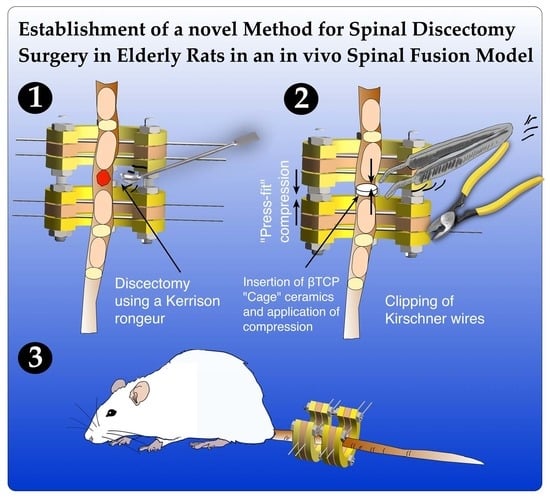
Establishment of a Novel Method for Spinal Discectomy Surgery in Elderly Rats in an In Vivo Spinal Fusion Model
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Fentanyl 0.05 mg/mL (Sintetica S.A., Mendrisio, Switzerland).
- Midazolam 5 mg/mL (Sintetica S.A., Mendrisio, Switzerland).
- Medetomidine 1 mg/mL (Orion Corporation, Espoo, Finland).
- Meloxicam 5 mg/mL, injection solution (Boehringer Ingelheim GmbH, Basel, Switzerland).
- Meloxicam 0.5 mg/mL, oral suspension (Boehringer Ingelheim GmbH, Basel, Switzerland).
- Buprenorphin 0.3 mg/mL (Streuli Pharma SA, Uznach, Switzerland).
- Flumazenil 0.1 mg/mL (CPS Cito Pharma Services GmbH, Uster, Switzerland).
- Naloxon 0.4 mg/mL (OrPha Swiss GmbH, Küsnacht, Switzerland).
- Atipamezol 5 mg/mL (Orion Corporation, Espoo, Finland).
- Ropivacain 7.5 mg/mL (Fresenius Kabi AG, Kriens, Switzerland).
- Sodiumchloride 0.9% (Bichsel AG, Interlaken, Switzerland).
- Glucose 5% (B. Braun Medical AG, Sempach, Switzerland).
- Pentobarbital 300 mg/mL (Streuli Pharma SA, Uznach, Switzerland).
- Isofluran 99.9% (Provet AG, Lyssach, Switzerland).
- O2 100%
- Sterile ophthalmic lubricant (Bausch & Lomb Swiss AG, Zug, Switzerland).
- Phosphate Buffered Solution (PBS).
- Bovine Serum Albumin (BSA) (Sigma–Aldrich Chemie GmbH, Buchs, Switzerland).
- OpSite transparent spray dressing (Smith & Nephew, Solothurn, Switzerland).
- Tissue culture-treated plate, 60 mm dish (TPP Techno Plastic Products AG, Schaffhausen, Switzerland).
- TOPIC spray (Vetoquinol AG, Bern, Switzerland).
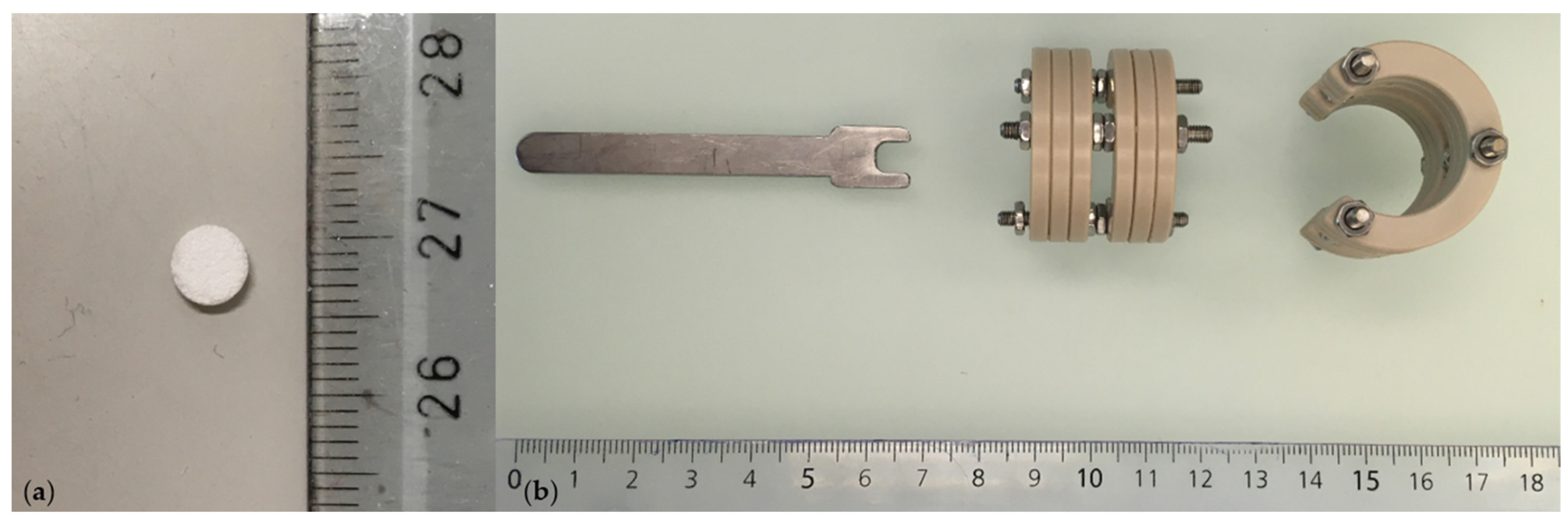
- β-TCP carrier, ∅ 0.5 mm, h = 1.5 mm, 75% porosity (produced by Prof Marc Bohner, Robert Mathys Foundation, Bettlach, Switzerland) (Figure 2a).
- Growth Factors, BMP-2 5 mg/mL and L51P 5 mg/mL. Growth factors were delivered lyophilized and sterile, and were kindly provided (produced by Prof Walter Sebald, Department of Physiological Chemistry, University of Würzburg, Würzburg, Germany).
- Customized external ring fixator (Urs Rohrer, SITEM, Inselspital, University Hospital Bern, Bern, Switzerland) (Figure 2b).
- Customized hex wrench (Urs Rohrer, ARTORG Center, Inselspital, University Hospital Bern, Bern, Switzerland) (Figure 2b).
- Surgical instruments: scalpel holder, needle holder, anatomical forceps, surgical forceps, suture scissors, curette, sharp pliersat, Kerrison rongeur (B. Braun Medical AG, Sempach, Switzerland).
- Kirschner wires 0.8 mm (Synthes GmbH, Oberdorf, Switzerland).
- Carbon steel surgical blades 15 mm (Swann–Morton, Sheffield, United Kingdom).
- Ethanol 70%, 80%, 96%, 100%.
- Sterilium disinfection (Paul Hartmann AG, Heidenheim, Germany).
- Octenisept spray disinfection (Schülke & Mayr GmbH, Norderstedt, Switzerland).
- Sterile surgical gloves (Mölnycke Health Care AB, Göteborg, Sweden).
- Leukosilk silk plasters 2.5 cm × 5 m (BSN medical GmbH, Hamburg, Germany).
- Ethilon Sutures 4.0 (Johnson & Johnson Medical Ltd., Livingston, United Kingdom).
- Vicryl Sutures 5.0 (Johnson & Johnson Medical Ltd., Livingston, United Kingdom).
- Sterile compresses 5 × 5 cm, 10 × 20 cm (IVF Hartmann AG, Neuhausen, Switzerland).
- Sterile covers 20 × 20 cm (IVF Hartmann AG, Neuhausen, Switzerland).
- Cling Film
- Syringes 1 mL, 2.5 mL, 5 mL, 10 mL (Terumo Corporation, Tokyo, Japan).
- 25 G 16 mm, 22 G 30 mm, 20 G 30 mm needles (B. Braun Melsungen AG, Melsungen, Germany).
- Falcon Tubes, 50 mL (BD Falcon, New York, NY, USA).
2.2. Equipment
- Biolaboratory safety class II
- Specific-pathogen-free (SPF) animal facility with rodent operation room
- Anaesthesia station (Vet Tech Solutions, Congleton, Great Britain).
- Rodent Surgical Monitor including heating pad, thermometer, and pulse oximeter, respiratory rate and heart rate (UNO B.V., PC Zevenaar, Netherlands).
- Bairhugger, heated air inflatable blanket (3 M Schweiz GmbH, Rüschlikon, Switzerland).
- Stryker System 8 cordless driver (Stryker, Selzach, Switzerland).
2.3. Animals
3. Procedure
3.1. Coating of β-TCP-Carrier
-
- CRITICAL STEP Do not vortex; shake by hand for approximately 10 min.
3.2. Anaesthesia
-
- CRITICAL STEP Anaesthetic mixture should be applied subcutaneously in loose restrained handling into the nuchal fold. Make sure to vent the needle prior to injection and use a different injection needle (22 G 30 mm) than the needle used for drawing up the anaesthetic mixture to avoid a blunt needle tip. Directly after injection, release loose restrained handling to avoid reflux of the fluid (Figure 3a,b).
3.3. Surgical Setup
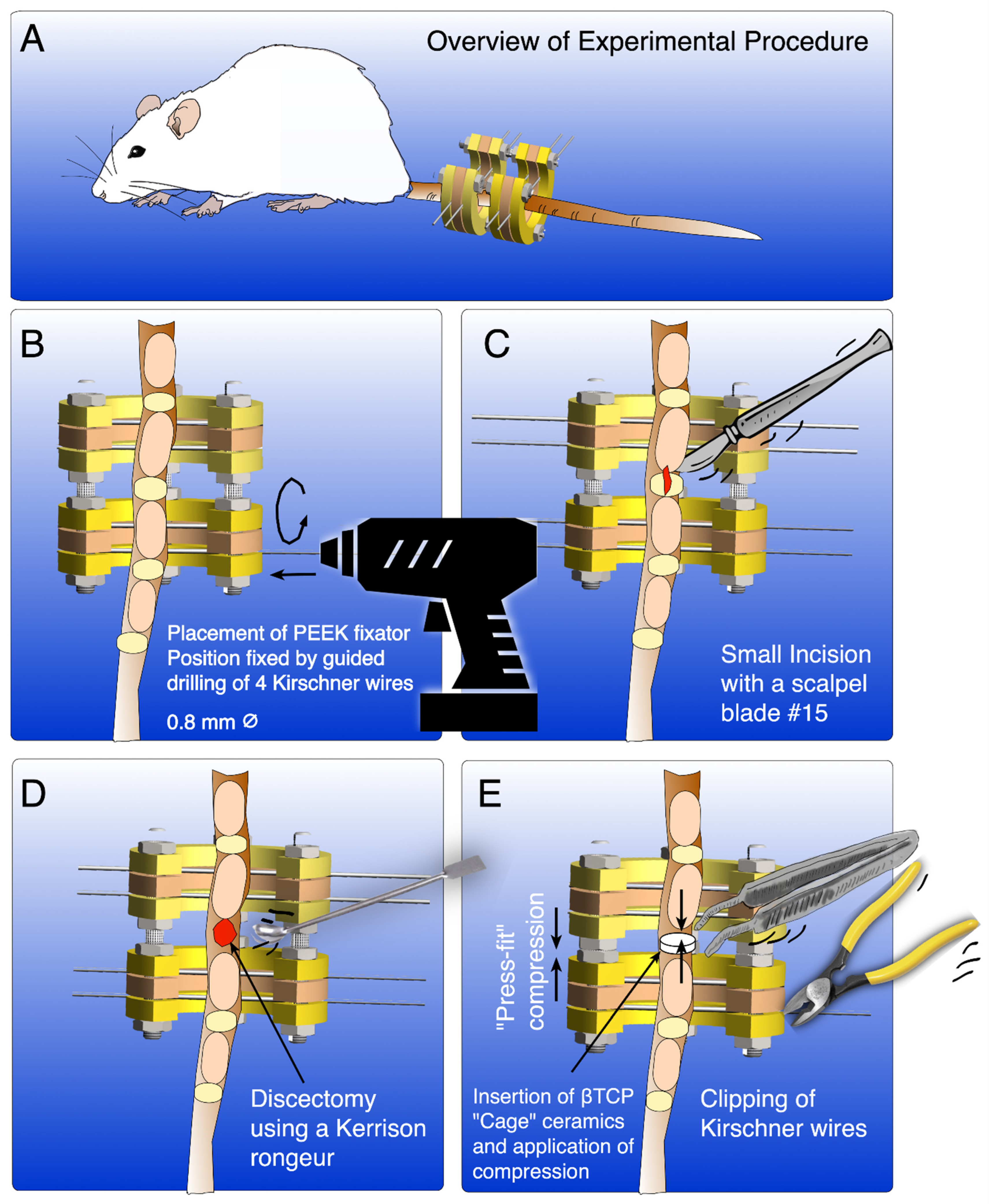
3.4. Surgical Procedure
-
- CRITICAL STEP Insert the k-wires in a slightly posterolateral direction to avoid damaging the vessels running laterally to the vertebrae.
-
- CRITICAL STEP Wound closure should be performed in a two-step process with subcutaneous closure using 4.0 Vycril and cutaneous closure with 5.0 Ethilone to ensure complete wound closure and prevent animal wound biting and wound infection.
3.5. Postoperative Regimen
- Analgesic water mixture (per rat for 72 h):
- 360 mL autoclaved H2O
- + 10 mL Glucose (5%)
- + 6 mL Buprenorphine (0.3 mg/mL)
-
- CRITICAL STEP Check on the correct positioning of the external ring fixator and closed wounds during the animal checkups.
3.6. Euthanasia
4. Expected Results
4.1. Effectiveness of the Protocol
4.1.1. Anaesthesia
4.1.2. Surgical Procedure
4.1.3. Pain Management
4.1.4. Complications
4.1.5. Euthanasia
4.2. Data Analysis and Interpretation
4.2.1. X-rays and µCT
4.2.2. PMMA Histology and Immunohistology
4.3. Data Application to Human Clinical Trials
4.4. Comparison of Surgical Technique
5. Reagents Setup
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, B.I.; Mirza, S.K.; Spina, N.; Spiker, W.R.; Lawrence, B.; Brodke, D.S. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine 2019, 44, 369–376. [Google Scholar] [CrossRef]
- Rajaee, S.S.; Bae, H.W.; Kanim, L.E.; Delamarter, R.B. Spinal fusion in the united states: Analysis of trends from 1998 to 2008. Spine 2012, 37, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, D.S.; Baker, K.; Hsu, W.K. Lumbar pseudarthrosis: A review of current diagnosis and treatment. Neurosurg. Focus 2015, 39, E10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, R.; Hanna, R. Non-Union Rate With Stand-Alone Lateral Lumbar Interbody Fusion. Medicine 2014, 93, e275. [Google Scholar] [CrossRef]
- Martin, B.I.; Mirza, S.K.; Comstock, B.A.; Gray, D.T.; Kreuter, W.; Deyo, R.A. Reoperation Rates Following Lumbar Spine Surgery and the Influence of Spinal Fusion Procedures. Spine 2007, 32, 382–387. [Google Scholar] [CrossRef]
- Argintar, E.; Edwards, S.; Delahay, J. Bone morphogenetic proteins in orthopaedic trauma surgery. Injury 2011, 42, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Parajón, A.; Alimi, M.; Navarro-Ramirez, R.; Christos, P.; Torres-Campa, J.M.; Moriguchi, Y.; Lang, G.; Härtl, R. Minimally Invasive Transforaminal Lumbar Interbody Fusion: Meta-analysis of the Fusion Rates. What is the Optimal Graft Material? Neurosurgery 2017, 81, 958–971. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, M.C.; Brown, J.V.E.; Heirs, M.K.; Higgins, J.; Mannion, R.J.; Rodgers, M.A.; Stewart, L.A. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: A meta-analysis of individual-participant data. Ann. Intern. Med. 2013, 158, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, C.; Hofstetter, W.; Sebald, H.-J.; Sebald, W.; Siebenrock, K.A.; Klenke, F.M. L51P—A BMP2 variant with osteoinductive activity via inhibition of Noggin. Bone 2012, 51, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Siegrist, M.; Keller, I.; Hofstetter, W. Healing of fractures in osteoporotic bones in mice treated with bisphosphonates—A transcriptome analysis. Bone 2018, 112, 107–119. [Google Scholar] [CrossRef]
- Khattab, H.; Ono, M.; Sonoyama, W.; Oida, Y.; Shinkawa, S.; Yoshioka, Y.; Maekawa, K.; Tabata, Y.; Sugama, K.; Sebald, W.; et al. The BMP2 antagonist inhibitor L51P enhances the osteogenic potential of BMP2 by simultaneous and delayed synergism. Bone 2014, 69, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Sebald, H.-J.; Klenke, F.M.; Siegrist, M.; Albers, C.E.; Sebald, W.; Hofstetter, W. Inhibition of endogenous antagonists with an engineered BMP-2 variant increases BMP-2 efficacy in rat femoral defect healing. Acta Biomater. 2012, 8, 3816–3820. [Google Scholar] [CrossRef]
- Ashinsky, B.G.; Gullbrand, S.E.; Bonnevie, E.D.; Wang, C.; Kim, D.H.; Han, L.; Mauck, R.L.; Smith, H.E. Sacrificial Fibers Improve Matrix Distribution and Micromechanical Properties in a Tissue-Engineered Intervertebral Disc. Acta Biomater. 2020, 111, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.T.; Milby, A.H.; Chiaro, J.A.; Kim, D.H.; Hebela, N.M.; Smith, L.J.; Elliott, D.M.; Mauck, R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014, 10, 2473–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckstein, J.C.; Sen, S.; Schaer, T.; Vresilovic, E.J.; Elliott, D.M. Comparison of animal discs used in disc research to human lumbar disc: Axial compression mechanics and glycosaminoglycan content. Spine 2008, 33, E166–E173. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Lane, J.M. Spinal fusion surgery: Animal models for tissue-engineered bone constructs. Biomaterials 2004, 25, 1475–1485. [Google Scholar] [CrossRef]
- Salamon, M.L.; Althausen, P.L.; Gupta, M.C.; Laubach, J. The Effects of BMP-7 in a Rat Posterolateral Intertransverse Process Fusion Model. J. Spinal Disord. Tech. 2003, 16, 90–95. [Google Scholar] [CrossRef]
- Schimandle, J.H.; Boden, S.D. Spine Update The Use of Animal Models to Study Spinal Fusion. Spine 1994, 19, 1998–2006. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Drespe, I.H.; Polzhofer, G.K.; Turner, A.S.; Grauer, J.N. Animal models for spinal fusion. Spine J. 2005, 5, S209–S216. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Praseedom, R.K.; Zheng, S.; Dong, J.; Chen, H. Establishment of Animal Model of Dual Liver Transplantation in Rat. PLoS ONE 2012, 7, e40818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimar, J.R.; Ante, W.A.; Zhang, Y.P.; Glassman, S.D. The Effects of Nonsteroidal Anti-inflammatory Drugs on Posterior Spinal Fusions in the Rat. Spine 1996, 21, 1870–1876. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, J.; Zhou, J.; Wu, X.; Huang, Z.; Chen, J.; Zhu, Q. The effects of osteoporosis and disc degeneration on vertebral cartilage endplate lesions in rats. Eur. Spine J. 2014, 23, 1848–1855. [Google Scholar] [CrossRef]
- Liao, J.-C. Cell Therapy Using Bone Marrow-Derived Stem Cell Overexpressing BMP-7 for Degenerative Discs in a Rat Tail Disc Model. Int. J. Mol. Sci. 2016, 17, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Dagostino, C.; De Gregori, M.; Gieger, C.; Manz, J.; Gudelj, I.; Lauc, G.; Divizia, L.; Wang, W.; Sim, M.; Pemberton, I.K.; et al. Validation of standard operating procedures in a multicenter retrospective study to identify -omics biomarkers for chronic low back pain. PLoS ONE 2017, 12, e0176372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridwell, K.H.; Lenke, L.G.; McEnery, K.W.; Baldus, C.; Blanke, K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine 1995, 20, 1410–1418. [Google Scholar] [CrossRef]
- Penney, D.P.; Powers, J.M.; Frank, M.; Willis, C.; Churukian, C. Analysis and testing of biological stains—The Biological Stain Commission Procedures. Biotech. Histochem. 2002, 77, 237–275. [Google Scholar] [CrossRef]
- MacNeal, W.J. Tetrachrome blood stain: An economical and satisfactory imitation of leishman’s stain. JAMA 1922, 78, 122-1. [Google Scholar]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.; Schindelin, J.E.; Cardona, A.; Seung, H.S. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Boden, S.D.; Titus, L.; Hair, G.; Liu, Y.; Viggeswarapu, M.; Nanes, M.S.; Baranowski, C. Lumbar spine fusion by local gene therapy with a cdna encoding a novel osteoinductive protein (lmp-1). Spine 1998, 23, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Khattab, H.M.; Kubota, S.; Takigawa, M.; Kuboki, T.; Sebald, W. The BMP-2 mutant L51P: A BMP receptor IA binding-deficient inhibitor of noggin. J. Bone Miner. Metab. 2019, 37, 199–205. [Google Scholar] [CrossRef] [PubMed]

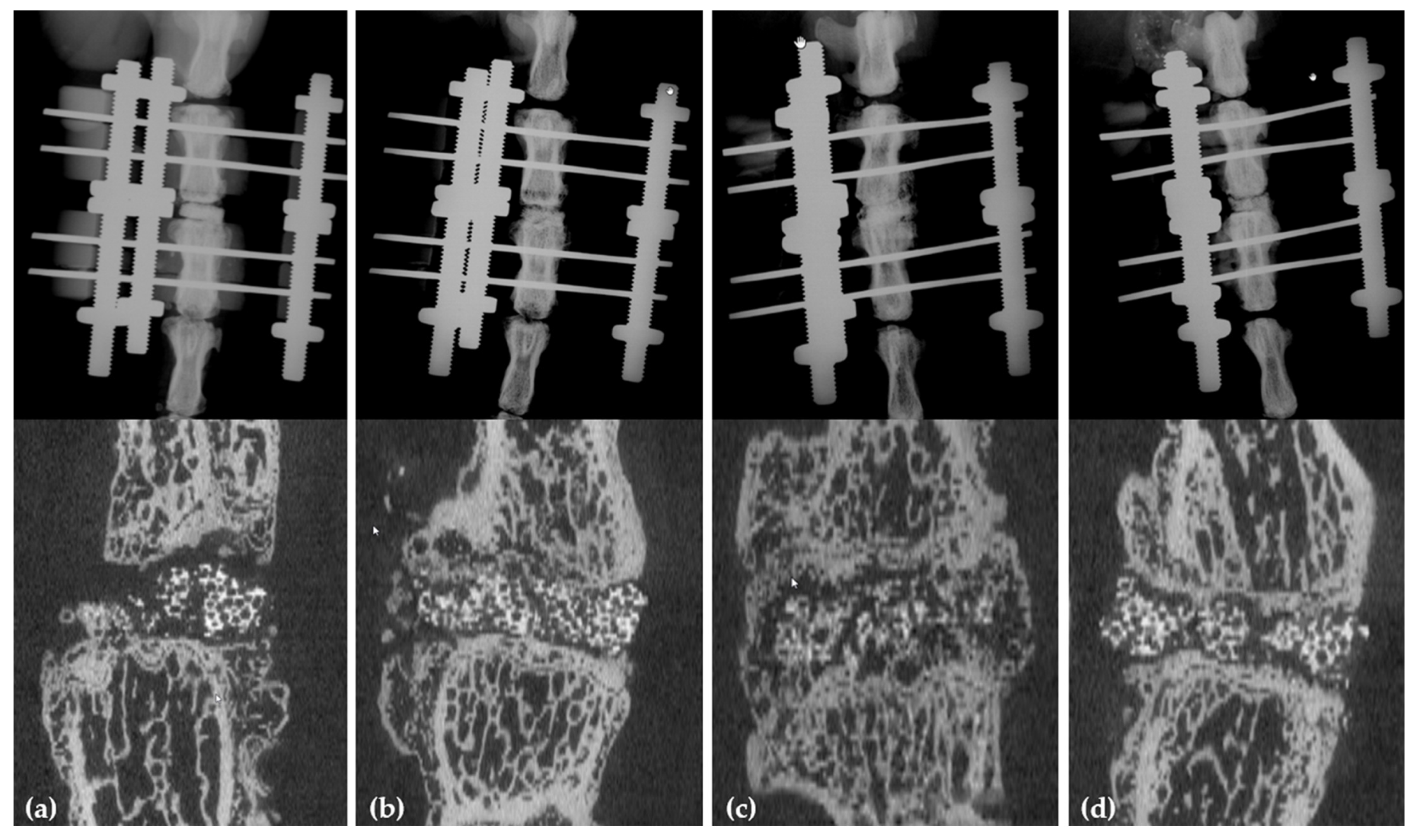
| Parameter | |||
|---|---|---|---|
| Fusion | none | incomplete | complete |
| On-growth on proximal endplate in % of endplate surface | none | <50 | >50 |
| On-growth on distal endplate in % of endplate surface | none | <50 | >50 |
| In-growth through carrier in % of carrier surface | none | <50 | >50 |
| Bridging callus formation in % of endplate surface | none | <50 | >50 |
| Carrier absorption in % of carrier surface | none | <50 | >50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oswald, K.A.C.; Bigdon, S.F.; Croft, A.S.; Bermudez-Lekerika, P.; Bergadano, A.; Gantenbein, B.; Albers, C.E. Establishment of a Novel Method for Spinal Discectomy Surgery in Elderly Rats in an In Vivo Spinal Fusion Model. Methods Protoc. 2021, 4, 79. https://doi.org/10.3390/mps4040079
Oswald KAC, Bigdon SF, Croft AS, Bermudez-Lekerika P, Bergadano A, Gantenbein B, Albers CE. Establishment of a Novel Method for Spinal Discectomy Surgery in Elderly Rats in an In Vivo Spinal Fusion Model. Methods and Protocols. 2021; 4(4):79. https://doi.org/10.3390/mps4040079
Chicago/Turabian StyleOswald, Katharina A. C., Sebastian F. Bigdon, Andreas S. Croft, Paola Bermudez-Lekerika, Alessandra Bergadano, Benjamin Gantenbein, and Christoph E. Albers. 2021. "Establishment of a Novel Method for Spinal Discectomy Surgery in Elderly Rats in an In Vivo Spinal Fusion Model" Methods and Protocols 4, no. 4: 79. https://doi.org/10.3390/mps4040079
APA StyleOswald, K. A. C., Bigdon, S. F., Croft, A. S., Bermudez-Lekerika, P., Bergadano, A., Gantenbein, B., & Albers, C. E. (2021). Establishment of a Novel Method for Spinal Discectomy Surgery in Elderly Rats in an In Vivo Spinal Fusion Model. Methods and Protocols, 4(4), 79. https://doi.org/10.3390/mps4040079