Monitoring Epithelial–Mesenchymal Transition of Pancreatic Cancer Cells via Investigation of Mitochondrial Dysfunction
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
- AsPC-1 (American Type Culture Collection, Manassas, VA, USA; Cat. no.: CRL-1682).
- RPMI1640 (Welgene, Daegu, Korea; Cat. no.: LM 011-02).
- Fetal bovine serum (Welgene, Daegu, Korea; Cat. no.: S 101-01).
- Penicillin-Streptomycin solution (Welgene, Daegu, Korea; Cat. no.: LS 202-02).
- Phosphate buffered saline (Welgene, Daegu, Korea; Cat. no.: LB 001-01).
- Trypsin-EDTA solution (Merck, Darmstadt, Hessen, Germany; Cat. no.: 59418C).
- 100 mm culture dishes (Sigma, St. Louis, MO, USA; CLS430167).
- 15 mL tubes (Sigma, St. Louis, MO, USA; CLS430791).
- 6-well plate (Sigma, St. Louis, MO, USA; SIAL0506).
- Mitochondrial superoxide indicator (Invitrogen, Carlsbad, CA, USA; M36008).
- Mitochondria tracking fluorogenic dye (Invitrogen, Carlsbad, CA, USA; M7514).
- bisBenzimide H 33342 trihydrochloride (Invitrogen, Carlsbad, CA, USA; H3570).
- Tetramethylrhodamine, methyl ester (Invitrogen, Carlsbad, CA, USA; T668).
- 1X Hanks’ Balanced Salt solution (Merck, Darmstadt, Hessen, Germany; Cat. no.: H1641).
- Paraformaldehyde (Biosesang, Seongnam, Korea; Cat. no.: PC2031-050-00).
- 5 mL flow cytometry and fluorescence-activated cell sorting tubes (Corning Life Sciences, Oneonta, NY, USA; Cat. no.: 38055).
- Cell imaging dishes (Effendorf, Hamburg, Germany; Cat. no.: 30780009).
- E-cadherin antibody (Invitrogen, Carlsbad, CA, USA; PA5-32178).
- N-cadherin antibody (Invitrogen, Carlsbad, CA, USA; PA5-85916).
- Bio-coated coverslip (BD Bioscience, San Jose, CA, USA; Cat. no.: 354085).
- streptavidin fluorescein-conjugated anti-rabbit secondary antibody (Santa Cruz Biotechnology, CA, USA; Cat. no.: sc-2359).
- Slide glass (Corning Life Sciences, Oneonta, NY, USA; Cat. no.: 2947-75X25).
- Mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA; Cat. no.: H-1500).
- 20 μL Pipette (Effendorf, Hamburg, Germany; Cat. no.: 3121000031).
- 200 μL Pipette (Effendorf, Hamburg, Germany; Cat. no.: 3121000082).
- 1000 μL Pipette (Effendorf, Hamburg, Germany; Cat. no.: 3121000120).
2.2. Equipment
- Hypoxia chamber (COY Laboratory, Grass Lake Charter Township, MI, USA; Cat. no.: 05150112019).
- 37 °C incubator (Benchmark Scientific, Edison, NJ, USA; Cat. no.: H2200-h).
- Inverted microscope (Nikon, Tokyo, Japan; Cat. no.: Ts2).
- Optical microscope (Leica, Wetzlar, Germany; Cat. no.: DM1000 LED).
- Centrifuge (Labogene, Seoul, Korea; Cat. no.: 1580MGR).
- CO2 Incubator (Thermo Fisher Scientific, Waltham, MA, USA; Cat. no.: 13-998-086PM).
- Flow cytometry and fluorescence-activated cell sorting machine (BD bioscience, Franklin Lakes, NJ, USA; Cat. no.: 342975).
- Confocal microscope (Nikon, Tokyo, Japan; Cat. no.: A1+).
3. Procedure
3.1. Cell Culture and Subculture
 The pancreatic cancer cells (AsPC-1) were cultured under two condition: a humidified atmosphere at 37 °C containing 19% oxygen, 5% carbon dioxide, and 76% nitrogen (normoxia) and hypoxia maintained a humidified atmosphere at 37 °C containing 1% oxygen, 5% carbon dioxide, and 94% nitrogen. The hypoxia chamber is designed to possible to handle materials inside.
The pancreatic cancer cells (AsPC-1) were cultured under two condition: a humidified atmosphere at 37 °C containing 19% oxygen, 5% carbon dioxide, and 76% nitrogen (normoxia) and hypoxia maintained a humidified atmosphere at 37 °C containing 1% oxygen, 5% carbon dioxide, and 94% nitrogen. The hypoxia chamber is designed to possible to handle materials inside.- AsPC-1 cells were grown in RPMI1640 media supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin.
- Seven days later, wash the cell with 1× phosphate-buffered saline (PBS) without Ca2+/Mg2+. Then, shake the plate gently.
- Pipette 1 mL trypsin-EDTA onto the washed cell monolayer. Gently rock the dishes so that the trypsin completely cover their surfaces.
- Incubate the culture dish at 37 °C for 5 min.
- Resuspend the cells in fresh serum-containing medium to inactivate the trypsin.
- Transfer the cell suspension to a 15 mL tube and centrifuge at 112× g for 3 min, and then remove the supernatant.
- Resuspend each cell pellet in 3 mL of pre-warmed culture medium.
- Dilute cell suspension to 1 × 105 per 100 mm culture dish containing pre-warmed culture medium.
- Incubate the culture dishes in a humidified atmosphere at 37 °C containing 5% carbon dioxide before each experiment.
3.2. Flow Cytometry and Fluorescence-Activated Cell Sorting Analysis for Measuring Mitochondrial ROS
 Pancreatic cancer cells were gated according to physical parameters and aggregates were removed from the analysis. All cells were included in the analysis without consideration of cell viability. The content of mitochondrial ROS was then measured in each condition (unstained, normoxia, hypoxia, and H2O2) by mitochondrial superoxide indicator.
Pancreatic cancer cells were gated according to physical parameters and aggregates were removed from the analysis. All cells were included in the analysis without consideration of cell viability. The content of mitochondrial ROS was then measured in each condition (unstained, normoxia, hypoxia, and H2O2) by mitochondrial superoxide indicator.- Culture 1 × 105 and 3 × 105 AsPC-1 cells in a 6-well plate (2 mL culture medium per well) and incubate under the normoxia or hypoxia for 48 h, respectively. And then remove the culture medium using suction.
- Wash the cell with 1 mL PBS per well.
- Add 5 μM fluorescent mitochondrial superoxide indicator in a serum-free growth medium at 37 °C in the dark for 10 min.
- Wash the cell with 1 mL PBS per well.
- Add 500 μL trypsin for 3 min at 37 °C in the dark.
- Resuspend the cells in 2 mL fresh serum-containing medium, transfer the cell suspension to a 15 mL tube, and centrifuge at 112× g for 3 min.
- Wash the collected cells twice with 10 mL PBS and then centrifuge at 112× g for 3 min.
- Remove supernatant and add 300 μL PBS.
- Transfer the cells to fluorescence-activated cell sorting tubes (5 mL round-bottom polystyrene tubes).
- Analyze the mitochondria ROS signal using the flow cytometry and fluorescence-activated cell sorting machine at the excitation wavelength of 582 nm (FL-2).
3.3. Confocal Imaging for Measuring Mitochondria ROS
- Culture 1 × 105 and 3 × 105 AsPC-1 cells in a cell imaging dish (2 mL culture medium per well) and incubate under the normoxia or hypoxia for 48 h, respectively.
- Wash the cells using 1 mL PBS per well.
- Remove the PBS from the cell imaging dish.
- Add 5 μM fluorescent mitochondrial superoxide indicator in serum-free growth medium at 37 °C in the dark for 10 min.
- Add 200 nM fluorescent mitochondria tracker and nuclear marker (bisBenzimide H 33342 trihydrochloride) for 15 min at 37 °C in the dark.
- Wash the cell with 1 mL 1× Hank’s Balanced Salt Solution (HBSS) per well and then shake the plate gently.
- Capture cell images consecutively for ROS fluorescent signal (emission range: 570–620 nm), mitochondria tracker (emission range: 500–550 nm), and bisBenzimide H 33342 trihydrochloride (Hoechst 33342; emission range: 425–475 nm) using the laser scanning confocal microscope.
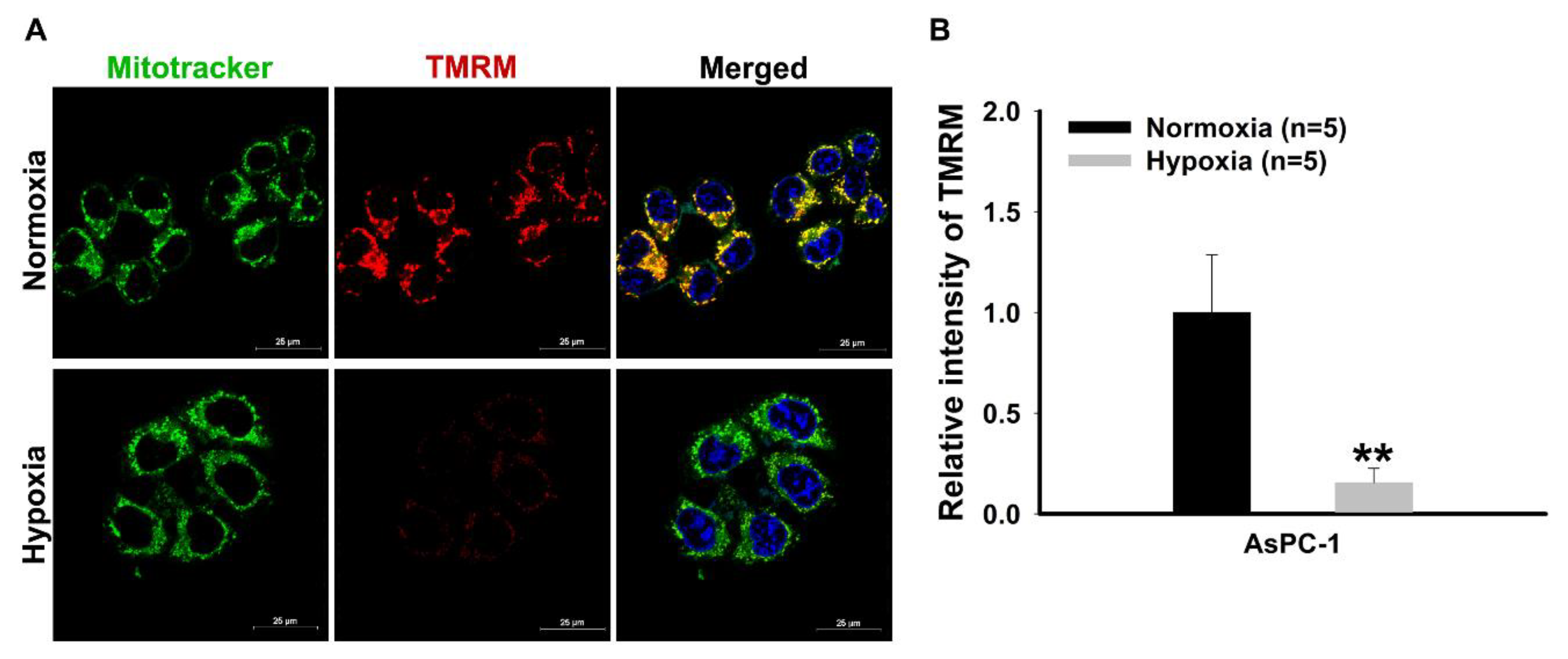
3.4. Confocal Imaging for Measuring Mitochondrial Membrane Potential
- Culture 1 × 105 and 3 × 105 AsPC-1 cells on a cell imaging dish (2 mL culture medium per dish) and incubate under the normoxia or hypoxia for 48 h, respectively.
- Wash the cell using 1 mL PBS per well and then shake the plate gently.
- Remove the PBS from the cell imaging dish.
- Add 200 nM tetramethylrhodamine, methyl ester (TMRM) in serum-free growth medium at 37 °C in the dark for 30 min.
- Wash the cell using 1 mL PBS per well and then shake the plate gently.
- Add 200 nM fluorescent mitochondria tracker and Hoechst 33342 for 15 min at 37 ℃ in the dark.
- Wash the cell with 1 mL 1× HBSS per dish and then shake the plate gently.
- Capture cell fluorescence consecutively for TMRM (emission range: 570–620 nm), mitochondria tracker (emission range: 500–550 nm), and Hoechst 33342 (emission range: 425–475 nm) using the laser scanning confocal microscope.
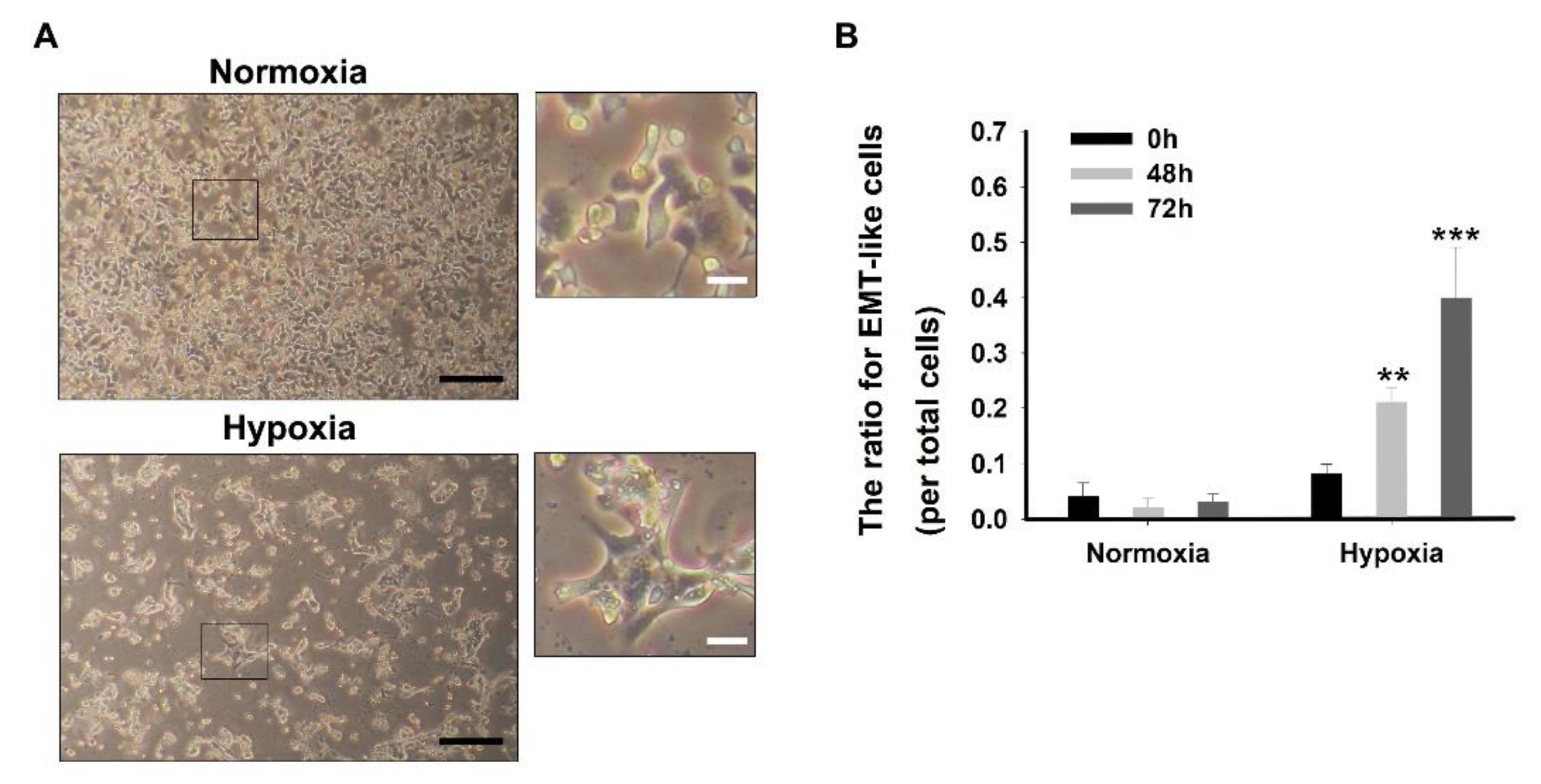
3.5. Cell Morphology and EMT-Like Cell Counting
- Culture 1 × 106 and 3 × 106 AsPC-1 cells in a 6-well plate and incubate under the normoxia or hypoxia for 72 h, respectively.
- After 72 h, add 2 mL 4% paraformaldehyde solution for cell fixation.
- After 20 min, take out the culture dishes from the normoxia and hypoxia incubator.
- Wash the cells three times with 3 mL PBS and then remove the PBS using suction.
- Add 3 mL fresh PBS to avoid drying.
- Capture cell morphology using an inverted microscope.
- Count the number of EMT-like cells using the optical microscope.
3.6. Confocal Analysis for Measuring E-Cadherin and N-Cadherin
 It is recommended to conduct experiments by blocking the light at the stage containing the fluorescent material.
It is recommended to conduct experiments by blocking the light at the stage containing the fluorescent material.- Culture 1 × 105 and 3 × 105 AsPC-1 cells on a bio-coated coverslip in a 6-well plate (2 mL culture medium per dish) and incubate under the normoxia or hypoxia for 48 h, respectively.
- After 48 h, add 2 mL 4% paraformaldehyde solution for cell fixation.
- After 20 min, take out the culture dishes from the normoxia and hypoxia incubator.
- Wash the cells three times with 3 mL PBS and then remove the PBS using suction.
- Incubate for 15 h at 4 °C with the E-Cadherin or N-Cadherin primary antibody (1:300 dilution using PBS).
- Wash the cells three times with 3 mL PBS and then remove the PBS using suction.
- Incubate for 15 h at 4 °C with the streptavidin fluorescein-conjugated anti-rabbit secondary antibody (1:1000 dilution using PBS).
- Wash the cells three times with 3 mL PBS and then remove the PBS using suction.
- Mount the coverslip on the slide glass with mounting medium.
- Capture cell fluorescence consecutively for E-Cadherin and N-Cadherin (emission range: 500–550 nm), and DAPI (emission range: 425–475 nm) using the laser scanning confocal microscope.
3.7. Statistical Analysis
4. Expected Results
Author Contributions
Funding
Conflicts of Interest
References
- Jóźwiak, P.; Forma, E.; Bryś, M.; Krześlak, A. O-GlcNAcylation and metabolic reprograming in cancer. Front. Endocrinol. 2014, 5, 145. [Google Scholar]
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 2017, 5, 225–253. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Brune, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, M.L.; Parliament, M.; Franko, A.; Allalunis-Turner, J. Variation in mitochondrial function in hypoxia-sensitive and hypoxia-tolerant human glioma cells. Br. J. Cancer 2002, 86, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y. Cancer-specific metabolism: Promising approaches for colorectal cancer treatment. World J. Gastrointest. Oncol. 2019, 11, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.; Park, S.; Phillips, P.; Santucci, N.; Goldstein, D.; Kumar, R.; Ramm, G.; Buchler, M.; Friess, H.; McCarroll, J.J.P. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Falasca, M.; Kim, M.; Casari, I. Pancreatic cancer: Current research and future directions. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2016, 1865, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial–mesenchymal transition. Sci. Signal 2014, 7, re8. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bueno, G.; Peinado, H.; Molina, P.; Olmeda, D.; Cubillo, E.; Santos, V.; Palacios, J.; Portillo, F.; Cano, A. The morphological and molecular features of the epithelial-to-mesenchymal transition. Nat. Protoc. 2009, 4, 1591–1613. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kang, Y. Epithelial–mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.J.; Wirth, T.; Beug, H. Epithelial–mesenchymal transition in pancreatic carcinoma. Cancers (Basel) 2010, 2, 2058–2083. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.J.; Jeong, K.-Y. Monitoring Epithelial–Mesenchymal Transition of Pancreatic Cancer Cells via Investigation of Mitochondrial Dysfunction. Methods Protoc. 2020, 3, 32. https://doi.org/10.3390/mps3020032
Sim JJ, Jeong K-Y. Monitoring Epithelial–Mesenchymal Transition of Pancreatic Cancer Cells via Investigation of Mitochondrial Dysfunction. Methods and Protocols. 2020; 3(2):32. https://doi.org/10.3390/mps3020032
Chicago/Turabian StyleSim, Jae Jun, and Keun-Yeong Jeong. 2020. "Monitoring Epithelial–Mesenchymal Transition of Pancreatic Cancer Cells via Investigation of Mitochondrial Dysfunction" Methods and Protocols 3, no. 2: 32. https://doi.org/10.3390/mps3020032
APA StyleSim, J. J., & Jeong, K.-Y. (2020). Monitoring Epithelial–Mesenchymal Transition of Pancreatic Cancer Cells via Investigation of Mitochondrial Dysfunction. Methods and Protocols, 3(2), 32. https://doi.org/10.3390/mps3020032




