Preconditioning of Bioactive Glasses before Introduction to Static Cell Culture: What Is Really Necessary?
Abstract
1. Introduction
2. Materials and Methods
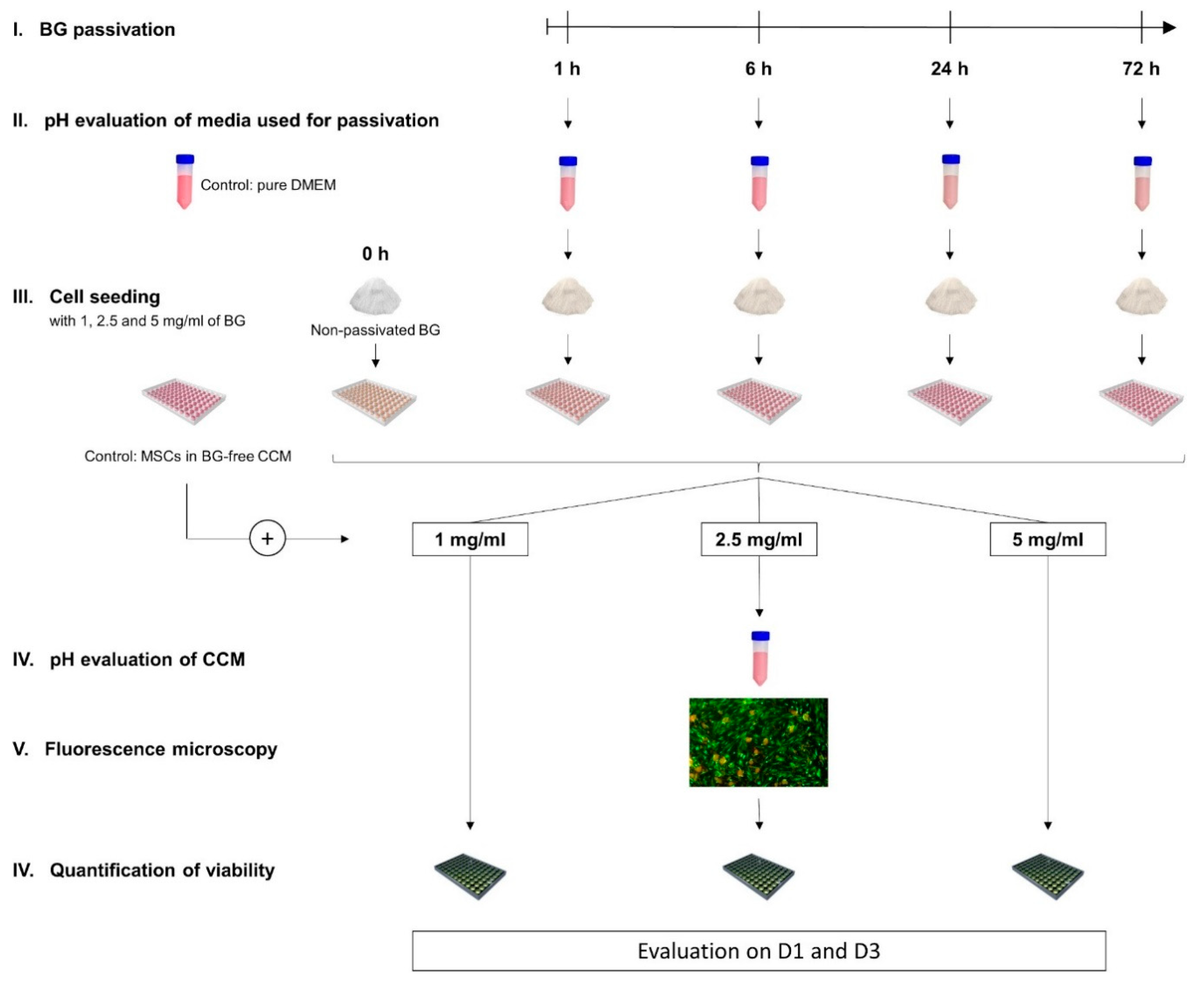
2.1. General Experimental Design: Overview
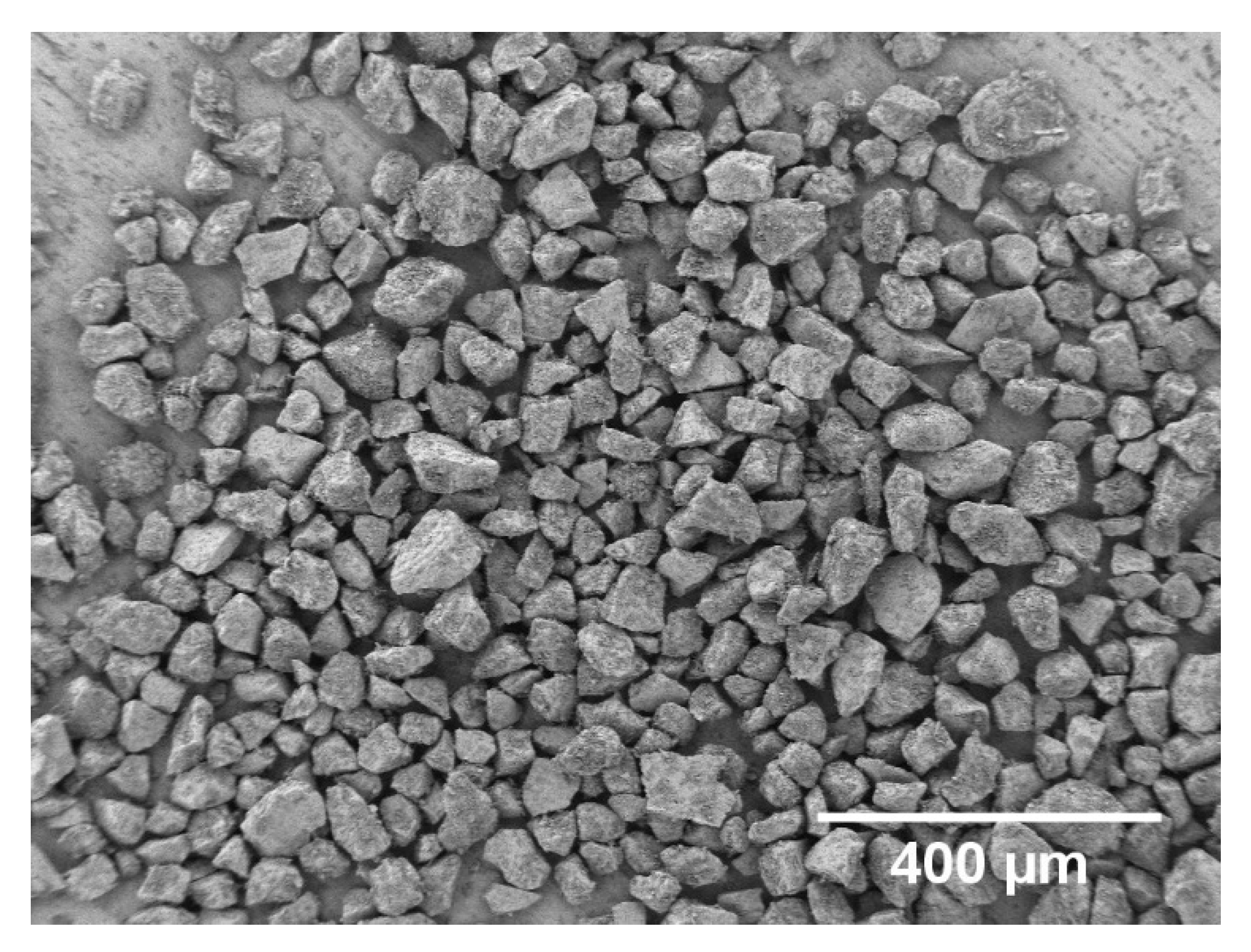
2.2. BG Production and Characterization
2.3. Passivation of the BG-Particles
2.4. Study Ethics and Cell Origin
2.5. BMSC Isolation, Cultivation, and Characterization
2.6. Evaluation of pH
2.7. Visual Assessment of Cell Morphology and Growth Patterns
2.8. Quantification of Cell Viability
2.9. Statistics
3. Results
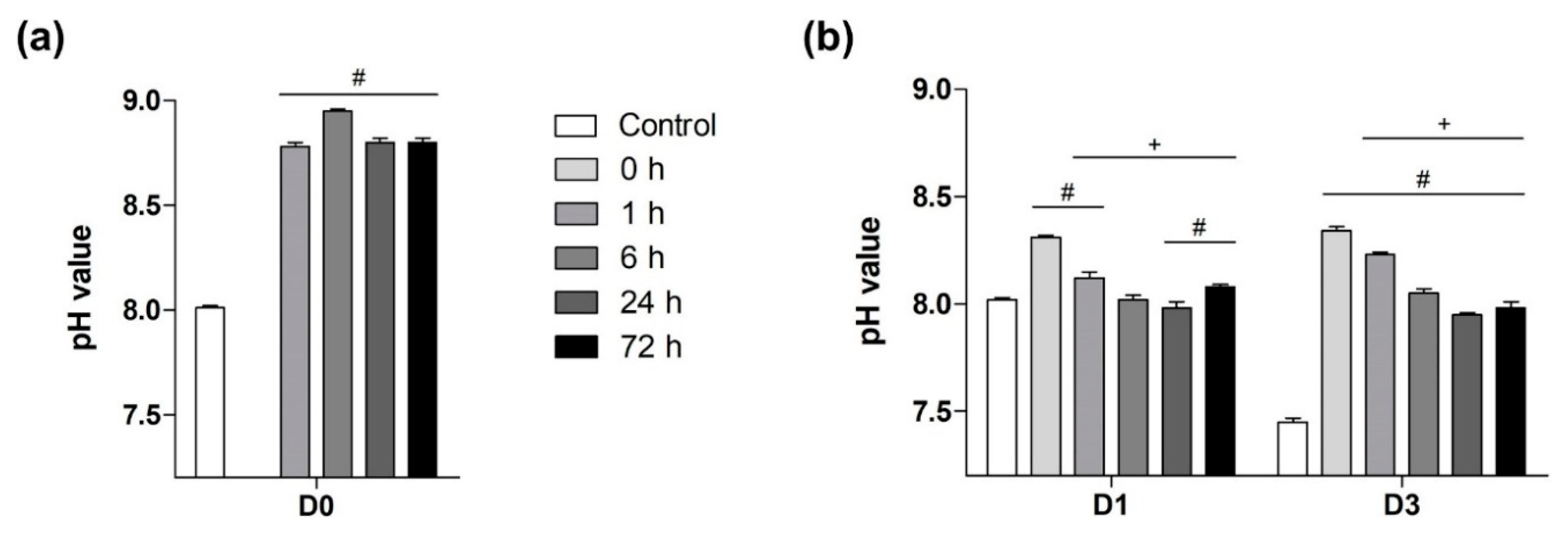
3.1. The Impact of Passivation Periods on BG-Induced pH Alkalization of Cell Medium
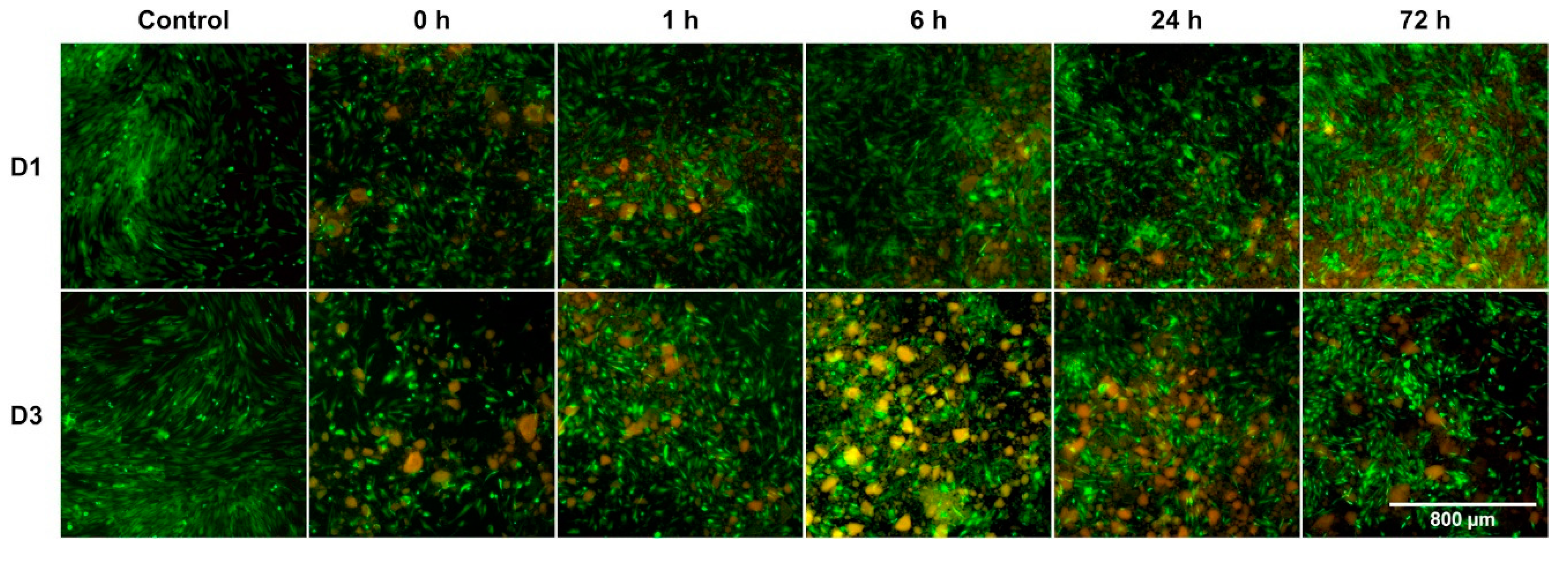
3.2. Impact on Cell Morphology and Growth Patterns Particularly Showed during Early Incubation Period
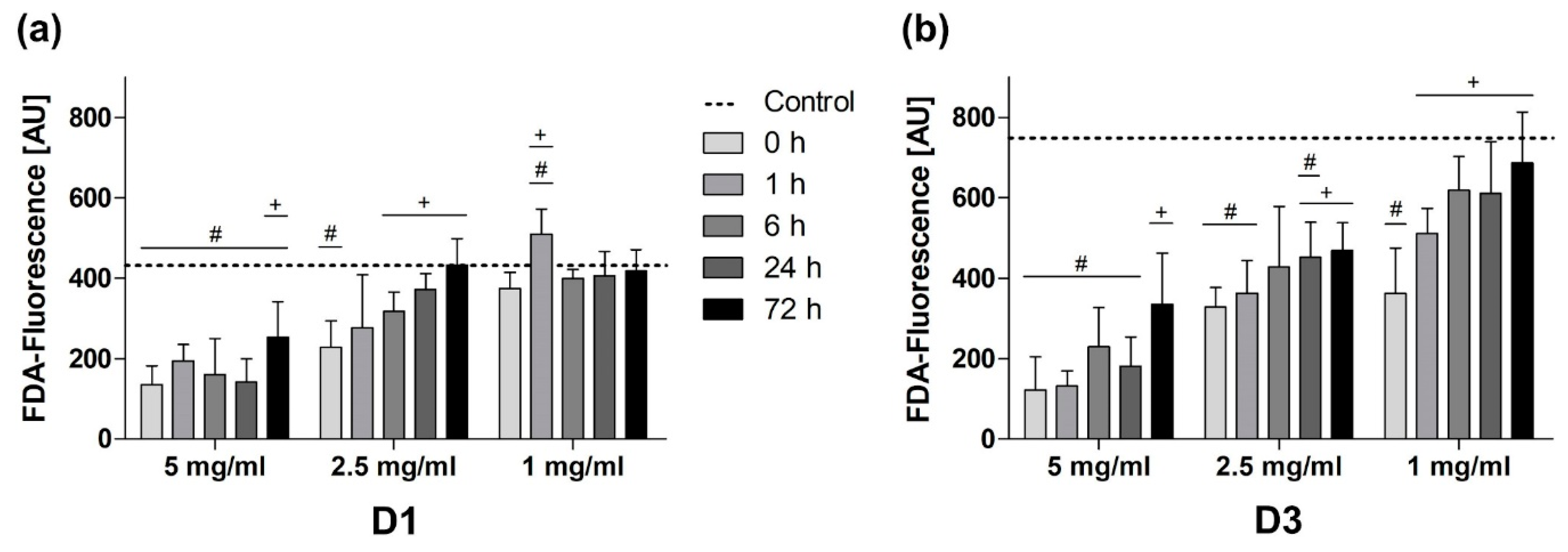
3.3. Passivation Mediated Reduction of BG-Induced Cytotoxicity on All Days
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BG | bioactive glass |
| BMSC | bone marrow-derived stromal cell |
| BTE | bone tissue engineering |
| CCM | cell culture medium |
| CO2 | carbon dioxide |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FDA | fluorescein diacetate |
| HCA | hydroxycarbonate apatite |
| NEAA | non-essential amino acids |
| PBS | phosphate buffered saline |
| PI | propidium iodide |
References
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Barberi, J.; Verne, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Paschall, H.A. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Wilkesmann, S.; Fellenberg, J.; Nawaz, Q.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Primary osteoblasts, osteoblast precursor cells or osteoblast-like cell lines: Which human cell types are (most) suitable for characterizing 45S5-Bioglass? J. Biomed. Mater. Res. A 2019, 108, 663–674. [Google Scholar] [CrossRef]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, D.C. Bioactive glass: Mechanisms of bone bonding. Tandläkartidningen Ǻrk 1999, 91, 1–32. [Google Scholar]
- Brito, A.F.; Antunes, B.; Dos Santos, F.; Fernandes, H.R.; Ferreira, J.M.F. Osteogenic capacity of alkali-free bioactive glasses. In vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jones, J.R.; Sepulveda, P. Bioactive Materials for Tissue Engineering Scaffolds. In Future Strategies for Tissue and Organ Replacement; Imperial College Press: London, UK, 2002. [Google Scholar] [CrossRef]
- Karadjian, M.; Essers, C.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Biological Properties of Calcium Phosphate Bioactive Glass Composite Bone Substitutes: Current Experimental Evidence. Int. J. Mol. Sci. 2019, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Widholz, B.; Essers, C.; Becker, M.; Tulyaganov, D.; Juan, I.; Westhauser, F. Superior biocompatibility and comparable osteoinductive properties: Sodium-reduced fluoride-containing bioactive glass belonging to the CaO-MgO-SiO2 system as a promising alternative to 45S5 bioactive glass. Bioact. Mater. 2020, 5, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Thavornyutikarn, B.; Feltis, B.; Wright, P.F.A.; Turney, T.W. Effect of pre-treatment of crystallized bioactive glass with cell culture media on structure, degradability, and biocompatibility. Mater. Sci. Eng. C 2019, 97, 188–197. [Google Scholar] [CrossRef]
- Westhauser, F.; Hohenbild, F.; Arango-Ospina, M.; Schmitz, S.I.; Wilkesmann, S.; Hupa, L.; Moghaddam, A.; Boccaccini, A.R. Bioactive Glass (BG) ICIE16 Shows Promising Osteogenic Properties Compared to Crystallized 45S5-BG. Int. J. Mol. Sci. 2020, 21, 1639. [Google Scholar] [CrossRef]
- Pryce, R.; Hench, L.L. Dissolution characteristics of bioactive glasses. Key Eng. Mater. 2003, 240–242, 201–204. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, W.; Bal, B.S.; Rahaman, M.N. Effect of copper-doped silicate 13–93 bioactive glass scaffolds on the response of MC3T3-E1 cells in vitro and on bone regeneration and angiogenesis in rat calvarial defects in vivo. Mater. Sci. Eng. C 2016, 67, 440–452. [Google Scholar] [CrossRef]
- Detsch, R.; Guillon, O.; Wondraczek, L.; Boccaccini, A.R. Initial Attatchment of rMSC and MG-63 Cells on Patterned Bioglass® Substrates. Adv. Eng. Mater. 2012, 14, B38–B44. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Prokscha, M.; Moghaddam, A.; Westhauser, F. Continuous stimulation with differentiation factors is necessary to enhance osteogenic differentiation of human mesenchymal stem cells in-vitro. Growth Factors 2017, 35, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Widholz, B.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Westhauser, F. Pooling of Patient-Derived Mesenchymal Stromal Cells Reduces Inter-Individual Confounder-Associated Variation without Negative Impact on Cell Viability, Proliferation and Osteogenic Differentiation. Cells 2019, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Westhauser, F.; Karadjian, M.; Essers, C.; Senger, A.S.; Hagmann, S.; Schmidmaier, G.; Moghaddam, A. Osteogenic differentiation of mesenchymal stem cells is enhanced in a 45S5-supplemented beta-TCP composite scaffold: An in-vitro comparison of Vitoss and Vitoss BA. PLoS ONE 2019, 14, e0212799. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ducheyne, P.; Shapiro, I.M. Formation of surface reaction products on bioactive glass and their effects on the expression of the osteoblastic phenotype and the deposition of mineralized extracellular matrix. Biomaterials 1997, 18, 295–303. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- McKee, T.J.; Komarova, S.V. Is it time to reinvent basic cell culture medium? Am. J. Physiol. Cell Physiol. 2017, 312, C624–C626. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Hupa, L.; Boccaccini, A.R. Bioactivity and dissolution behavior of boron-containing bioactive glasses under static and dynamic conditions in different media. Biomed. Glasses 2019, 5, 124–139. [Google Scholar] [CrossRef]
- Nuschke, A.; Rodrigues, M.; Wells, A.W.; Sylakowski, K.; Wells, A. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem. Cell Res. Ther. 2016, 7, 179. [Google Scholar] [CrossRef]
- Wilkesmann, S.; Westhauser, F.; Fellenberg, J. Combined Fluorescence-Based in Vitro Assay for the Simultaneous Detection of Cell Viability and Alkaline Phosphatase Activity during Osteogenic Differentiation of Osteoblast Precursor Cells. Methods Protoc. 2020, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Galow, A.-M.; Rebl, A.; Koczan, D.; Bonk, S.M.; Baumann, W.; Gimsa, J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem. Biophys. Rep. 2017, 10, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Monfoulet, L.-E.; Becquart, P.; Marchat, D.; Vandamme, K.; Bourguignon, M.; Pacard, E.; Viateau, V.; Petite, H.; Logeart-Avramoglou, D. The pH in the Microenvironment of Human Mesenchymal Stem Cells Is a Critical Factor for Optimal Osteogenesis in Tissue-Engineered Constructs. Tissue Eng. Part A 2014, 20, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. The Sequential Expression Profiles of Growth Factors from Osteroprogenitors to Osteoblasts In Vitro. Tissue Eng. 2007, 13, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cell Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Alcaide, M.; Portolés, P.; López-Noriega, A.; Arcos, D.; Vallet-Regí, M.; Portolés, M.T. Interaction of an ordered mesoporous bioactive glass with osteoblasts, fibroblasts and lymphocytes, demonstrating its biocompatibility as a potential bone graft material. Acta Biomater. 2010, 6, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, G.; Goudouri, O.; Kontonasaki, E.; Chatzistavrou, X.; Papadopoulou, L.; Kantiranis, N.; Paraskevopoulos, K. Comparative Bioactivity Study of 45S5 and 58S Bioglasses in Organic and Inorganic Environment. Bioceram. Dev. Appl. 2011, 1. [Google Scholar] [CrossRef]
- LutiŠAnovÁ, G.; Palou, M.; Kozankova, J. Comparison of bioactivity in vitro of glass and glass ceramic materials during soaking in SBF and DMEM medium. Ceram. Silik. 2011, 55, 199–207. [Google Scholar]
- Bellucci, D.; Cannillo, V.; Sola, A.; Chiellini, F.; Gazzarri, M.; Migone, C. Macroporous Bioglass®-derived scaffolds for bone tissue regeneration. Ceram. Int. 2011, 37, 1575–1585. [Google Scholar] [CrossRef]
- Hoppe, A.; Meszaros, R.; Stähli, C.; Romeis, S.; Schmidt, J.; Peukert, W.; Marelli, B.; Nazhat, S.N.; Wondraczek, L.; Lao, J.; et al. In vitro reactivity of Cu doped 45S5 Bioglass® derived scaffolds for bone tissue engineering. J. Mater. Chem. B 2013, 1, 5659–5674. [Google Scholar] [CrossRef]
- Mohd Zain, N.S.; Tajudin, S.S.; Mohd Noor, S.N.F.; Mohamad, H. Biocompatibility Assessment of Newly Fabricated Melt Derived 45S5 Bioactive Glass on Dental Cells Proliferation. J. Biomed. Clin. Sci. 2017, 1, 26–32. [Google Scholar]
- Yun, H.-S.; Park, J.-W.; Kim, S.-H.; Kim, Y.-J.; Jang, J.-H. Effect of the pore structure of bioactive glass balls on biocompatibility in vitro and in vivo. Acta Biomater. 2011, 7, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Westhauser, F.; Widholz, B.; Nawaz, Q.; Tsitlakidis, S.; Hagmann, S.; Moghaddam, A.; Boccaccini, A.R. Favorable angiogenic properties of the borosilicate bioactive glass 0106-B1 result in enhanced in vivo osteoid formation compared to 45S5 Bioglass. Biomater. Sci. 2019, 7, 5161–5176. [Google Scholar] [CrossRef] [PubMed]
- Haro Durand, L.A.; Vargas, G.E.; Romero, N.M.; Vera-Mesones, R.; Porto-López, J.M.; Boccaccini, A.R.; Zago, M.P.; Baldi, A.; Gorustovich, A. Angiogenic effects of ionic dissolution products released from a boron-doped 45S5 bioactive glass. J. Mater. Chem. B 2015, 3, 1142–1148. [Google Scholar] [CrossRef]
- Xie, F.; Gonzalo-Juan, I.; Arango Ospina, M.; Riedel, R.; Boccaccini, A.; Ionescu, E. Apatite Forming Ability and Dissolution Behavior of Boron- and Calcium-Modified Silicon Oxycarbides in Comparison to Silicate Bioactive Glass. ACS Biomater. Sci. Eng. 2019, 5, 5337–5347. [Google Scholar] [CrossRef]
- Chen, S.; Michálek, M.; Galuskova, D.; Michálková, M.; Švančárek, P.; Talimian, A.; Kaňková, H.; Kraxner, J.; Zheng, K.; Liverani, L.; et al. Multi-targeted B and Co co-doped 45S5 bioactive glass with angiogenic potential for bone regeneration. Mater. Sci. Eng. C 2020, 112, 110909. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Zhang, Y.; Mizuno, M.; Yanagisawa, M.; Takadama, H. Bioactive Behaviors of Porous Apatite- and Wollastonite-Containing Glass-Ceramic in Two Kinds of Simulated Body Fluid. J. Mater. Res. 2003, 18, 433–441. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohenbild, F.; Arango-Ospina, M.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Preconditioning of Bioactive Glasses before Introduction to Static Cell Culture: What Is Really Necessary? Methods Protoc. 2020, 3, 38. https://doi.org/10.3390/mps3020038
Hohenbild F, Arango-Ospina M, Moghaddam A, Boccaccini AR, Westhauser F. Preconditioning of Bioactive Glasses before Introduction to Static Cell Culture: What Is Really Necessary? Methods and Protocols. 2020; 3(2):38. https://doi.org/10.3390/mps3020038
Chicago/Turabian StyleHohenbild, Frederike, Marcela Arango-Ospina, Arash Moghaddam, Aldo R. Boccaccini, and Fabian Westhauser. 2020. "Preconditioning of Bioactive Glasses before Introduction to Static Cell Culture: What Is Really Necessary?" Methods and Protocols 3, no. 2: 38. https://doi.org/10.3390/mps3020038
APA StyleHohenbild, F., Arango-Ospina, M., Moghaddam, A., Boccaccini, A. R., & Westhauser, F. (2020). Preconditioning of Bioactive Glasses before Introduction to Static Cell Culture: What Is Really Necessary? Methods and Protocols, 3(2), 38. https://doi.org/10.3390/mps3020038






