of 83.7 % (BPA) and 87.4 % (t-OP), for water extraction of Bisphenol-A and alkylphenols from zooplankton samples using Oasis HLB glass cartridges [27]. Our results for BPA and t-OCT extraction are in accordance with a recently published report on a water-acetonitrile extraction of bisphenols and octylphenols from fish and solid matrices, in which affinity chromatography clean-up instead of the reversed phase C18-SPE was employed and lower concentrations of 1 ng/g were used [79], resulting in recoveries of up to 101.1% for BPA and to 87.9 for t-octylphenol.

Table 2.
Linearity, accuracy (bias), precision, and recovery of BPA and t-OCT determination in the tissue and the culture medium of Artemia.
3.2. Method Validation
The results of the linear regression analysis performed are presented below (Table 2). The use of an internal standard was deemed necessary since sample matrix complexity, due to the presence of proteins and lipids in both samples, required an extraction protocol for both tissues and culture medium.
To this end, 4-n-octylphenol was selected, since it has a similar structure to both bisphenol and tert-octylphenol, is well resolved from both analytes, and is not present in the blank samples (Figure S1).
Linearity of the standard curves in the Artemia culture medium and Artemia metanauplii (Table 2) was excellent, as indicated by the R2 values of 0.999 for BPA and t-OCT. The Relative Bias estimated as the percentage of ((calculated- nominal)/nominal]) was within the accepted range of ±10%. Intra-day precision was calculated by three analyses performed on the same day, while inter-day precision was calculated by performing six analyses over three days and estimated as % RSD, never exceeding 5.0% for all analytes. Our results indicate that the method is precise and repeatable with excellent recovery rates. The nominal concentrations of 1.0 μg/mL BPA and 1.0 μg/mL t-OCT in the culture medium were below the calculated LOD values (Table 3) and were, thus, excluded from the estimation of recovery results described above. The values for LOD and LOQ calculation were based on the response and the slope of the calibration curve.

Table 3.
LOD, LOQ, and RT values for BPA and t-OCT.
The value of a LOD of 0.21 μg/mL and 0.17 μg/mL were estimated for BPA and t-OCT, respectively, in Artemia tissue corresponding to 10.5 ng/g dry weight and 8.6 ng/g dry weight, respectively, and of 0.63 μg/mL and 0.41 μg/mL for Artemia culture medium (Table 3). Although LOD values of 0.07 μg/kg were reported for BPA in mussels’ tissue [80], estimations were made with σ taken as the standard deviation of three samples spiked at the experimental estimated LOQ, and S as the slope of the corresponding calibration curve. On the other hand, LOD values of 5.6 μg/mL and 6.3 μg/mL, were reported following liquid–liquid extraction of water samples [81]. The previously reported LOQ values in zooplankton amounted to 2 ng/g dry weight for BPA and 0.8 ng/g dry weight for t-OCT, and to 5 and 1 ng/L, respectively, for water samples, determined as a tenfold signal-to-noise ratio for a sample with a very low analyte content [27], employing different methods for the biological and water samples. Variable results were obtained from the computation of LOD and LOQ values, depending on the statistical approach used. The residual standard deviation or error of intercept of calibration line was recommended instead of the mean blank signal value, as it is considered that it provides a more accurate estimate [82].
Since the LOD and LOQ values are calculated based on the calibration curve, they greatly depend on its range and lower values are expected when a range of lower concentrations are used. However, the range of the calibration curve should extend over the range of expected analytes’ concentrations in the samples [83]. In the present work, the expected concentrations of Bisphenol-A and t-octylphenol were within the range of the LC50 values. These values were acquired from the toxicity experiments that allowed the estimation of the amount that could be administered to Artemia metanauplii without leading to high mortality rates.
Typical HPLC chromatograms of Artemia and culture medium samples spiked with BPA, t-OCT, and n-OCT are presented in Figure 1. The peaks of the analytes BPA, t-OCT, and of the internal standard n-OCT, showed with a retention time (Rt) of 15.200 ± 0.12, 21.876 ± 0.11 26.241 ± 0.1 min, respectively. The peak appearing from 0 to 6 min, in all samples, possibly corresponds to the solvents, while the peak appearing at approximately 7.0 ± 0.5 min only in the medium samples possibly corresponds to medium constituents. Similar peaks also appear in the blank samples without, however, interfering with analysis (Figure S1).
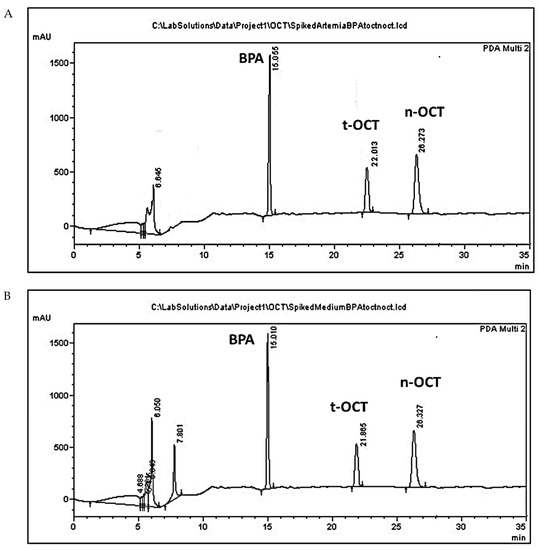
Figure 1.
HPLC chromatograms of (A) Artemia tissue; and (B) culture medium samples containing BPA (50 μg/mL), t-OCT (10 μg/mL) and the internal standard n-OCT (75 μg/mL).
3.3. Administration of Pollutants
BPA was administered at a concentration of 10 μg/mL, while t-OCT was administered at 0.5 μg/mL, since their LOD values amounted to15.6 and 0.73 μg/mL, respectively (Figure S2). The simultaneous administration of the two pollutants at LC50 levels was not applicable, since it resulted in higher mortality values, possible due to synergistic effects. Hence, each pollutant, namely BPA or t-OCT, was administered separately. Acute toxicity and inhibition of growth of the early developmental stages of Artemia nauplii (Instar I-II) have been reported after a 24 h exposure to BPA with an LC50 of 44.8 mg/L [43] for Artemia franciscana. The LC50 values for Artemia sinica nauplii amounted to 47.5 mg/L for BPA and to 2.27 mg/L for t-OCT [84]. On the other hand, the LC50 values of 70.1 μg/L and 7.7 μg/L were reported for adult (15 days old) Artemia sinica individuals exposed to BPA and n-hexylphenol [45], illustrating that the advanced developmental stages present greater sensitivity to these pollutants. The increased sensitivity of subsequent developmental stages to pollutants was attributed to the fact that the organisms appear more ravenous compared to the earlier developmental stages, during which the animals depend on reserved yolk for their energy requirements [85]. In addition to the importance of the developmental stage employed, the origin of cysts and abiotic parameters regarding, among others, the pH, hatching temperature, photoperiod duration, and saltwater treatments are also of great importance [42]. Considering that bacteria are capable of BPA biotransformation [18], the use of axenic cultures in the autoclaved medium were used in the present study. Representative chromatograms of the pollutants following administration of 10 μg/mL BPA or 0.5 μg/mL t-OCT to Artemia, that are below their estimated LC50 values, are presented in Figure 2 for Artemia, and in Figure 3 for its culture medium.
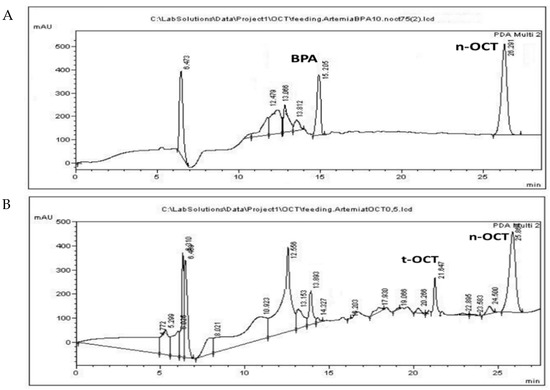
Figure 2.
HPLC chromatograms of Artemia tissue following administration of (A) 10 μg/mL BPA; and (B) 0.5 μg/mL t-OCT.
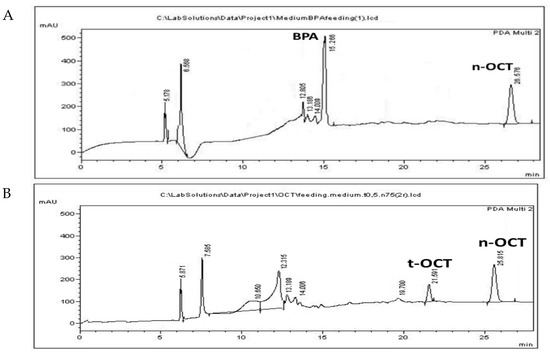
Figure 3.
HPLC chromatograms of culture medium following the administration of (A) 10 μg/mL BPA; and (B) 0.5 μg/mL t-OCT.
The peaks appearing at 12.5 ± 0.18, 13.1 ± 0.08 and 13.8 ± 0.2 and apparent following administration of either pollutant (Figure 2A,B) might correspond to products produced during their biotransformation by Artemia. Similar peaks were observed following BPA microbial biotransformation corresponding to 4-Hydroxyacetophenone, 4-Hydroxybenzoic acid, and hydroquinone [18]. Moreover, biodegradation of tert-octylphenol by the fungus Thielavia sp. HJ22 has been reported to result in the formation of 4-hydroxybenzoic acid hydroquinone [86]. Although UV spectra presented a good match with standard compounds, the identity of these peaks was not confirmed by MS in the present work. The peak that appears at 19.7 (Figure 2B and Figure 3B) is evident only following administration of t-OCT and might correspond to a photodegradation product, since similar peaks were observed during the photodegradation experiment (Figure S3D). Photodegradation via photolysis has been confirmed for BPA [25,27] and for t-OCT [87], and the products of solar transformation presented higher toxicity than the parent compound [88]. The feeding experiments were conducted under normal light and, hence, photodegradation losses were estimated to examine whether any pollutant concentration decline is due to photolysis. In the photolysis experiments, the initial BPA and t-OCT concentration were calculated at 49.15 μg/mL and 9.81 μg/mL, respectively, and their concentration following a 24 h incubation amounted to 44.63 μg/mL and 7.87 μg/mL, while after 48 h BPA amounted to 37.94 and t-OCT to only 1.7 μg/mL. Hence, the reduction in the analyte concentration induced by photodegradation was 9.2% for BPA and 19.7% for t-OCT at 24 h (Table 4).

Table 4.
Bisphenol A and t-octylphenol levels in administration experiments.
These values are in accordance with those reported for bisphenol A degradation [89] but higher than the previously reported absence of t-OCT degradation under one hour of direct solar irradiation [90]. Although part of the pollutants is degraded via photolysis, this does not compensate for the observed reduction in the total levels of both pollutants (Table 4). The amounts detected in the processed medium samples were 66.8 and 3.0 μg and the total amounts in a 1000 mL culture corresponded for BPA to 66.8% and for t-OCT to 63.8% of the initially added amounts. In addition, the amounts detected in Artemia tissue amounted to 4.1% and 0.7% of the initially added amounts for BPA and t-OCT, respectively, and a reduction of up to approximately 32% in total BPA and of 35% in total t-OCT was observed in our experiments. The accumulation of BPA in zooplankton has been confirmed in an eco-system bioreactor during incubation of phytoplankton (Nannochloropsis sp.) and zooplankton (Artemia sp. or Brachinous sp.) in the presence of the pollutant [91].
The occurrence of high levels of BPA and alkylphenols in the marine environment has been documented in aquaculture facilities which are in the vicinity of industrial or urban activity [92]. The BPA values amounted to 37 ng/L in mussels cultured in Thailand [92], to 1.5 ng/g d. w. in fish cultured in Taiwan [93], to 4.2 ng/g in the muscle of fish cultured in Malaysia [94], or 272 ng/g d. w. in the North Atlantic Ocean [24]. The relevant t- octylphenol concentration was 16 ng/ g d. w. in mussels cultured in Malaysia [94], while the occurrence of the alkylphenol nonylphenol has been documented in the sediments of the Northern Aegean, indicating a risk for the organisms that live in it [95]. The cultured fish fry fed on Artemia have a weight of 15 mg and consume about 300 nauplii per day [96], corresponding to a wet weight of 5 mg, indicating that these pollutants, if present in Artemia, may accumulate and possibly pose a health risk to the fish larvae. Recently the accumulation of bisphenols in farmed seabream muscle following exposure to microplastics, amounting to 0.3 μg/g muscle, was reported and the authors consider aquaculture equipment as a source of such microplastics [97]. Considering the TDI for BPA amounting to 0.04 ng/kg b. w. daily and the suggested TDI for t-OCT for men of 0.067 ng/kg b. w. daily (Table 1), it becomes apparent that the exposure of cultured organisms to the pollutants might also pose a possible hazard to human health. The use of marine phytoplanktons and zooplanktons has been suggested for the recovery of BPA from seawater [91]. The uncompensated decline in both BPA and t-OCT levels observed in the present study indicates that, although these compounds might accumulate in Artemia, their biotransformation is possible. Since the toxicity of the possible biotransformation products has not been investigated and Artemia is used as a live feed for cultured marine larvae, the monitoring of its pollutants levels is of extreme importance.
4. Conclusions
The developed analytical method provides a simple approach for the simultaneous detection and quantification of bisphenol A and t-octylphenol in the live fish feed Artemia and in its culture medium. The presented extraction protocol and the use of 4-n-octylphenol as the internal standard provided a detection limit of 0.21 for BPA and 0.17 ng/μL for t-OCT. The method was applied in the analysis following administration of the pollutants to Artemia metanauplii at levels close to their corresponding LC50. The administration experiments revealed that Artemia is capable to uptake bisphenol and t-octylphenol from the culture medium. Experimental efforts to reduce the pollutant load of Artemia are currently under investigation. Further experiments are needed to evaluate the transfer of the pollutants to the fish larvae that feed on Artemia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/mps5030038/s1, Figure S1. HPLC chromatograms of blank Artemia tissue (A) and culture medium (B) samples; Figure S2. Linear Regression analysis for the determination of LC50 values of BPA and t-OCT; Figure S3. Photodegradation of BPA and t-OCT. HPLC chromatograms of medium with (A)BPA at 0 h; (B) BPA at 48 h; (C) t-OCT at 0 h; (D) t-OCT at 48 h.
Author Contributions
M.T. and V.F.S. conceived and designed the experiments; M.T., writing—original draft preparation; V.F.S., review and editing; D.G., formal analysis in BPA experiments; K.D., formal analysis in the t-OCT experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
No additional funding was received in support of the present research work. The authors wish to thank INVE HELLAS S.A., for the provision of Artemia cysts and Sorgeloos for the provision of yeast.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koelmans, A.A.; Besseling, E.; Foekema, E.M. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014, 187, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Chemical Components of Plastics as Endocrine Disruptors: Overview and Commentary. Birth Defects Res. 2020, 112, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef] [PubMed]
- López-Cervantes, J.; Sánchez-Machado, D.; Paseiro-Losada, P.; Simal-Lozano, J. Effects of Compression, Stacking, Vacuum Packing and Temperature on the Migration of Bisphenol A from Polyvinyl Chloride Packaging Sheeting into Food Simulants. Chromatographia 2003, 58, 327–330. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Candidate List of Substances of Very High Concern for Authorization (2020). Available online: https://echa.europa.eu/candidate-list-table (accessed on 3 April 2022).
- EFSA. Bisphenol, A. 2017. Available online: https://www.efsa.europa.eu/en/topics/topic/bisphenol (accessed on 2 April 2022).
- Available online: https://plasticseurope.org/wp-content/uploads/2021/10/20210301-BPA-Judgment-appeal.pdf (accessed on 1 April 2022).
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 99 (Suppl. S3), S559–S566. [Google Scholar] [CrossRef]
- Ying, G.G.; Williams, B.; Kookana, R. Environmental fate of alkylphenols and alkylphenol ethoxylates—A review. Environ. Int. 2002, 28, 215–226. [Google Scholar] [CrossRef]
- Octylphenol. Available online: https://echa.europa.eu/el/substance-information/-/substanceinfo/100.060.634 (accessed on 2 April 2022).
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- White, R.; Jobling, S.; Hoare, S.; Sumpter, J.; Parker, M. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 1994, 135, 175–182. [Google Scholar] [CrossRef]
- Olaniyan, L.; Okoh, O.O.; Mkwetshana, N.T.; Okoh, A.I. Environmental Water Pollution, Endocrine Interference and Ecotoxicity of 4-tert-Octylphenol: A Review. Rev. Environ. Contam. Toxicol. 2020, 248, 81–109. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, E. Environmental Effects of BPA: Focus on Aquatic Species. Dose Response 2015, 13, 1559325815598304. [Google Scholar] [CrossRef] [PubMed]
- Nachman, R.M.; Hartle, J.C.; Lees, P.S.; Groopman, J.D. Early Life Metabolism of Bisphenol A: A Systematic Review of the Literature. Curr. Environ. Health Rep. 2014, 1, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kyrila, G.; Katsoulas, A.; Schoretsaniti, V.; Rigopoulos, A.; Rizou, E.; Doulgeridou, S.; Sarli, V.; Samanidou, V.; Touraki, M. Bisphenol A removal and degradation pathways in microorganisms with probiotic properties. J. Hazard. Mater. 2021, 413, 125363. [Google Scholar] [CrossRef]
- Hayashi, O.; Kameshiro, M.; Masuda, M.; Satoh, K. Bioaccumulation and metabolism of [14C] bisphenol A in the brackish water bivalve Corbicula japonica. Biosci. Biotechnol. Biochem. 2008, 72, 3219–3224. [Google Scholar] [CrossRef][Green Version]
- Certa, H.; Fedtke, N.; Wiegand, H.J.; Müller, A.M.; Bolt, H.M. Toxicokinetics of p-tert-octylphenol in male Wistar rats. Arch. Toxicol. 1996, 71, 112–122. [Google Scholar] [CrossRef]
- Jing, X.; Bing, S.; Xiaoyan, W.; Xiaojie, S.; Yongning, W. A study on bisphenol A, nonylphenol, and octylphenol in human urine samples detected by SPE-UPLC-MS. Biomed. Environ. Sci. 2011, 24, 40–46. [Google Scholar] [CrossRef]
- Rajendran, R.K.; Huang, S.L.; Lin, C.C.; Kirschner, R. Biodegradation of the endocrine disrupter 4-tert-octylphenol by the yeast strain Candida rugopelliculosa RRKY5 via phenolic ring hydroxylation and alkyl chain oxidation pathways. Bioresour. Technol. 2017, 226, 55–64. [Google Scholar] [CrossRef]
- Pop, C.-E.; Draga, S.; Măciucă, R.; Niță, R.; Crăciun, N.; Wolff, R. Bisphenol A Effects in Aqueous Environment on Lemna minor. Processes 2021, 9, 1512. [Google Scholar] [CrossRef]
- Barboza, L.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef]
- Baralla, E.; Pasciu, V.; Varoni, M.V.; Nieddu, M.; Demuro, R.; Demontis, M.P. Bisphenols’ occurrence in bivalves as sentinel of environmental contamination. Sci. Total Environ. 2021, 785, 147263. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.; van der Hoeven, N.; Clark, K.; Mihaich, E.; Woelz, J.; Hentges, S. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 2018, 201, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Falkowska, L.; Grabowski, P.; Kwaśniak, J.; Mudrak-Cegiołka, S.; Reindl, A.R.; Sokołowski, A.; Szumiło, E.; Zgrundo, A. Bisphenol A, 4-tert-octylphenol, and 4-nonylphenol in the Gulf of Gdańsk (Southern Baltic). Arch. Environ. Contam. Toxicol. 2014, 67, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro-Gonzalez, N.; Turnes-Carou, I.; Besada, V.; Muniategui-Lorenzo, S.; Lopez-Mahia, P.; Prada-Rodriguez, D. Occurrence, distribution and bioaccumulation of endocrine disrupting compounds in water, sediment and biota samples from a European river basin. Sci. Total Environ. 2015, 529, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; Chen, Q.; Wang, R.; Sun, D.; Cai, Z.; Wu, H.; Duan, S. Phenolic endocrine-disrupting compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci. Total Environ. 2017, 584–585, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Little, D.C.; Newton, R.W.; Beveridge, M.C. Aquaculture: A rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc. 2016, 75, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Hollman, P.C.H.; Mendoza-Hill, J.J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper. No. 615; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; ISBN 978-92-5-109882-0. Available online: https://www.fao.org/3/i7677e/i7677e.pdf (accessed on 20 March 2022).
- Vázquez-Rowe, I.; Ita-Nagy, D.; Kahhat, R. Microplastics in fisheries and aquaculture: Implications to food sustainability and safety. Curr. Opin. Green Sust. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Kolkovski, S.; Curnow, J.; King, J. Intensive rearing system for fish larvae research II. Artemia hatching and enriching system. Aquac. Eng. 2004, 31, 309–317. [Google Scholar] [CrossRef]
- Turcihan, G.; Turgay, E.; Yardımcı, R.E.; Eryalçın, K.M. The effect of feeding with different microalgae on survival, growth, and fatty acid composition of Artemia franciscana metanauplii and on predominant bacterial species of the rearing water. Aquacult. Int. 2021, 29, 2223–2241. [Google Scholar] [CrossRef]
- Sargent, J.; McEvoy, L.; Estevez, A.; Bell, G.; Bell, M.; Henderson, J.; Tocher, D. Lipid nutrition of marine fish during early development: Current status and future directions. Aquaculture 1999, 179, 217–229. [Google Scholar] [CrossRef]
- Triantaphyllidis, G.V.; Abatzopoulos, T.J.; Sorgeloos, P. Review of the biogeography of the genus Artemia (Crustacea. Anostraca). J. Biogeogr. 1998, 25, 213–226. [Google Scholar] [CrossRef]
- Maldonado-Montiel, T.D.N.J.; Rodriguez-Canche, L.G.; Olveranova, M.A. Evaluation of Artemia biomass production in San Crisanto, Yucatan, Mexico, with the use of poultry manure as organic fertilizer. Aquaculture 2003, 219, 573–584. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Kunej, U.; Skalar, T. Screening study of four environmentally relevant microplastic pollutants: Uptake and effects on Daphnia magna and Artemia franciscana. Chemosphere 2018, 208, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, H.; Wang, J.; Li, J.; Liu, S.; Tu, J.; Chen, Y.; Zong, Y.; Zhang, P.; Wang, Z.; et al. Influence of Microplastics on the Growth and the Intestinal Microbiota Composition of Brine Shrimp. Front. Microbiol. 2021, 12, 717272. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; Van Stappen, G. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 2006, 144, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G. The case of Artemia spp. in nanoecotoxicology. Mar. Environ. Res. 2014, 101, 38–43. [Google Scholar] [CrossRef]
- Castritsi-Catharios, J.; Syriou, V.; Miliou, H.; Zouganelis, G.D. Toxicity effects of bisphenol A to the nauplii of the brine shrimp Artemia franciscana. J. Biol. Res. Thessalon. 2013, 19, 38–45. [Google Scholar]
- Ekonomou, G.; Lolas, A.; Castritsi-Catharios, J.; Neofitou, C.; Zouganelis, G.D.; Tsiropoulos, N.; Exadactylos, A. Mortality and Effect on Growth of Artemia franciscana Exposed to Two Common Organic Pollutants. Water 2019, 11, 1614. [Google Scholar] [CrossRef]
- Shaukat, A.; Liu, G.; Li, Z.; Xu, D.; Huang, T.; Chen, H. Toxicity of five phenolic compounds to brine shrimp Artemia sinica (Crustacea: Artemiidae). J. Ocean Univ. China 2014, 13, 141–145. [Google Scholar] [CrossRef]
- Kidd, K.A.; Paterson, M.J.; Rennie, M.D.; Podemski, C.L.; Findlay, D.L.; Blanchfield, P.J.; Liber, K. Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130578. [Google Scholar] [CrossRef]
- Jeong, T.Y.; Simpson, M.J. Endocrine Disruptor Exposure Causes Infochemical Dysregulation and an Ecological Cascade from Zooplankton to Algae. Environ. Sci. Technol. 2021, 55, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Gómez, A.; Rubio, S.; Pérez-Bendito, D. Analytical methods for the determination of bisphenol A in food. J. Chromatogr. A 2009, 1216, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Li, Y.; Ouyang, H.; Xu, P.; Wu, D. High-performance liquid chromatographic analysis of bisphenol A and 4-nonylphenol in serum, liver and testis tissues after oral administration to rats and its application to toxicokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 830, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Frysali, M.A.; Papadoyannis, I.N. Matrix solid phase dispersion for the extraction of bisphenol A from human breast milk prior to HPLC analysis. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 247–258. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Kerezoudi, C.; Tolika, E.; Palaghias, G. A Simple Isocratic HPLC Method for the Simultaneous Determination of the Five Most Common Residual Monomers Released from Resin-Based Dental Restorative Materials. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 740–749. [Google Scholar] [CrossRef]
- Samanidou, V.; Hadjicharalampous, M.; Palaghias, G.; Papadoyannis, I. Development and validation of an isocratic HPLC method for the simultaneous determination of residual monomers released from dental polymeric materials in artificial saliva. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 511–523. [Google Scholar] [CrossRef]
- Diamantopoulou, E.I.; Plastiras, O.E.; Mourouzis, P.; Samanidou, V. Validation of a Simple HPLC-UV Method for the Determination of Monomers Released from Dental Resin Composites in Artificial Saliva. Methods Protoc. 2020, 3, 35. [Google Scholar] [CrossRef]
- Liu, J.; Pan, X.; Huang, B.; Fang, K.; Wang, Y.; Gao, J. An improved method for simultaneous analysis of steroid and phenolic endocrine disrupting chemicals in biological samples. J. Environ. Anal. Chem. 2012, 92, 1135–1149. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Thomaidis, N.S.; Koupparis, M.A. Recent trends in biomonitoring of bisphenol A, 4-t-octylphenol, and 4-nonylphenol. Toxicol. Lett. 2012, 210, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Preindl, K.; Braun, D.; Aichinger, G.; Sieri, S.; Fang, M.; Marko, D.; Warth, B. A Generic Liquid Chromatography-Tandem Mass Spectrometry Exposome Method for the Determination of Xenoestrogens in Biological Matrices. Anal. Chem. 2019, 91, 11334–11342. [Google Scholar] [CrossRef] [PubMed]
- Aznar, R.; Albero, B.; Pérez, R.A.; Sánchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Analysis of emerging organic contaminants in poultry manure by gas chromatography-tandem mass spectrometry. J. Sep. Sci. 2018, 41, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Koniecko, I.; Falkowska, L.; Krzymyk, E. Occurrence and distribution of bisphenol A and alkylphenols in the water of the gulf of Gdansk (southern Baltic). Mar. Pollut. Bull. 2015, 91, 372–379. [Google Scholar] [CrossRef]
- Staniszewska, M.; Graca, B.; Sokołowski, A.; Nehring, I.; Wasik, A.; Jendzul, A. Factors determining accumulation of bisphenol A and alkylphenols at a low trophic level as exemplified by mussels Mytilus trossulus. Environ. Pollut. 2017, 220, 1147–1159. [Google Scholar] [CrossRef]
- Staniszewska, M.; Nehring, I.; Mudrak- Cegiołka, S. Changes of concentrations and possibility of accumulation of bisphenol A and alkylphenols, depending on biomass and composition, in zooplankton of the Southern Baltic (Gulf of Gdansk). Environ. Pollut. 2016, 10, 489–501. [Google Scholar] [CrossRef]
- Rigopoulos, A.T.; Samanidou, V.F.; Touraki, M. Development and Validation of an HPLC-DAD Method for the Simultaneous Extraction and Quantification of Bisphenol-A, 4-Hydroxybenzoic Acid, 4-Hydroxyacetophenone and Hydroquinone in Bacterial Cultures of Lactococcus lactis. Separations 2018, 5, 12. [Google Scholar] [CrossRef]
- Samat, N.A.; Yusoff, F.M.; Rasdi, N.W.; Karim, M. Enhancement of Live Food Nutritional Status with Essential Nutrients for Improving Aquatic Animal Health: A Review. Animals 2020, 10, 2457. [Google Scholar] [CrossRef]
- Varó, I.; Serrano, R.; Pitarch, E.; Amat, F.; López, F.J.; Navarro, J.C. Bioaccumulation of chlorpyrifos through an experimental food chain: Study of protein HSP70 as biomarker of sublethal stress in fish. Arch. Environ. Contam. Toxicol. 2002, 42, 229–235. [Google Scholar] [CrossRef]
- Correa-Reyes, G.; Viana, M.T.; Marquez-Rocha, F.J.; Licea, A.F.; Ponce, E.; Vazquez-Duhalt, R. Nonylphenol algal bioaccumulation and its effect through the trophic chain. Chemosphere 2007, 68, 662–670. [Google Scholar] [CrossRef]
- Dorn, P.B.; Chou, C.S.; Getempo, J.J. Degradation of Bisphenol A in Natural Waters. Chemosphere 1987, 16, 1501–1507. [Google Scholar] [CrossRef]
- Chapin, R.E.; Adams, J.; Boekelheide, K.; Gray, L.E., Jr.; Hayward, S.W.; Lees, P.S.; McIntyre, B.S.; Portier, K.M.; Schnorr, T.M.; Selevan, S.G.; et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. B Dev. Reprod. Toxicol. 2008, 83, 157–395. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8814, 4-tert-Octylphenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-tert-Octylphenol (accessed on 14 February 2022).
- Jonsson, B. Risk Assessment on Butylphenol, Octylphenol and Nonylphenol, and Estimated Human Exposure of Alkylphenols from Swedish Fish. Uppsala Uppsala University. 2006. Available online: https://www.uu.se/digitalAssets/177/c_177024-l_3-k_jonsson-beatrice-report.pdf (accessed on 22 April 2022).
- Profiles of the Initial Environmental Risk Assessment of Chemicals Vol. 7, Ministry of the Environment, Government. Available online: https://www.env.go.jp/en/chemi/chemicals/profile_erac/index.html#vol7 (accessed on 22 April 2022).
- Touraki, M.; Karamanlidou, G.; Karavida, P.; Chrysi, K. Evaluation of the probiotics Bacillus subtilis and Lactobacillus plantarum bioencapsulated in Artemia nauplii against vibriosis in European sea bass larvae (Dicentrarchus labrax, L.). World J. Microbiol. Biotechnol. 2012, 28, 2425–2433. [Google Scholar] [CrossRef]
- Giarma, E.; Amanetidou, E.; Toufexi, A.; Touraki, M. Defense systems in developing Artemia franciscana nauplii and their modulation by probiotic bacteria offer protection against a Vibrio anguillarum challenge. Fish Shellfish Immunol. 2017, 66, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.N.; Lindholst, C. Quantification of the xenoesterogens 4-tert-octylphenol and bisphenol A in water and in fish tissue based on microwave assisted extraction, solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 1999, 864, 17–24. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A.; Malik, A.K. Recent advances in sample preparation methods for analysis of endocrine disruptors from various matrices. Crit. Rev. Anal. Chem. 2014, 44, 255–269. [Google Scholar] [CrossRef]
- Cerkvenik-Flajs, V.; Fonda, I.; Gombač, M. Analysis and Occurrence of Bisphenol A in Mediterranean Mussels (Mytilus galloprovincialis) Sampled from the Slovenian Coastal Waters of the North Adriatic Sea. Bull. Environ. Contam. Toxicol. 2018, 101, 439–445. [Google Scholar] [CrossRef]
- Völkel, W.; Colnot, T.; Csanady, G.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Chen, C.F. Seasonal and spatial distribution of 4-nonylphenol and 4-tert-octylphenol in the sediment of Kaohsiung Harbor, Taiwan. Chemosphere 2015, 134, 588–597. [Google Scholar] [CrossRef]
- Pisciottano, I.D.M.; Mita, G.D.; Gallo, P. Bisphenol A, octylphenols and nonylphenols in fish muscle determined by LC/ESI-MS/MS after affinity chromatography clean up. Food Addit. Contam. Part B 2020, 13, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, R.; Garrido Gamarro, E.; Garcinuño Martínez, R.M.; Paniagua González, G.; Fernández Hernando, P. Occurrence of common plastic additives and contaminants in mussel samples: Validation of analytical method based on matrix solid-phase dispersion. Food Chem. 2021, 349, 129169. [Google Scholar] [CrossRef] [PubMed]
- Basheer, C.; Lee, H.K.; Tan, K.S. Endocrine disrupting alkylphenols and bisphenol-A in coastal waters and supermarket seafood from Singapore. Mar. Pollut. Bull. 2004, 48, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J. Principles of analytical validation. In Proteomic Profiling and Analytical Chemistry: The Crossroads, 2nd ed.; Ciborowski, P., Silberring, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–215. [Google Scholar] [CrossRef]
- Shaukat, A.; Guangxing, L.; Zhengyan, L. The Acute Toxicity of Phenolic Compounds and Heavy Metals on Brine Shrimp Artemia sinica (Crustacea: Artemiidae). Sindh Univ. Res. J. 2013, 45, 21–24. [Google Scholar]
- Rajasree, S.R.R.; Kumar, V.G.; Abraham, L.S.; Manoharan, N. Assessment on the toxicity of engineered nanoparticles on the life stages of marine aquatic invertebrate Artemia salina. Int. J. Nanosci. 2011, 10, 1153–1159. [Google Scholar] [CrossRef]
- Mtibaà, R.; Ezzanad, A.; Aranda, E.; Pozo, C.; Ghariani, B.; Moraga, J.; Nasri, M.; Manuel Cantoral, J.; Garrido, C.; Mechichi, T. Biodegradation and toxicity reduction of nonylphenol, 4-tert-octylphenol and 2,4-dichlorophenol by the ascomycetous fungus Thielavia sp HJ22: Identification of fungal metabolites and proposal of a putative pathway. Sci. Total Environ. 2020, 708, 135129. [Google Scholar] [CrossRef]
- Olaniyan, L.W.B.; Okoh, A.I. Determination and ecological risk assessment of two endocrine disruptors from River Buffalo, South Africa. Environ. Monit. Assess. 2020, 192, 750. [Google Scholar] [CrossRef]
- Gurban, A.M.; Burtan, D.; Rotariu, L.; Bala, C. Manganese oxide based screen-printed sensor for xenooestrogens detection. Sens. Actuators B Chem. 2015, 210, 273–280. [Google Scholar] [CrossRef]
- Neamtu, M.; Frimmel, F.H. Degradation of endocrine disrupting bisphenol A by 254 nm irradiation in different water matrices and effect on yeast cells. Water Res. 2006, 40, 3745–3750. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, H.; Wei, G.; Zhang, S.; Li, H.; Dong, W. Photodegradation of 4-tert octylphenol in aqueous solution promoted by Fe (III). Environ. Sci. Pollut. Res. Int. 2013, 20, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Nakajima, N. Improvement of marine environmental pollution using eco-system: Decomposition and recovery of endocrine disrupting chemicals by marine phyto- and zooplanktons. J. Mol. Catal. B 2003, 23, 419–424. [Google Scholar] [CrossRef]
- Ocharoen, Y.; Boonphakdee, C.; Boonphakdee, T.; Shinn, A.P.; Moonmangmee, S. High levels of the endocrine disruptors bisphenol-A and 17β-estradiol detected in populations of green mussel, Perna viridis, cultured in the Gulf of Thailand. Aquaculture 2018, 497, 348–356. [Google Scholar] [CrossRef]
- Lu, I.-C.; Chao, H.-R.; Mansor, W.-N.-W.; Peng, C.-W.; Hsu, Y.-C.; Yu, T.-Y.; Chang, W.-H.; Fu, L.-M. Levels of Phthalates, Bisphenol-A, Nonylphenol, and Microplastics in Fish in the Estuaries of Northern Taiwan and the Impact on Human Health. Toxics 2021, 9, 246. [Google Scholar] [CrossRef]
- Yap, C.K. Contamination of Heavy Metals and Other Organic Pollutants in Perna Viridis from the Coastal Waters of Malaysia: A Review Based On 1998 Data. J. Sci. Res. Rep. 2014, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Arditsoglou, A.; Voutsa, D. Occurrence and partitioning of endocrine-disrupting compounds in the marine environment of Thermaikos Gulf, Northern Aegean Sea, Greece. Mar. Pollut. Bull. 2012, 64, 2443–2452. [Google Scholar] [CrossRef]
- Giebichenstein, J.; Giebichenstein, J.; Hasler, M.; Schulz, C.; Ueberschär, B. Comparing the performance of four commercial microdiets in an early weaning protocol for European seabass larvae (Dicentrarchus labrax). Aquac. Res. 2022, 53, 544–558. [Google Scholar] [CrossRef]
- Capó, X.; Alomar, C.; Compa, M.; Sole, M.; Sanahuja, I.; Soliz Rojas, D.L.; González, G.P.; Garcinuño Martínez, R.M.; Deudero, S. Quantification of differential tissue biomarker responses to microplastic ingestion and plasticizer bioaccumulation in aquaculture reared sea bream Sparus aurata. Environ. Res. 2022, 211, 113063. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).