Validation of In Vivo Nodal Assessment of Solid Malignancies with USPIO-Enhanced MRI: A Workflow Protocol
Abstract
1. Introduction
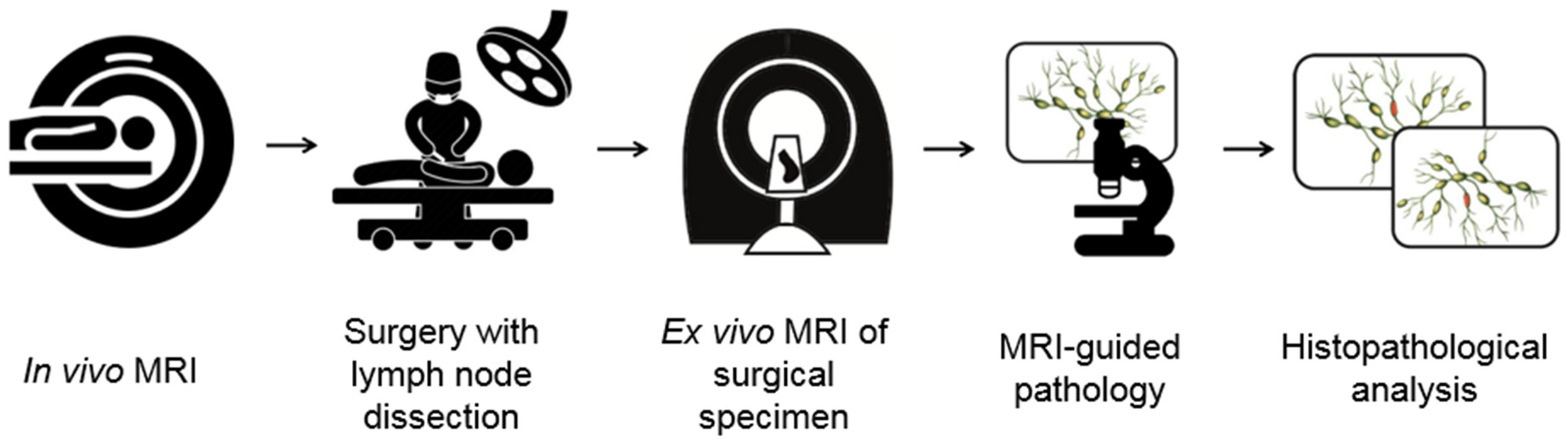
2. Materials and Methods
2.1. Subjects
2.2. Surgery and Ex Vivo MR Examination
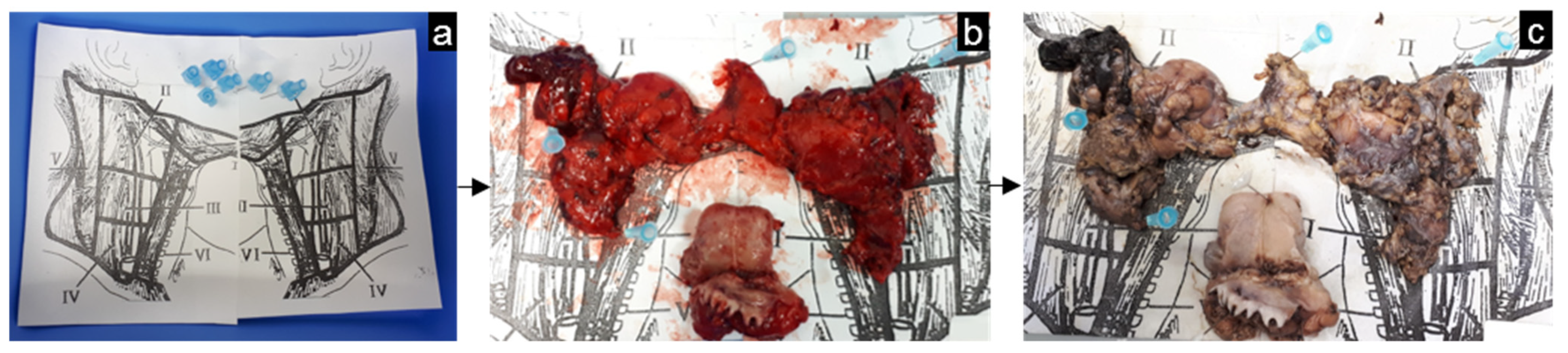
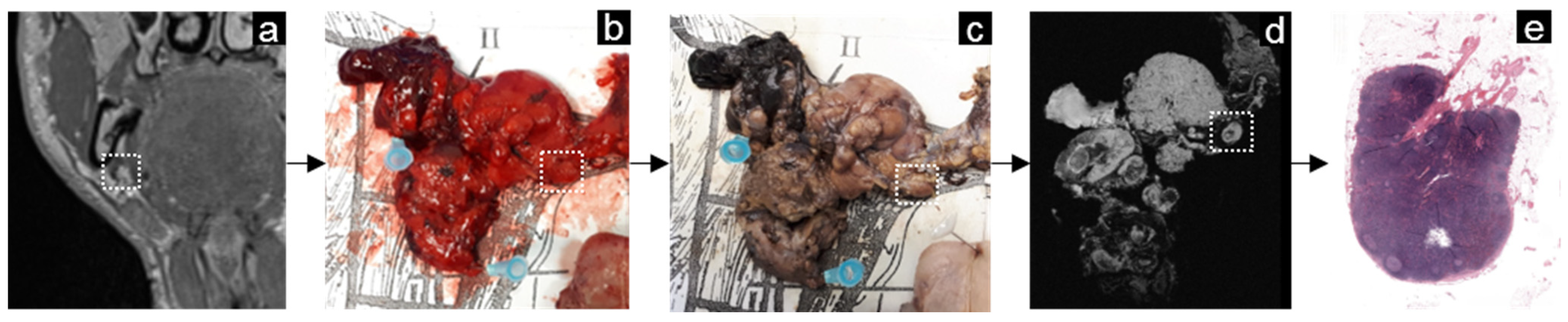
2.3. MRI-Guided Pathology and Node-to-Node Correlation
3. Results
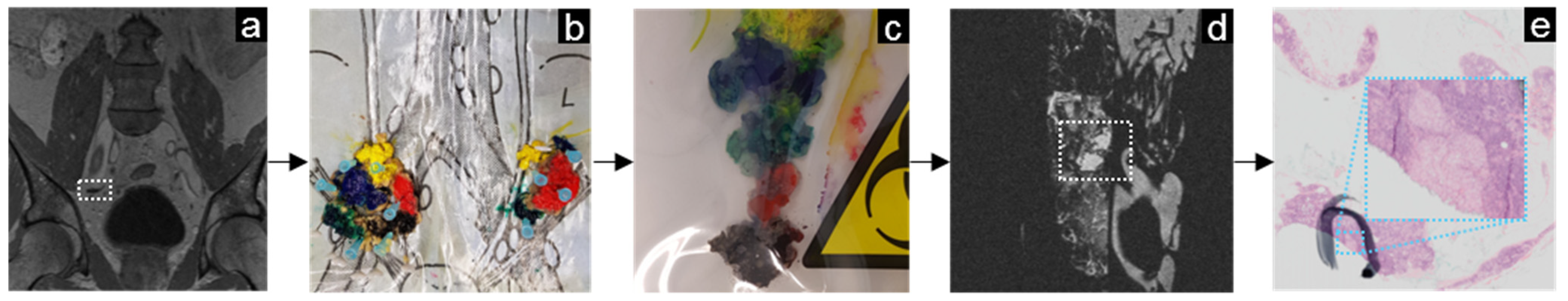
3.1. Prostate Cancer
3.2. Rectal Cancer
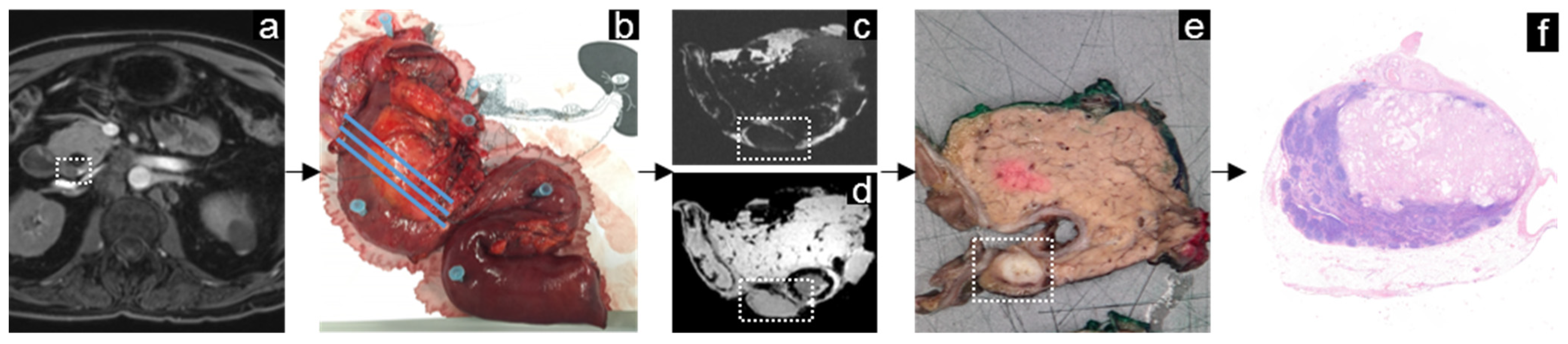
3.3. Periampullary Cancer
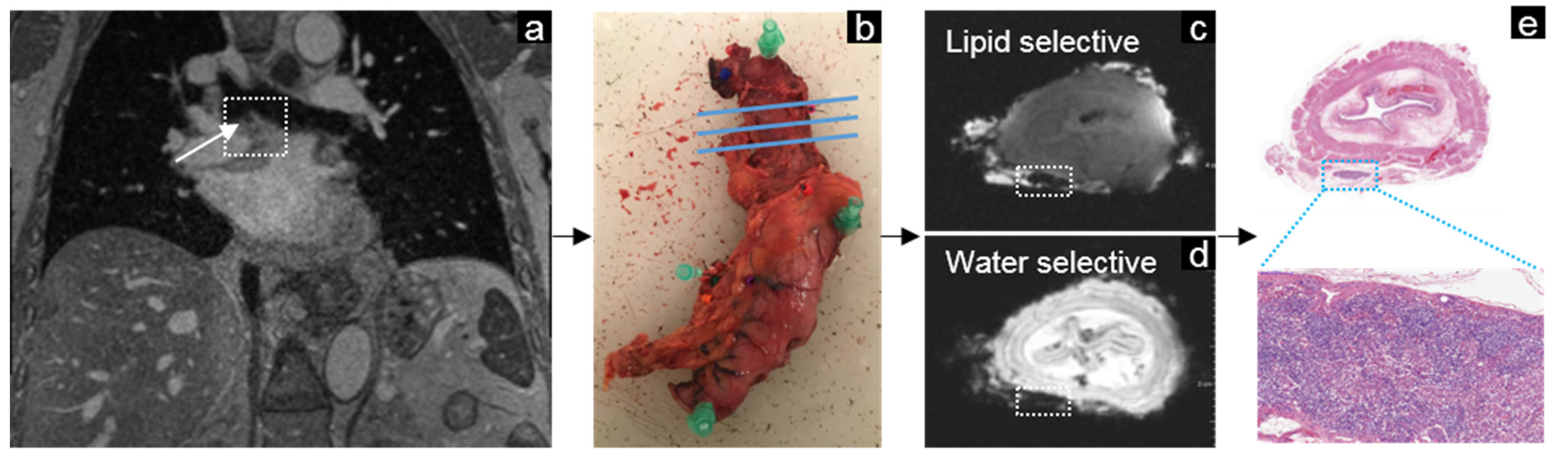
3.4. Esophageal Cancer
3.5. Head-and-Neck Cancer
4. Discussion
4.1. Comparison with the Literature
4.2. Strengths and Limitations
4.3. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilczak, W.; Wittmer, C.; Clauditz, T.; Minner, S.; Steurer, S.; Buscheck, F.; Krech, T.; Lennartz, M.; Harms, L.; Leleu, D.; et al. Marked Prognostic Impact of Minimal Lymphatic Tumor Spread in Prostate Cancer. Eur. Urol. 2018, 74, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.; Venkatraman, E.; Park, B.; Flores, R.; Bains, M.S.; Rusch, V.; American Joint Committee on Cancer staging, s. The prognostic importance of the number of involved lymph nodes in esophageal cancer: Implications for revisions of the American Joint Committee on Cancer staging system. J. Thorac Cardiovasc. Surg. 2006, 132, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Driessen, D.; Dijkema, T.; Weijs, W.L.J.; Takes, R.P.; Pegge, S.A.H.; Zamecnik, P.; van Engen-van Grunsven, A.C.H.; Scheenen, T.W.J.; Kaanders, J. Novel Diagnostic Approaches for Assessment of the Clinically Negative Neck in Head and Neck Cancer Patients. Front. Oncol. 2020, 10, 637513. [Google Scholar] [CrossRef] [PubMed]
- Korteweg, M.A.; Zwanenburg, J.J.; van Diest, P.J.; van den Bosch, M.A.; Luijten, P.R.; van Hillegersberg, R.; Mali, W.P.; Veldhuis, W.B. Characterization of ex vivo healthy human axillary lymph nodes with high resolution 7 Tesla MRI. Eur. Radiol. 2011, 21, 310–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fortuin, A.S.; Bruggemann, R.; van der Linden, J.; Panfilov, I.; Israel, B.; Scheenen, T.W.J.; Barentsz, J.O. Ultra-small superparamagnetic iron oxides for metastatic lymph node detection: Back on the block. WIREs Nanomed. Nanobi. 2017, 10, 10. [Google Scholar] [CrossRef]
- Heesakkers, R.A.; Hovels, A.M.; Jager, G.J.; van den Bosch, H.C.; Witjes, J.A.; Raat, H.P.; Severens, J.L.; Adang, E.M.; van der Kaa, C.H.; Futterer, J.J.; et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: A prospective multicohort study. Lancet Oncol. 2008, 9, 850–856. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef]
- Schilham, M.G.; Zamecnik, P.; Prive, B.M.; Israel, B.; Rijpkema, M.; Scheenen, T.; Barentsz, J.O.; Nagarajah, J.; Gotthardt, M. Head-to-head comparison of (68)Ga-prostate-specific membrane antigen PET/CT and ferumoxtran-10 enhanced MRI for the diagnosis of lymph node metastases in prostate cancer patients. J. Nucl. Med. 2021, 62, 1258–1263. [Google Scholar] [CrossRef]
- De Gouw, D.; Maas, M.C.; Slagt, C.; Muhling, J.; Nakamoto, A.; Klarenbeek, B.R.; Rosman, C.; Hermans, J.J.; Scheenen, T.W.J. Controlled mechanical ventilation to detect regional lymph node metastases in esophageal cancer using USPIO-enhanced MRI; comparison of image quality. Magn. Reson. Imaging 2020, 74, 258–265. [Google Scholar] [CrossRef]
- Scheenen, T.W.J.; Zamecnik, P. The Role of Magnetic Resonance Imaging in (Future) Cancer Staging: Note the Nodes. Investig. Radiol. 2020, 56, 42–49. [Google Scholar] [CrossRef]
- Anzai, Y.; Brunberg, J.A.; Lufkin, R.B. Imaging of nodal metastases in the head and neck. J. Magn. Reson. Imaging 1997, 7, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Elizondo, G.; Wittenberg, J.; Rabito, C.A.; Bengele, H.H.; Josephson, L. Ultrasmall superparamagnetic iron oxide: Characterization of a new class of contrast agents for MR imaging. Radiology 1990, 175, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cao, Y.; Liao, C.; Huang, J.; Gao, F. Diagnostic performance of USPIO-enhanced MRI for lymph-node metastases in different body regions: A meta-analysis. Eur. J. Radiol. 2011, 80, 582–589. [Google Scholar] [CrossRef]
- Thoeny, H.C.; Triantafyllou, M.; Birkhaeuser, F.D.; Froehlich, J.M.; Tshering, D.W.; Binser, T.; Fleischmann, A.; Vermathen, P.; Studer, U.E. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur. Urol. 2009, 55, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, M.; Studer, U.E.; Birkhauser, F.D.; Fleischmann, A.; Bains, L.J.; Petralia, G.; Christe, A.; Froehlich, J.M.; Thoeny, H.C. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur. J. Cancer 2013, 49, 616–624. [Google Scholar] [CrossRef]
- Park, J.S.; Jang, Y.J.; Choi, G.S.; Park, S.Y.; Kim, H.J.; Kang, H.; Cho, S.H. Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: Node-for-node matched histopathology validation of MRI features. Dis. Colon Rectum 2014, 57, 32–38. [Google Scholar] [CrossRef]
- Luciani, A.; Pigneur, F.; Ghozali, F.; Dao, T.H.; Cunin, P.; Meyblum, E.; De Baecque-Fontaine, C.; Alamdari, A.; Maison, P.; Deux, J.F.; et al. Ex vivo MRI of axillary lymph nodes in breast cancer. Eur. J. Radiol. 2009, 69, 59–66. [Google Scholar] [CrossRef]
- Stijns, R.C.H.; Philips, B.W.J.; Nagtegaal, I.D.; Polat, F.; de Wilt, J.H.W.; Wauters, C.A.P.; Zamecnik, P.; Futterer, J.J.; Scheenen, T.W.J. USPIO-enhanced MRI of lymph nodes in rectal cancer: A node-to-node comparison with histopathology. Eur. J. Radiol. 2021, 138, 109636. [Google Scholar] [CrossRef]
- Philips, B.W.J.; Fortuin, A.S.; Orzada, S.; Scheenen, T.W.J.; Maas, M.C. High resolution MR imaging of pelvic lymph nodes at 7 Tesla. Magn. Reson. Med. 2017, 78, 1020–1028. [Google Scholar] [CrossRef]
- Stijns, R.; Philips, B.; Wauters, C.; de Wilt, J.; Nagtegaal, I.; Scheenen, T. Can Ex Vivo Magnetic Resonance Imaging of Rectal Cancer Specimens Improve the Mesorectal Lymph Node Yield for Pathological Examination? Investig. Radiol. 2019, 54, 645–652. [Google Scholar] [CrossRef]
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef]
- Quirke, P.; Palmer, T.; Hutchins, G.G.; West, N.P. Histopathological work-up of resection specimens, local excisions and biopsies in colorectal cancer. Dig. Dis. 2012, 30 (Suppl. 2), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S. Resection margins in pancreatic cancer. Surg. Clin. North Am. 2013, 93, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Hagens, E.R.C.; van Berge Henegouwen, M.I.; van Sandick, J.W.; Cuesta, M.A.; van der Peet, D.L.; Heisterkamp, J.; Nieuwenhuijzen, G.A.P.; Rosman, C.; Scheepers, J.J.G.; Sosef, M.N.; et al. Distribution of lymph node metastases in esophageal carcinoma [TIGER study]: Study protocol of a multinational observational study. BMC Cancer 2019, 19, 662. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.D.; Lapi, S.E. Designing the magic bullet? The advancement of immuno-PET into clinical use. J. Nucl. Med. 2013, 54, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.W.J.; Stijns, R.C.H.; Rietsch, S.H.G.; Brunheim, S.; Barentsz, J.O.; Fortuin, A.S.; Quick, H.H.; Orzada, S.; Maas, M.C.; Scheenen, T.W.J. USPIO-enhanced MRI of pelvic lymph nodes at 7-T: Preliminary experience. Eur. Radiol. 2019, 29, 6529–6538. [Google Scholar] [CrossRef]


| Patient | Sex | Age | Cancer Type | TNM-Stage | Therapy | USPIO Infusion-Surgery Interval | Study Registration Number |
|---|---|---|---|---|---|---|---|
| 1. | Male | 67 | Prostate | cT3N1M0 | Pelvic lymphadenectomy followed by post-operative radiotherapy | 1 day | None |
| 2. | Male | 76 | Rectum | cT2N0 | Total mesorectal excision | 9 days | NCT02751606 |
| 3. | Male | 58 | Periampullary | p T2N2M1 * | Pancreaticoduodenectomy with lymphadenectomy followed by postoperative chemotherapy | 8 days | NCT04311047 |
| 4. | Female | 65 | Esophagus | cT2-3N1 | Neoadjuvant chemoradiotherapy followed byesophagectomy | 1 day | NTR6072 |
| 5. | Male | 51 | Head-and-neck | cT3N0M0 | Primary tumor resection and cervical lymphadenectomy followed by postoperative radiotherapy | 5 days | NCT03817307 |
| Head-and-Neck Cancer | Esophageal Cancer | Periampullary Cancer | Rectal Cancer | Prostate Cancer | |
|---|---|---|---|---|---|
| Sequence | VIBE Dixon | VIBE Dixon | VIBE Dixon | VIBE Dixon | VIBE |
| Voxel size (mm3) | 0.8 × 0.8 × 0.8 | 1.4 × 1.4 × 1.4 | 1.4 × 1.4 × 1.7 | 0.9 × 0.9 × 0.9 | 0.73 × 0.89 × 0.91 |
| FOV (mm3) | 260 × 260 × 154 | 450 × 394 × 168 | 394 × 450 × 177 | 350 × 350 × 173 | 328 × 328 × 175 |
| Acquisition mode | 3D | Breath-hold 3D | Breath-hold 3D | 3D | 3D |
| TE (ms) | 2.57, 3.8 | 1.23, 2.46 | 1.23, 2.46 | 1.2, 2.5 | 2.5 |
| TR (ms) | 6.02 | 4.21 | 3.86 | 5.8 | 6.5 |
| Bandwidth (Hz) | 300 | 20 | 20 | 305 | 510 |
| Acquisition time (min) | 4:53 | 18 s for each breath-hold | 12 s for each breath-hold | 4:56 | 9:13 |
| Flip angle (°) | 10 | 9 | 9 | 10 | 10 |
| Head-and-Neck Cancer | Esophageal Cancer | Periampullary Cancer | Rectal Cancer | Prostate Cancer | |
|---|---|---|---|---|---|
| Sequence | mGRE | mGRE | mGRE | mGRE | MEDIC |
| Voxel size (mm3) | 0.8 × 0.8 × 0.7 | 1.5 × 1.5 × 1.5 | 1.3 × 1.3 × 1.3 | 0.85 × 0.85 × 0.85 | 0.73 × 0.73 × 0.73 |
| FOV (mm3) | 260 × 260 × 154 | 336 × 279 × 30 | 291 × 291 × 211 | 328 × 328 × 190 | 328 × 328 × 164 |
| Acquisition mode | 3D | Breath-hold 3D | Breath-hold 3D | 3D | 3D |
| Acquired TEs (ms) | 2.5–27.1 | 2.7–16.7 | 2.7–16.7 | 3.6–17.2 | 12 |
| Reconstructed TEs (ms) | 12 | 12 | 12 | 12 | n.a. |
| TR (ms) | 31 | 20 | 20 | 590 | 21 |
| Bandwidth (Hz) | 360 | 380 | 380 | 3 × 1 | 172 |
| Acquisition time (min) | 11:08 | 19 s each Breath-hold | 20 s eachBreath-hold | 11:28 | 12:27 |
| Flip angle (°) | 10 | 10 | 10 | 10 | 10 |
| Ex Vivo MRI | 3D Lipid Excitation | 3D Water Excitation |
|---|---|---|
| Acquisition time (min) | 13 | 20 |
| TR (ms) | 15 | 30 |
| TE (ms) | 3.0 | 6.2 |
| Resolution (mm3) | 0.29 × 0.29 × 0.29 | 0.29 × 0.29 × 0.29 |
| Flip angle (°) | 10 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driessen, D.A.J.J.; de Gouw, D.J.J.M.; Stijns, R.C.H.; Litjens, G.; Israël, B.; Philips, B.W.J.; Hermans, J.J.; Dijkema, T.; Klarenbeek, B.R.; van der Post, R.S.; et al. Validation of In Vivo Nodal Assessment of Solid Malignancies with USPIO-Enhanced MRI: A Workflow Protocol. Methods Protoc. 2022, 5, 24. https://doi.org/10.3390/mps5020024
Driessen DAJJ, de Gouw DJJM, Stijns RCH, Litjens G, Israël B, Philips BWJ, Hermans JJ, Dijkema T, Klarenbeek BR, van der Post RS, et al. Validation of In Vivo Nodal Assessment of Solid Malignancies with USPIO-Enhanced MRI: A Workflow Protocol. Methods and Protocols. 2022; 5(2):24. https://doi.org/10.3390/mps5020024
Chicago/Turabian StyleDriessen, Daphne A. J. J., Didi J. J. M. de Gouw, Rutger C. H. Stijns, Geke Litjens, Bas Israël, Bart W. J. Philips, John J. Hermans, Tim Dijkema, Bastiaan R. Klarenbeek, Rachel S. van der Post, and et al. 2022. "Validation of In Vivo Nodal Assessment of Solid Malignancies with USPIO-Enhanced MRI: A Workflow Protocol" Methods and Protocols 5, no. 2: 24. https://doi.org/10.3390/mps5020024
APA StyleDriessen, D. A. J. J., de Gouw, D. J. J. M., Stijns, R. C. H., Litjens, G., Israël, B., Philips, B. W. J., Hermans, J. J., Dijkema, T., Klarenbeek, B. R., van der Post, R. S., Nagtegaal, I. D., van Engen-van Grunsven, A. C. H., Brosens, L. A. A., Veltien, A., Zámecnik, P., & Scheenen, T. W. J. (2022). Validation of In Vivo Nodal Assessment of Solid Malignancies with USPIO-Enhanced MRI: A Workflow Protocol. Methods and Protocols, 5(2), 24. https://doi.org/10.3390/mps5020024






