KidneyCare Guided Immuno-Optimization in Renal Allografts: The KIRA Protocol
Abstract
1. Introduction
2. The KidneyCare Platform-Three-Pronged, Multimodality Testing
2.1. AlloSure®-Donor-Derived-cfDNA (dd-cfDNA)
2.2. AlloMap Kidney®-Gene Expression Profiling (GEP)
2.3. iBox-Allograft Prognostic Score
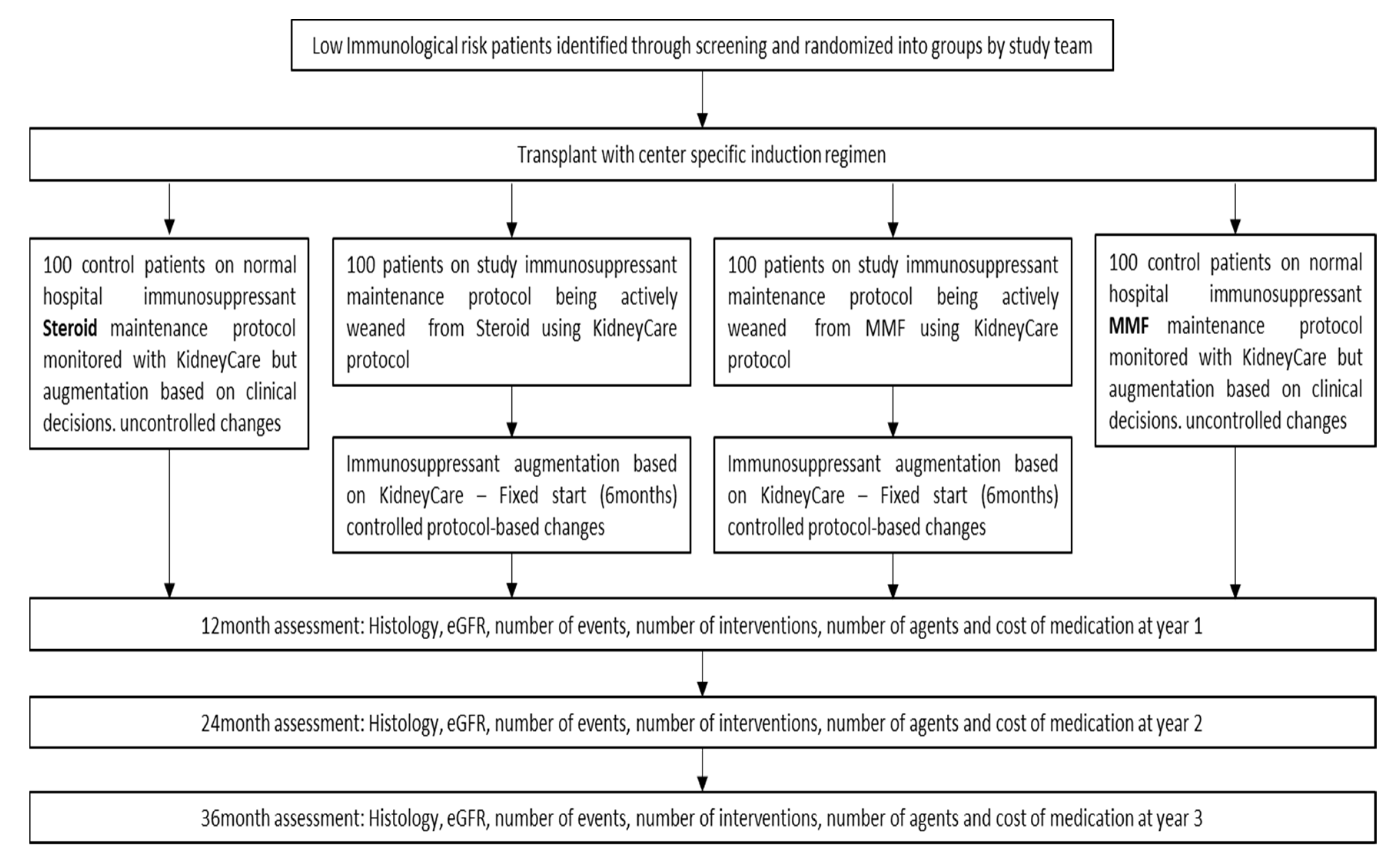
3. Methods
Abnormal Biopsy Result
4. Results/Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shapiro, R.; Young, J.B.; Milford, E.L.; Trotter, J.F.; Bustami, R.; Leichtman, A.B. Immunosuppression: Evolution in practice and trends, 1993–2003. Am. J. Transpl. 2005, 5, 874–886. [Google Scholar] [CrossRef]
- Axelrod, D.A.; Naik, A.S.; Schnitzler, M.A.; Segev, D.L.; Dharnidharka, V.R.; Brennan, D.C.; Bae, S.; Chen, J.; Massie, A.; Lentine, K.L. National Variation in use of immunosuppression for kidney transplantation: A call for evidence-based regimen selection. Am. J. Transpl. 2016, 16, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Westfall, A.O.; Allison, J.; Bijlsma, J.W.; Freeman, A.; George, V.; Kovac, S.H.; Spettell, C.M.; Saag, K.G. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006, 55, 420. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, E.; Fernández-Fresnedo, G.; Valero, R.; Ruiz, J.C.; Piñera, C.; Palomar, R.; González-Cotorruelo, J.; Gómez-Alamillo, C.; Arias, M. New-Onset Diabetes after Kidney Transplantation: Risk Factors. JASN 2006, 17, S291–S295. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.S.; Dharnidharka, V.R.; Schnitzler, M.A.; Brennan, D.C.; Segev, D.L.; Axelrod, D.; Xiao, H.; Kucirka, L.; Chen, J.; Lentine, K.L. Clinical and economic consequences of first-year urinary tract infections, sepsis, and pneumonia in contemporary kidney transplantation practice. Transpl. Int. 2016, 29, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Dharnidharka, V.R.; Naik, A.S.; Axelrod, D.A.; Schnitzler, M.A.; Zhang, Z.; Bae, S.; Segev, D.L.; Brennan, D.C.; Alhamad, T.; Ouseph, R.; et al. Center practice drives variation in choice of US kidney transplant induction therapy: A retrospective analysis of contemporary practice. Transpl. Int. 2018, 31, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Maintenance immunosuppression regimens: Conversion, minimization, withdrawal and avoidance. Am. J. Kidney Dis. 2006, 47, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Quereda, C.; Zamora, J.; Hernández, D.; Spanish Group for Evidence-Based Medicine in Renal Transplantation. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: A meta-analysis of randomized, controlled trials. Transplantation 2004, 78, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Rev. Clin. J. Am. Soc. Nephrol. 2018, 13, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.S.; Brennan, D.C.; Poggio, E.; Bunnapradist, S.; Langone, A.; Sood, P.; Matas, A.J.; Mannon, R.B.; Mehta, S.; Sharfuddin, A. Biological Variation of Donor-Derived Cell-Free DNA in Renal Transplant Recipients: Clinical Implications. J. Appl. Lab. Med. 2017, 2, 309–321. [Google Scholar] [CrossRef]
- Weir, M.R.; Wali, R.K. Minimizing the Risk of Chronic Allograft Nephropathy. Transplantation 2009, 87, S14–S18. [Google Scholar] [CrossRef] [PubMed]
- Hricik, D.E.; Formica, R.N.; Nickerson, P.; Rush, D.; Fairchild, R.L.; Poggio, E.D.; Gibson, I.W.; Wiebe, C.; Tinckam, K.; Bunnapradist, S.; et al. Adverse Outcomes of Tacrolimus Withdrawal in Immune-Quiescent Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2015, 26, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Krämer, B.K.; Klinger, M.; Vítko, Š.; Glyda, M.; Midtvedt, K.; Stefoni, S.; Citterio, F.; Pietruck, F.; Squifflet, J.P.; Segoloni, G.; et al. Tacrolimus-based, steroid-free regimens in renal transplantation: 3-year follow-up of the ATLAS trial. Transplantation 2012, 94, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Grskovic, M.; Hiller, D.J.; Eubank, L.A.; Sninsky, J.J.; Christopherson, C.; Collins, J.P.; Thompson, K.; Song, M.; Wang, Y.S.; Ross, D.; et al. Validation of a Clinical-Grade Assay to Measure Donor-Derived Cell-Free DNA in Solid Organ Transplant Recipients. J. Mol. Diagn. 2016, 18, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. JASN 2017, 28, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.M.; Williams, A.N.; Gelbart, T.; Campbell, D.T.; Mondala, S.; Head, S.R.; Horvath, S.; Gaber, L.; Thompson, R.; Whisenant, T.; et al. Molecular classifiers for acute kidney transplant rejection in peripheral blood by whole genome gene expression profiling. Am. J. Transpl. 2014, 14, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Aubert, O.; Orandi, B.K.; Naesens, M.; Bouatou, Y.; Raynaud, M.; Divard, G.; Jackson, A.M.; Viglietti, D.; Giral, M.; et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 2019, 366, l4923. [Google Scholar] [CrossRef] [PubMed]

| Post-Tx Regimen | Week 1 | Mon 1 | Mon 2 | Mon 3 | Mon 4 | Mon 5 | Mon 6 | Mon 7 | Mon 8 | Mon 9 | Mon 10 | Mon 11 | Mon 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prednisone | Center protocol | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg |
| MMF q 12 h | 1000 mg | 1000 mg | 1000 mg | 1000 mg | 750 mg | 750 mg | 500 mg | 500 mg | 500 mg | 250 mg | 250 mg | 0 mg | 0 mg |
| Tacrolimus goal (ng/mL) | 8–12 | 8–12 | 8–12 | 8–12 | 8–12 | 8–12 | 8–12 | 6–8 | 6–8 | 6–8 | 6–8 | 6–8 | 6–8 |
| DSA | X | X | X | X | X | ||||||||
| BK screening | X | X | X | X | X | X | X | X | X | X | X | X | |
| KidneyCare | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Protocol Bx | X | X |
| Post Tx Regimen | 13 Month | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSA | X | X | X | X | ||||||||
| BK screening | X | X | X | X | X | X | X | X | X | X | X | X |
| KidneyCare | X | X | X | X | X | X | X | X | X | X | X | X |
| Cystatin C | X | X | ||||||||||
| Protocol Bx | X |
| Post Tx Regimen | Week 1 | Mon 1 | Mon 2 | Mon 3 | Mon 4 | Mon 5 | Mon 6 | Mon 7 | Mon 8 | Mon 9 | Mon 10 | Mon 11 | Mon 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prednisone | 30 mg | 10 mg | 5 mg | 5 mg | 0 mg | 0 mg | 0 mg | 0 mg | 0 mg | 0 mg | 0 mg | 0 mg | 0 mg |
| MMF q 12 h | 1000 mg | 1000 mg | 1000 mg | 1000 mg | 750 mg | 750 mg | 500 mg | 500 mg | 500 mg | 500 mg | 500 mg | 500 mg | 500 mg |
| Tacrolimus goal (ng/mL) | 8–12 | 8–12 | 8–12 | 8–11 | 8–12 | 8–12 | 8–12 | 8–12 | 8–12 | 6–8 | 6–8 | 6–8 | 6–8 |
| DSA | X | X | X | X | X | ||||||||
| BK screening | X | X | X | X | X | X | X | X | X | X | X | X | |
| Kidney Care | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Protocol Bx | X | X |
| Post Tx Regimen | 13 Month | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSA | X | X | X | X | ||||||||
| BK screening | X | X | X | X | X | X | X | X | X | X | X | X |
| KidneyCare | X | X | X | X | X | X | X | X | X | X | X | X |
| Cystatin C | X | X | ||||||||||
| Protocol Bx | X |
| New DSA | AlloSure (%) | 3 yr iBox Score | AlloMap Kidney (RUO) | Action |
|---|---|---|---|---|
| Negative | ≤0.2 | > 85% | <10 | Proceed/Minimize |
| Negative | >0.2 ≤ 0.5 | >85% | <10 | Proceed/Minimize |
| Negative | >0.5 + ≤ 61% change | >85% | <10 | Proceed/Minimize |
| Positive | >0.2 ≤ 0.5 | <85% | Any value | Repeat |
| Negative | >0.5 + >61% change | >85% | Any value | Repeat |
| Positive | >0.5 + ≤61% change | <85% | Any value | Not Safe to Proceed |
| Positive | >0.5 + >61% change | <85% | Any value | Not Safe to Proceed |
| Positive | ≤0.2 | <85% | >10 | Not Safe to proceed |
| Positive | >1 | <85% | Any value | Not Safe to Proceed |
| Negative | >1 | >85% | Any value | Not Safe to Proceed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gray, J.N.; Wolf-Doty, T.; Sulejmani, N.; Gaber, O.; Axelrod, D.; Abdalla, B.; Danovitch, G. KidneyCare Guided Immuno-Optimization in Renal Allografts: The KIRA Protocol. Methods Protoc. 2020, 3, 68. https://doi.org/10.3390/mps3040068
Gray JN, Wolf-Doty T, Sulejmani N, Gaber O, Axelrod D, Abdalla B, Danovitch G. KidneyCare Guided Immuno-Optimization in Renal Allografts: The KIRA Protocol. Methods and Protocols. 2020; 3(4):68. https://doi.org/10.3390/mps3040068
Chicago/Turabian StyleGray, Jennifer N., Theresa Wolf-Doty, Nimisha Sulejmani, Osama Gaber, David Axelrod, Basmah Abdalla, and Gabriel Danovitch. 2020. "KidneyCare Guided Immuno-Optimization in Renal Allografts: The KIRA Protocol" Methods and Protocols 3, no. 4: 68. https://doi.org/10.3390/mps3040068
APA StyleGray, J. N., Wolf-Doty, T., Sulejmani, N., Gaber, O., Axelrod, D., Abdalla, B., & Danovitch, G. (2020). KidneyCare Guided Immuno-Optimization in Renal Allografts: The KIRA Protocol. Methods and Protocols, 3(4), 68. https://doi.org/10.3390/mps3040068





