Time Mating Guinea Pigs by Monitoring Changes to the Vaginal Membrane throughout the Estrus Cycle and with Ultrasound Confirmation
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Female and male Hartley guinea pigs, between 2–4 months of age (Charles River, Wilmington, MA, USA).
- Standard Guinea Pig Chow with stabilized Vitamin C. Example: LabDiet 5025 (LabDiet, St. Louis, MI, USA; Cat. no. 5025).
- Ultrasound coupling gel. Example: Aquasonic Clear (Parker Laboratories Inc, Fairfield, NJ, USA; Cat. no. 03-08).
2.2. Equipment
- Ultrasound machine. Example: Voluson I Portable Ultrasound Machine (GE Healthcare, Boston, MA, USA).
- Ultrasound Probe capable of transmitting at depths of 3–10 cm. Example: 25 E 12 MHz vascular probe (GE Healthcare, Boston, MA, USA).
3. Procedure
3.1. Animal Husbandry and Daily Estrus Checks
- Purchase guinea pigs at 500–550 g.
- Group house guinea pigs of same sex on a 12:12 h light on/off cycle at 22 °C/72 °F and 50% humidity.
- Provide water and food ad libitum.
CRITICAL STEP: Ensure vitamin C is stabilized within the feed, otherwise, vitamin C will need to be supplemented in the drinking water (400 mg/L).
PAUSE STEP: Allow guinea pigs to acclimatize in the new location with minimal handling for at least 1 week.
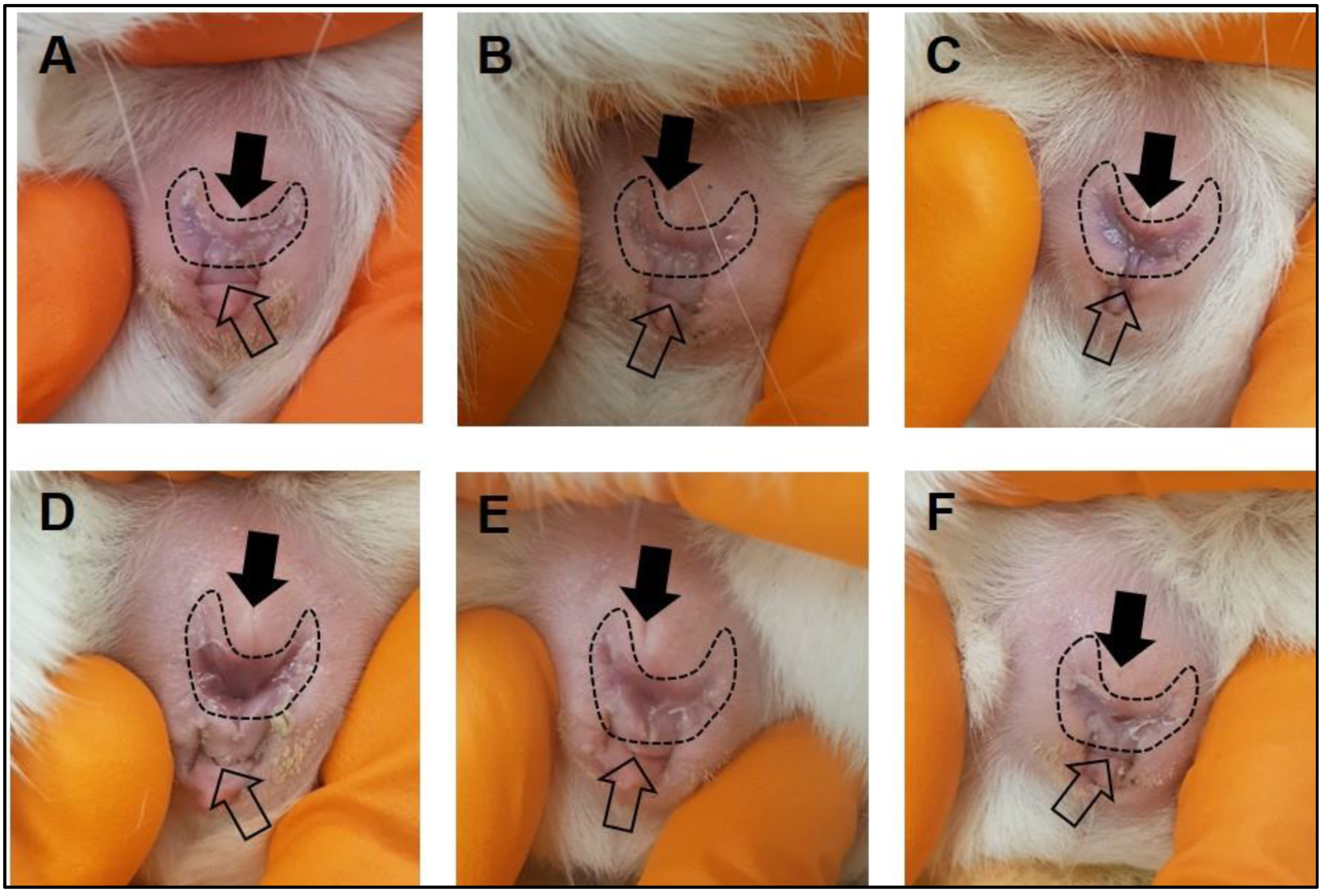
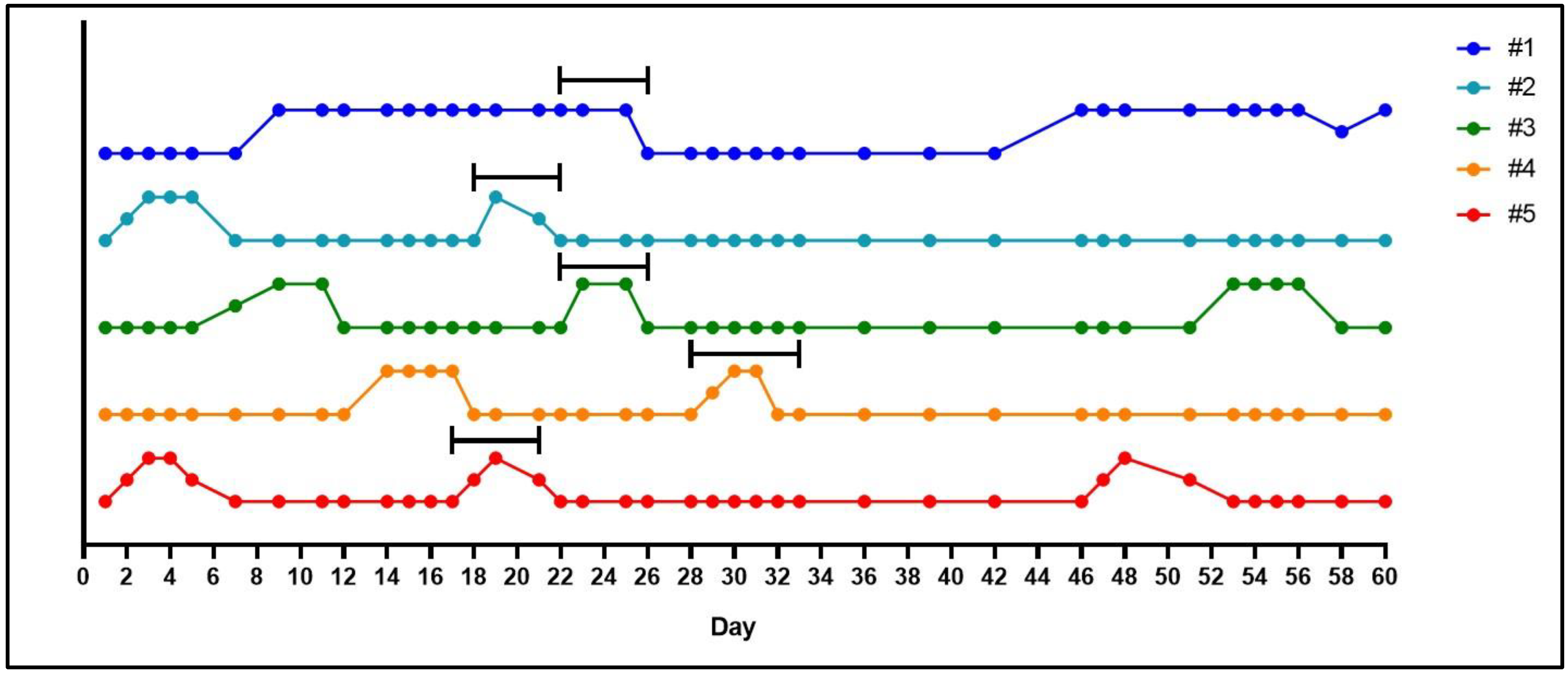
- After the acclimatization period, make daily observations of the female vaginal membrane by holding the animal secure and using the thumb and index finger to gently manipulate the vaginal opening (Figure 1). Record whether the vaginal membrane appears “closed”, “open”, “opening” or “closing”.
- Repeat observations daily, in the morning at approximately the same time of day until the vaginal membrane is observed as perforated (Figure 1). Record the date that vaginal membrane is first observed as perforated.
3.2. Time Mating Guinea Pigs
- If housed together, 2–3 days prior to starting the mating, separate male guinea pigs into individual cages.
- Fourteen days after the vaginal membrane is first observed as perforated, place the female with the male. This will allow the breeding pair to become acquainted before ovulation occurs, leading to a more successful mating. The cage size allowing, up to two females can be placed with a single male.
- Continue to monitor the vaginal membrane daily, as outlined in Section 3.1 of Step 4.
- The morning when the vaginal membrane is observed as perforated, record this date as gestational day (GD) 1. Ovulation is presumed to have occurred overnight and is often associated with the presence of ‘estrus fluid’ in the vaginal opening [14]. Record any ‘signs of mating’ observations such as a pale crustiness around the vaginal opening or a vaginal plug.
- Keep the female(s) with the male until the vaginal membrane closes, females will typically be with males 5–6 days in total.
- Return the female to the group housing.
3.3. Ultrasound Pregnancy Confirmation
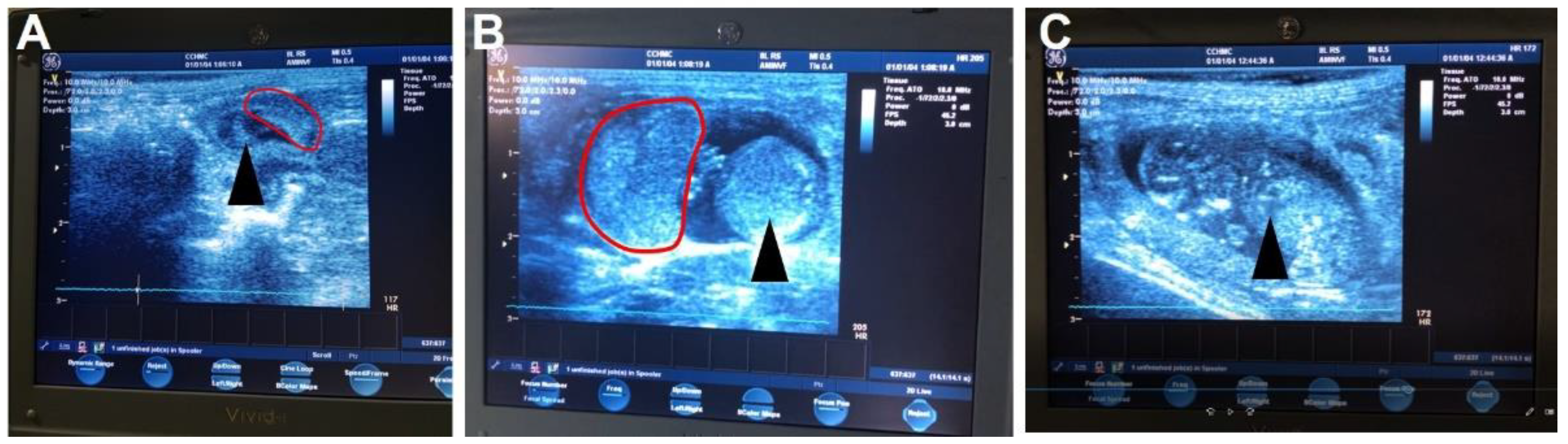
- Ultrasound pregnancy confirmation can be performed without anesthesia [20] between GD21-30.
- Whilst carefully restraining the guinea pig, shave the female guinea pig’s lower abdomen.
- Set the ultrasound to a depth between 6 and 10 cm and a frequency between 8 and 10 MHz.
- Place enough ultrasound coupling gel on the end of the ultrasound probe to cover the probe and perform the ultrasound. Scan from the cervix up to each uterine horn until a conceptus is found. Multiple conceptuses may be observed, but only one is needed to confirm the pregnancy.
- Once the pregnancy is confirmed, return the animal to the group housing.
- OPTIONAL STEP: Ultrasound pregnancy confirmation can be performed under anesthesia using 4–5% isoflurane mixed with 2 L/min oxygen and maintenance at 1 L/min.
- If the female is not confirmed pregnant, repeat the process until a successful pregnancy is established.
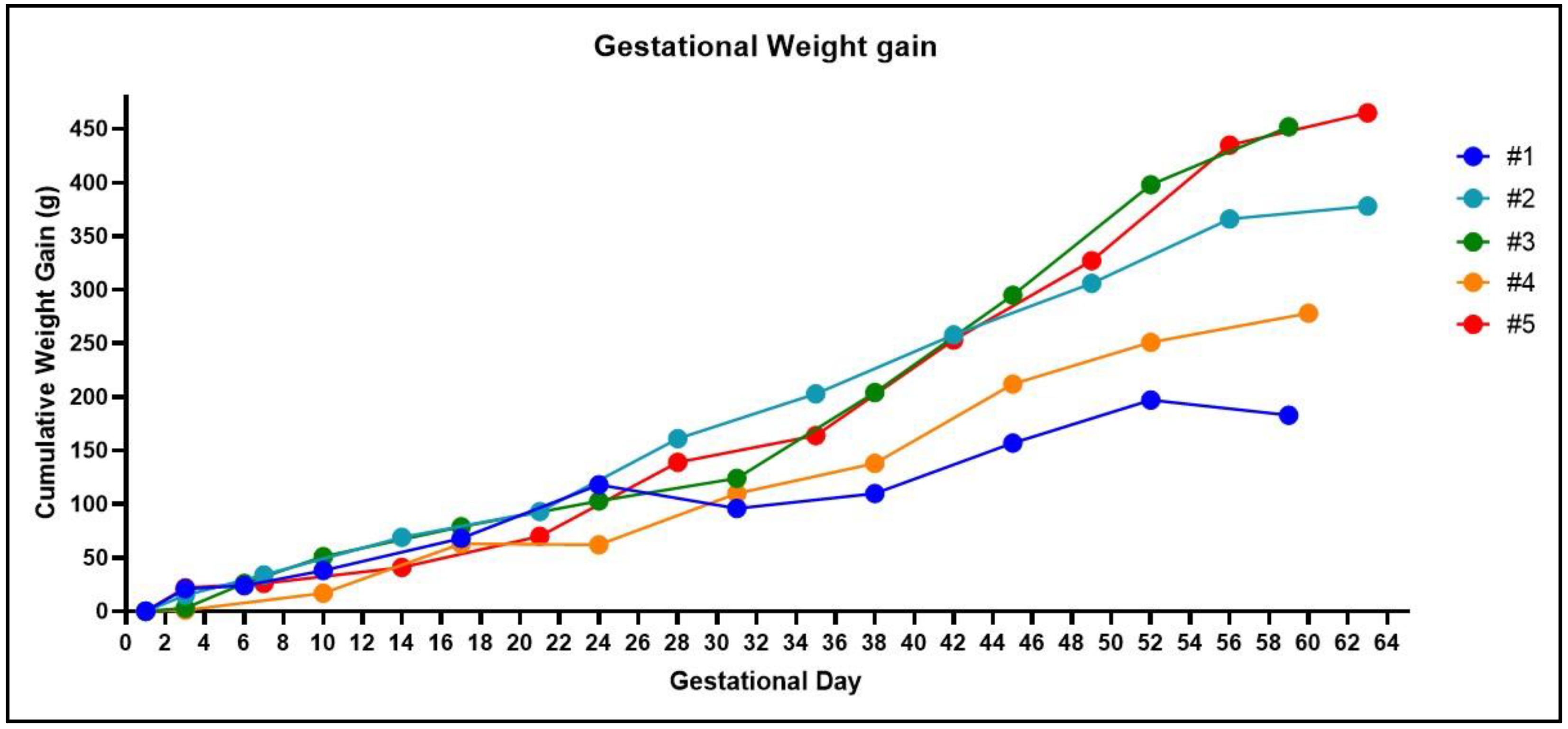
4. Expected Results
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef]
- Roberts, C.T. IFPA Award in Placentology Lecture: Complicated interactions between genes and the environment in placentation, pregnancy outcome and long term health. Placenta 2010, 31, S47–S53. [Google Scholar] [CrossRef]
- Kunkele, J.; Trillmich, F. Are precocial young cheaper? Lactation energetics in the guinea pig. Physiol. Zool. 1997, 70, 589–596. [Google Scholar] [CrossRef]
- Morrison, J.L.; Botting, K.J.; Darby, J.R.T.; David, A.L.; Dyson, R.M.; Gatford, K.L.; Gray, C.; Herrera, E.A.; Hirst, J.J.; Kim, B.; et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol. 2018, 596, 5535–5569. [Google Scholar] [CrossRef]
- Mace, K.; Shahkhalili, Y.; Aprikian, O.; Stan, S. Dietary fat and fat types as early determinants of childhood obesity: A reappraisal. Int. J. Obes. 2006, 30 (Suppl. S4), S50–S57. [Google Scholar] [CrossRef] [Green Version]
- Hunt, A.N.; Kelly, F.J.; Postle, A.D. Developmental variation in whole human lung phosphatidylcholine molecular species: A comparison with guinea pig and rat. Early Hum. Dev. 1991, 25, 157–171. [Google Scholar] [CrossRef]
- Welling, L.W.; Evan, A.P.; Gattone, V.H., 2nd; Rollins, S.; Saunders, R.; Kaskel, F.J.; Spitzer, A. Correlation of structure and function in developing proximal tubule of guinea pig. Am. J. Physiol. 1989, 256, F13–F17. [Google Scholar] [CrossRef] [PubMed]
- Rolph, T.P.; Jones, C.T.; Parry, D. Ultrastructural and enzymatic development of fetal guinea pig heart. Am. J. Physiol. 1982, 243, H87–H93. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.C.; Blankenship, T.N. Comparative placental structure. Adv. Drug Deliv. Rev. 1999, 38, 3–15. [Google Scholar] [CrossRef]
- Mess, A. The Guinea pig placenta: Model of placental growth dynamics. Placenta 2007, 28, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, J. Corpus luteum function in guinea pigs, hamsters, rats, mice and rabbits. Biol. Reprod. 1973, 8, 203–221. [Google Scholar] [CrossRef] [Green Version]
- Selle, R.M. Changes in the vaginal epithelium of the guinea-pig during the oestrous cycle. Am. J. Anat. 1922, 30, 429–449. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, P. Guinea Pig. In Comparative Placentation; Benirschke, K., Ed.; San Diego Zoo Institute for Conservation Research: Escondido, CA, USA, 2004. [Google Scholar]
- Stockard, C.R.; Papanicolaou, G.N. The vaginal closure membrane, copulation, and the vaginal plug in the guinea-pig, with further considerations of the oestrous rhythm. Biol. Bull. 1919, 37, 222–245. [Google Scholar] [CrossRef]
- Bierle, C.J.; Fernandez-Alarcon, C.; Hernandez-Alvarado, N.; Zabeli, J.C.; Janus, B.C.; Putri, D.S.; Schleiss, M.R. Assessing Zika virus replication and the development of Zika-specific antibodies after a mid-gestation viral challenge in guinea pigs. PLoS ONE 2017, 12, e0187720. [Google Scholar] [CrossRef]
- Takeda, M.; Watanabe, S.; Katano, H.; Noguchi, K.; Sato, Y.; Kojima, S.; Miura, T.; Majima, R.; Yamada, S.; Inoue, N. Roles of GP33, a guinea pig cytomegalovirus-encoded G protein-coupled receptor homolog, in cellular signaling, viral growth and inflammation in vitro and in vivo. PLoS Pathog. 2018, 14, e1007487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, D.H.; Young, W.C. The role of progesterone in the production of cyclic vaginal changes in the female guinea pig. Endocrinology 1951, 49, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Lilley, K.G.; Epping, R.J.; Hafner, L.M. The guinea pig estrous cycle: Correlation of vaginal impedance measurements with vaginal cytologic findings. Lab. Anim. Sci. 1997, 47, 632–637. [Google Scholar]
- Elvidge, H. Production of dated pregnant guinea pigs without post-partum matings. Inst. Anim. Tech. J. 1972, 23, 111–117. [Google Scholar]
- Turner, A.J.; Trudinger, B.J. Ultrasound measurement of biparietal diameter and umbilical artery blood flow in the normal fetal guinea pig. Comp. Med. 2000, 50, 379–384. [Google Scholar] [PubMed]
- Matthews, P.J.; Jackson, J. Pregnancy diagnosis in the guinea pig. Lab. Anim. Sci. 1977, 27, 248–250. [Google Scholar]
- Ford, D.H.; Webster, R.C.; Young, W.C. Rupture of the vaginal closure membrane during pregnancy in the guinea pig. Anat. Rec. 1951, 109, 707–714. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, R.L.; Lampe, K.; Matushewski, B.J.; Regnault, T.R.H.; Jones, H.N. Time Mating Guinea Pigs by Monitoring Changes to the Vaginal Membrane throughout the Estrus Cycle and with Ultrasound Confirmation. Methods Protoc. 2021, 4, 58. https://doi.org/10.3390/mps4030058
Wilson RL, Lampe K, Matushewski BJ, Regnault TRH, Jones HN. Time Mating Guinea Pigs by Monitoring Changes to the Vaginal Membrane throughout the Estrus Cycle and with Ultrasound Confirmation. Methods and Protocols. 2021; 4(3):58. https://doi.org/10.3390/mps4030058
Chicago/Turabian StyleWilson, Rebecca L., Kristin Lampe, Brad J. Matushewski, Timothy R. H. Regnault, and Helen N. Jones. 2021. "Time Mating Guinea Pigs by Monitoring Changes to the Vaginal Membrane throughout the Estrus Cycle and with Ultrasound Confirmation" Methods and Protocols 4, no. 3: 58. https://doi.org/10.3390/mps4030058
APA StyleWilson, R. L., Lampe, K., Matushewski, B. J., Regnault, T. R. H., & Jones, H. N. (2021). Time Mating Guinea Pigs by Monitoring Changes to the Vaginal Membrane throughout the Estrus Cycle and with Ultrasound Confirmation. Methods and Protocols, 4(3), 58. https://doi.org/10.3390/mps4030058








