Analytical Scheme for Simultaneous Determination of Phthalates and Bisphenol A in Honey Samples Based on Dispersive Liquid–Liquid Microextraction Followed by GC-IT/MS. Effect of the Thermal Stress on PAE/BP-A Levels
Abstract
1. Introduction
2. Experimental Design
2.1. Chemicals
- Dimethyl phthalate (Sigma-Aldrich, Milan, Italy; Cat. no.: 41320; purity ≥99.5%)
- Diethyl phthalate (Sigma-Aldrich, Milan, Italy; Cat. no.: 53008; purity ≥99.5%)
- Diisobutyl phthalate (Sigma-Aldrich, Milan, Italy; Cat. no.: 43540; purity ≥99.9%)
- Dibutyl phthalate (Sigma-Aldrich, Milan, Italy; Cat. no.: 36736; purity ≥98%)
- Bis(2-ethylhexyl) phthalate (Sigma-Aldrich, Milan, Italy; Cat. no.: 36735; purity ≥99.9%)
- Di-n-octyl-phthalate (Sigma-Aldrich, Milan, Italy; Cat. no.: 80153; purity ≥98.0%)
- Bisphenol-A (Sigma-Aldrich, Milan, Italy; Cat. no.: 42088; grade certified reference material)
- Phenanthrene (C14H10; Lab Service Analytica, Anzola Emilia, Bologna, Italy; Cat. no.: CILDLM3711)
- n-Heptane (Carlo Erba, Milan, Italy; Cat. no.: 446841)
- iso-Octane (Carlo Erba, Milan, Italy; Cat. no.: 456734)
- Toluene (Carlo Erba, Milan, Italy; Cat. no.: P0710540)
- Benzene (Carlo Erba, Milan, Italy; Cat. no.: 426113)
- Acetone (Carlo Erba, Milan, Italy; Cat. no.: 508200)
- Sodium chloride (Carlo Erba, Milan, Italy; Cat. no.: 368257000)
2.2. Standard Solutions
- Preparation of stock solution for each analyte, 1000 µg g−1:
- ○
- weigh 10 mg of each PAE/BPA;
- ○
- make up to volume with 10 mL of acetone.
- Preparation of diluted PAE/BPA mix solution, 10 µg g−1:
- ○
- appropriate dilution of the mother solutions with acetone to set up a PAE/BPA mix solution.
- Preparation of the solutions for the calibration curves:
- ○
- appropriate dilution of the PAE/BPA mix solution, 10 µg g−1, with acetone to obtain seven solutions of known concentrations (0.03, 0.05, 0.1, 0.25, 0.5, 1.0, and 5.0 µg g−1).
- Preparation of the phenanthrene (Internal Standard, I.S.) stock solution:
- ○
- weigh 1 mg of phenanthrene;
- ○
- make up to volume, 10 mL, with acetone (100 µg g−1);
- ○
- appropriate dilution for obtaining 0.5 and 0.05 µg g−1 I.S. solutions.
- All solutions were stored in darkness vials at 4 °C.
2.3. Equipment
- Gas chromatograph (GC) TraceGC (ThermoFischer, Milan, Italy; Cat. no.: MS210649)
- Ion Trap Mass Spectrometry (IT/MS) PolarisQ (ThermoFischer, Milan, Italy; Cat. no.: MS210649)
- Software Xcalibur, version 1.4.1 (ThermoFischer, Milan, Italy; Cat. no.: 1.4.1 SR1)
- Fused-silica capillary column, 95% dimethylpolysiloxane - 5% phenyl, 30 m × 0.25 mm × 0.25 μm (Teknokroma, Rome, Italy; Cat. no.: TRB-5MS)
- Centrifuge Neya 8 (Giorgio Bormac S.r.L., Carpi, Italy; Cat. no.: ZBDN-04729)
- Ultrasounds Starsonic 18-35 (Liarre s.r.l., Casalfiumanese, Italy)
- Vortex ZX3 (VELP Scientific, Usmate, Milan, Italy; Cat. no.: F202A0176)
3. Procedure
3.1. Protocol for Phthalates and Bisphenol A (PAEs/BPA) Analysis in Honey Samples
- Weigh 2.5 g of honey.
- Add distilled water up to 10 g.
- Solubilize the honey in the solution.
- Check pH and adjust at pH 4.
CRITICAL STEP: the pH choice is decisive for the successful procedure. PAEs are better extracted at pH alkaline but they are only recovered at pH 4, BPA is extracted at acid pH whereas at alkaline pH its recovery is very low (between 20–30%): pH 4 allows for the quantitative recovery of all compounds.
- Add 7.5 µL of phenanthrene as I.S.
- Vortex 15 s.
- Add the extraction solvent, 75 µL of benzene.
- Vortex 5 min: formation of the macroemulsion.
- Ultrasound 6 min.
CRITICAL STEP: This step is fundamental for the formation of the microemulsion. The ultrasound give the power for the microemulsion.
PAUSE STEP: the microemulsion formation is essential for the procedure. If it does not form, the analytical procedure can be stopped because the extraction has not occurred.
- Add NaCl 10 g L−1 to break the microemulsion. OPTIONAL STEP: the addition of NaCl helps the microemulsion break.
- Centrifugation at 4000 rpm per 30 min.
- Withdraw 1 µL of the supernatant.
- Inject into gas chromatography equipped with ion trap mass spectrometry (GC-IT/MS).
3.2. Thermal Stress Procedure
- 24 h at 40 °C, withdrawing 2.5 g of honey and processing like in Section 3.1.
- 48 h at 40 °C, withdrawing 2.5 g of honey and processing like in Section 3.1.
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamini, Y.; Rezazadeh, M.; Seidi, S. Liquid-phase microextraction. The different principles and configurations. Trends Anal. Chem. 2019, 112, 264–272. [Google Scholar] [CrossRef]
- Fontanals, N.; Marcé, R.M.; Borrull, F. Materials for solid-phase extraction of organic compounds. Separations 2019, 6, 56. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2020, 152, 104319. [Google Scholar] [CrossRef]
- Ridgway, K.; Lalljie, S.P.D.; Smith, R.M. Sample preparation techniques for the determination of trace residues and contaminants in foods. J. Chromatogr. A 2007, 1153, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Zang, X.-H.; Wu, Q.-H.; Zhang, M.-Y.; Xi, G.-H.; Wang, Z. Developments of dispersive liquid-liquid microextraction technique. Chin. J. Anal. Chem. 2009, 37, 161–168. [Google Scholar] [CrossRef]
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and opportunities of experimental design for the dispersive liquid–liquid microextraction method—A review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef]
- Sajid, M.; Alhooshani, K. Dispersive liquid-liquid microextraction based binary extraction techniques prior to chromatographic analysis: A review. Trends Anal. Chem. 2018, 108, 167–182. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Huang, S.-D. Dispersive liquid-liquid microextraction with little solvent consumption combined with gas chromatography-mass spectrometry for the pretreatment of organochlorine pesticides in aqueous samples. J. Chromatogr. A 2009, 1216, 5171–5175. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, T.; Ramil, M.; Rodríguez, I.; Cela, R. Dispersive liquid-liquid microextraction with non-halogenated extractants for trihalomethanes determination in tap and swimming pool water. Talanta 2012, 99, 846–852. [Google Scholar] [CrossRef]
- Yana, H.; Wanga, H. Recent development and applications of dispersive liquid-liquid microextraction. J. Chromatogr. A 2013, 1295, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Aghamohammadhassan, M.; Chamsaz, M.; Akhlaghi, H.; Pedramrad, T. Dispersive solid phase microextraction. Trends Anal. Chem. 2019, 118, 793–809. [Google Scholar] [CrossRef]
- Ahmadi, F.; Assadi, Y.; Hosseini, S.M.R.M.; Rezaee, M. Determination of organophosphorus pesticides in water samples by single drop microextraction and gas chromatography-flame photometric detector. J. Chromatogr. A 2006, 1101, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Herrera, A.V.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.A. Dispersive liquid-liquid microextraction for determination of organic analytes. Trends Anal. Chem. 2010, 29, 728–751. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, B.; Zheng, K.; Zhang, W.; Meng, P. Ultrasound-assisted low-density solvent dispersive liquid-liquid microextraction for the determination of eight drugs in biological samples by gas chromatography-triple quadrupole mass spectrometry. Se Pu 2015, 33, 304–308. [Google Scholar] [CrossRef]
- Sereshti, H.; Khorram, P.; Nouri, N. Recent trends in replacement of disperser solvent in dispersive liquid-liquid microextraction methods. Sep. Purif. Rev. 2019, 48, 159–178. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Utilization of deep eutectic solvents in dispersive liquid-liquid micro-extraction. Trends Anal. Chem. 2019, 120, 115651. [Google Scholar] [CrossRef]
- Liang, P.; Xu, J.; Li, Q. Application of dispersive liquid-liquid microextraction and high-performance liquid chromatography for the determination of three phthalate esters in water samples. Anal. Chim. Acta 2008, 609, 53–58. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, E.; Zhu, W.; Gao, H.; Zhou, Z. Determination of four heterocyclic insecticides by ionic liquid dispersive liquid–liquid microextraction in water samples. J. Chromatogr. 2009, 1216, 885–891. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A. Application of DLLME to isolation and concentration of non-steroidal anti-inflammatory drugs in environmental water samples. Chromatographia 2010, 72, 671–678. [Google Scholar] [CrossRef]
- Notardonato, I.; Salimei, E.; Russo, M.V.; Avino, P. Simultaneous determination of organophosphorus pesticides and phthalates in baby food samples by ultrasound-vortex-assisted liquid-liquid microextraction and GC–IT/MS. Anal. Bioanal. Chem. 2018, 410, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. Available online: https:// www.cdc.gov/exposurereport/ (accessed on 14 March 2020).
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.R.; Flaws, J.A. Bisphenol A and phthalates: How environmental chemicals are reshaping toxicology. Toxicol. Sci. 2018, 166, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.V.; Avino, P.; Perugini, L.; Notardonato, I. Extraction and GC-MS analysis of phthalate esters in food matrices: A review. RSC Adv. 2015, 5, 37023–37043. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, D.; Wang, T.; Wang, X.-M.; Xinzhen Du, X. Dispersive liquid-liquid microextraction followed by high performance liquid chromatography for determination of phthalic esters in environmental water samples. Anal. Methods-UK 2014, 6, 1121–1127. [Google Scholar] [CrossRef]
- Russo, M.V.; Notardonato, I.; Cinelli, G.; Avino, P. Evaluation of an analytical method for determining phthalate esters in wine samples by solid-phase extraction and gas chromatography coupled with ion-trap mass spectrometer detector. Anal. Bioanal. Chem. 2012, 402, 1373–1381. [Google Scholar] [CrossRef]
- Russo, M.V.; Notardonato, I.; Avino, P.; Cinelli, G. Determination of phthalate esters at trace levels in light alcoholic drinks and soft drinks by XAD-2 adsorbent and gas chromatography coupled with ion trap-mass spectrometry detection. Anal. Methods-UK 2014, 6, 7030–7037. [Google Scholar] [CrossRef]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Study of XAD-2 adsorbent for the enrichment of trace levels of phthalate esters in hydroalcoholic food beverages and analysis by gas chromatography coupled with flame ionization and ion-trap mass spectrometry detectors. Food Chem. 2014, 146, 181–187. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Notardonato, I. PAH residues in honey by ultrasound-vortex-assisted liquid-liquid micro-extraction followed by GC-FID/IT-MS. Food Anal. Methods 2017, 10, 2132–2142. [Google Scholar] [CrossRef]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Rapid analysis of six phthalate esters in wine by ultrasound-vortex-assisted dispersive liquid-liquid micro-extraction coupled with gas chromatography-flame ionization detector or gas chromatography-ion trap mass spectrometry. Anal. Chim. Acta 2013, 769, 72–78. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Notardonato, I. Fast analysis of phthalates in freeze-dried baby foods by ultrasound-vortex-assisted liquid-liquid microextraction coupled with gas chromatography-ion trap/mass spectrometry. J. Chromatogr. A 2016, 1474, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Notardonato, I.; Protano, C.; Vitali, M.; Bhattacharya, B.; Avino, P. A method validation for simultaneous determination of phthalates and bisphenol A released from plastic water containers. Appl. Sci. 2019, 9, 2945. [Google Scholar] [CrossRef]
- Notardonato, I.; Protano, C.; Vitali, M.; Avino, P. Phthalates and bisphenol-A determination and release from different beverage plastic containers by dispersive liquid-liquid microextraction and GC-IT/MS analysis. Food Anal. Methods 2019, 12, 2562–2571. [Google Scholar] [CrossRef]
- Notardonato, I.; Avino, P.; Cinelli, G.; Russo, M.V. Trace determination of acaricides in honey samples using XAD-2 adsorbent and gas chromatography coupled with an ion trap mass spectrometer detector. RSC Adv. 2014, 4, 42424–42431. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1). International Conference on Harmonisation of Technical Requirement for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 10 February 2020).
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- European Commission. Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) No 10/2011 as regards the use of that substance in plastic food contact materials. Off. J. Eur. Union 2018, 41, 6–12. [Google Scholar]
- Herrero-Hernández, E.; Carabias-Martínez, R.; Rodríguez-Gonzalo, E. Use of a bisphenol-A imprinted polymer as a selective sorbent for the determination of phenols and phenoxyacids in honey by liquid chromatography with diode array and tandem mass spectrometric detection. Anal. Chim. Acta 2009, 21, 195–201. [Google Scholar] [CrossRef]
- Rodríguez-Gonzalo, E.; García-Gómez, D.; Carabias-Martínez, R. A confirmatory method for the determination of phenolic endocrine disruptors in honey using restricted-access material-liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 1239–1247. [Google Scholar] [CrossRef]
- Rodríguez-Gonzalo, E.; Domínguez-Alvarez, J.; García-Gómez, D.; García-Jiménez, M.G.; Carabias-Martínez, R. Determination of endocrine disruptors in honey by CZE-MS using restricted access materials for matrix cleanup. Electrophoresis 2010, 31, 2279–2288. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Y.; Wu, H.; Diao, Q.; Tian, F.; Li, Y. Simultaneous determination of trace migration of phthalate esters in honey and royal jelly by GC-MS. J. Sep. Sci. 2014, 37, 650–657. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Abbaspour, M.; Mogaddam, M.R.A.; Ghorbanpour, H. Determination of some synthetic phenolic antioxidants and bisphenol a in honey using dispersive liquid–liquid microextraction followed by gas chromatography-flame ionization detection. Food Anal. Methods 2015, 8, 2035–2043. [Google Scholar] [CrossRef]
- Lo Turco, V.; Di Bella, G.; Potortì, A.G.; Tropea, A.; Casale, E.K.; Fede, M.R.; Dugo, G. Determination of plasticisers and BPA in Sicilian and Calabrian nectar honeys by selected ion monitoring GC/MS. Food Addit. Contam. A 2016, 33, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Lambropoulou, D.; Laimou-Geraniou, M.; Kosjek, T.; Blaznik, U.; Heath, D.; Heath, E. Determination of bisphenols and related compounds in honey and their migration from selected food contact materials. J. Agric. Food Chem. 2016, 64, 8866–8875. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.P.; Yahaya, N.; Omar, W.A.W. Analysis of dibutyl phthalate and oleamide in stingless bee honey harvested from plastic cups. Sains Malays. 2017, 46, 449–455. [Google Scholar] [CrossRef]
- Sahebzadeh, N.; Ebadi, R.; Khajehali, J. Effect of selected repellent chemicals on honey bees in canola and alfalfa fields. J. Apic. Res. 2009, 48, 29–33. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.M.; García-Valcárcel, A.I.; Tadeo, J.L.; Fernández-Alba, A.R.; Hernando, M.D. Screening of environmental contaminants in honey bee wax comb using gas chromatography-high-resolution time-of-flight mass spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 4609–4620. [Google Scholar] [CrossRef]
- Muturi, E.J.; Doll, K.; Berhow, M.; Flor-Weilera, L.B.; Rooney, A.P. Honeysuckle essential oil as a potential source of ecofriendly larvicides for mosquito control. Pest Manag. Sci. 2019, 75, 2043–2048. [Google Scholar] [CrossRef]

| Compound (Abbrev.—CAS#) | Formula | MW SIM | Solubility a log Kow b | LD50 (g kg−1 mouse) | ADI (ng kg−1 bw) |
|---|---|---|---|---|---|
| Di-methyl phthalate (DMP—131-11-3) | 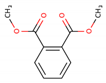 | 194.18 163, 194 | 4000 mg L−1 1.60 | 8–10 | 79.1 |
| Di-ethyl phthalate (DEP—84-66-2) | 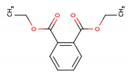 | 222.24 149, 177 | 1080 mg L−1 2.42 | 8–10 | 1.4–28.2 |
| Di-isobutyl phthalate (DIBP—84-69-5) | 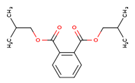 | 278.34 149, 223 | 1 mg L−1 4.11 | 8–10 | 105 |
| Di-n-butyl phthalate (DBP—84-74-2) | 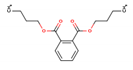 | 278.35 149, 205 | 11.2 mg L−1 4.50 | 8–10 | 191.8 |
| Bisphenol-A (BP-A—80-05-7) |  | 228.29 213, 228 | 300 mg L−1 3.44 | 5 | 69 × 106 |
| Bis-(2-ethylhexyl) phthalate (DEHP—118-81-7) | 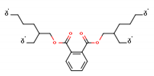 | 390.56 149, 167 | 0.09 mg L−1 8.39 | 14 | 1458 |
| Di-n-octyl phthalate (DNOP—117-84-0) | 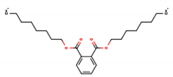 | 390.56 149, 279 | 0.022 mg L−1 8.06 | 13 | 37 × 106 |
| n-Heptane | iso-Octane | Benzene | Toluene | Tol. + Ac 1 | |
|---|---|---|---|---|---|
| DMP | 18.6 ± 8.4 | 17.5 ± 8.1 | 92.2 ± 6.2 | 91.0 ± 5.7 | 112.9 ± 4.8 |
| DEP | 89.3 ± 6.4 | 64.4 ± 10.5 | 97.6 ± 5.7 | 96.7 ± 1.9 | 87.9 ± 7.2 |
| DiBP | 109.6 ± 3.7 | 109.8 ± 2.2 | 106.9 ± 2.6 | 110.8 ± 2.9 | 118.9 ± 6.8 |
| DBP | 116.7 ± 4.6 | 108.6 ± 2.6 | 101.3 ± 3.4 | 109.3 ± 0.9 | 95.6 ± 6.4 |
| BP-A | 17.4 ± 3.0 | 23.5 ± 3.1 | 74.2 ± 2.7 | 50.2 ± 5.3 | 35.6 ± 4.1 |
| DEHP | 117.3 ± 7.9 | 112.6 ± 1.8 | 105.6 ± 9.9 | 108.8 ± 4.6 | 64.0 ± 11.6 |
| DNOP | 123.1 ± 6.3 | 113.4 ± 8.6 | 103.6 ± 8.8 | 105.0 ± 7.4 | 66.0 ± 9.6 |
| 25 μL | 50 μL | 75 μL | 100 μL | 200 μL | |
|---|---|---|---|---|---|
| DMP | 64.8 ± 3.4 | 88.8 ± 5.4 | 101.3 ± 4.5 | 100.8 ± 9.6 | 92.2 ± 6.2 |
| DEP | 74.5 ± 5.3 | 87.8 ± 4.8 | 103.5 ± 4.4 | 108.1 ± 8.2 | 97.6 ± 5.7 |
| DiBP | 82.6 ± 4.9 | 100.1 ± 5.1 | 104.8 ± 7.0 | 106.3 ± 9.0 | 106.9 ± 2.6 |
| DBP | 98.8 ± 2.5 | 109.7 ± 2.3 | 107.0 ± 6.5 | 91.0 ± 4.1 | 101.3 ± 3.4 |
| BP-A | 24.2 ± 5.1 | 40.3 ± 6.2 | 80.6 ± 6.5 | 70.8 ± 9.1 | 74.2 ± 2.7 |
| DEHP | 87.7 ± 6.8 | 104.3 ± 5.7 | 99.5 ± 5.0 | 96.1 ± 11.8 | 105.6 ± 9.9 |
| DNOP | 84.7 ± 5.1 | 98.7 ± 7.2 | 98.5 ± 6.1 | 91.5 ± 6.4 | 103.6 ± 8.8 |
| pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | |
|---|---|---|---|---|---|
| DMP | 98.0 | 92.0 | 81.7 | 84.3 | 74.6 |
| DEP | 102.3 | 97.8 | 88.3 | 82.1 | 76.8 |
| DiBP | 104.9 | 100.6 | 95.4 | 88.3 | 85.4 |
| DBP | 102.2 | 98.7 | 98.7 | 87.4 | 82.7 |
| BP-A | 73.1 | 54.1 | 44.7 | 30.5 | 26.0 |
| DEHP | 97.6 | 93.9 | 91.4 | 92.1 | 91.5 |
| DNOP | 103.1 | 94.3 | 92.2 | 89.7 | 89.4 |
| Regr. eq. a | R2 | LOD | LOQ | Intra-Day | Inter-Day | Recovery b | ||
|---|---|---|---|---|---|---|---|---|
| (m, q) | 50 ng g−1 | 500 ng g−1 | ||||||
| DMP | 0.255, 0.008 | 0.9973 | 10 | 22 | 3.1 | 6.4 | 97.1 (8) | 99.8 (5) |
| DEP | 0.263, 0.015 | 0.9970 | 7 | 17 | 4.7 | 7.7 | 96.2 (7) | 98.4 (4) |
| DiBP | 0.539, 0.079 | 0.9963 | 3 | 7 | 5.9 | 8.2 | 94.2 (9) | 98.6 (6) |
| DBP | 0.625, 0.087 | 0.9972 | 4 | 8 | 2.4 | 4.5 | 93.5 (9) | 97.9 (4) |
| BP-A | 0.321, 0.009 | 0.9996 | 11 | 22 | 7.2 | 9.3 | 71.5 (8) | 76.2 (5) |
| DEHP | 0.448, 0.062 | 0.9951 | 10 | 19 | 3.6 | 7.3 | 94.6 (8) | 99.2 (5) |
| DNOP | 0.720, 0.019 | 0.9984 | 13 | 22 | 4.5 | 6.9 | 97.0 (5) | 100.4 (3) |
| Methodology | Compounds | Recovery | LOD/LOQ | RSD | Ref. |
|---|---|---|---|---|---|
| (%) | (ng g−1) | (%) | |||
| MISPE-LC-DAD-UV a | BPA | N/A | 2000 | 7–12.5 | [39] |
| RAM-LC-MS/MS b | BPA | 100–112 | 9.6/16.6 | 2.5–11 | [40] |
| RAM-SPE-CE-ESI-MS | BPA | 96–103 | 7.5/N/A | <22 | [41] |
| LLE-GC-MS | DBP, DMP, DEP, DEHP, DiBP, DNOP | 80.1–110.9 | 0.3–6.0/10–17.5 | <11.8 | [42] |
| DLLME-GC-FID | BPA | 91–101 | 16–47/14–48 | <7.5 | [43] |
| SPE-GC-MS | BPA, DBP, DMP, DEP, DEHP, DiBP | 81.2–119.8 | 5–303/12–1013 | 1.5–4.1 | [44] |
| SPE-GC-MS | BPA | 103 | 0.128/0.428 | 4.9–10.2 | [45] |
| ST-DLLME-HPLC-PAD c | DBP | N/A | 150/500 | N/A | [46] |
| This study | DMP, DEP, DiBP, DBP, BPA, DEHP, DNOP | 71.5–100.4 | 2–13/7–22 | 3–9 |
| Sample 1 | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| DMP | <LOD | 0.03 | 0.04 | <LOD | <LOD | <LOD |
| DEP | <LOD | 0.02 | 5.05 | <LOD | <LOD | <LOD |
| DiBP | 0.02 | <LOD | 0.26 | 0.05 | <LOD | 0.01 |
| DBP | 0.08 | <LOD | 0.28 | 0.05 | <LOD | 0.05 |
| BP-A | 0.06 | 0.15 | 0.02 | 0.04 | 0.19 | 0.27 |
| DEHP | 0.20 | 0.10 | 0.84 | 0.30 | <LOD | 0.19 |
| DNOP | 0.20 | 0.02 | 0.72 | 0.18 | <LOD | 0.21 |
| Sample 1 | B | C | D | E |
|---|---|---|---|---|
| DMP | <LOD | <LOD | <LOD | <LOD |
| DEP | <LOD | 5.05 | 0.71 | <LOD |
| DiBP | <LOD | 0.26 | <LOD | 0.02 |
| DBP | <LOD | 0.28 | <LOD | 0.03 |
| BP-A | 0.54 | 0.02 | 0.26 | 0.19 |
| DEHP | 0.18 | 0.84 | 0.36 | 0.24 |
| DNOP | 0.04 | 0.72 | <LOD | 0.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notardonato, I.; Passarella, S.; Ianiri, G.; Di Fiore, C.; Russo, M.V.; Avino, P. Analytical Scheme for Simultaneous Determination of Phthalates and Bisphenol A in Honey Samples Based on Dispersive Liquid–Liquid Microextraction Followed by GC-IT/MS. Effect of the Thermal Stress on PAE/BP-A Levels. Methods Protoc. 2020, 3, 23. https://doi.org/10.3390/mps3010023
Notardonato I, Passarella S, Ianiri G, Di Fiore C, Russo MV, Avino P. Analytical Scheme for Simultaneous Determination of Phthalates and Bisphenol A in Honey Samples Based on Dispersive Liquid–Liquid Microextraction Followed by GC-IT/MS. Effect of the Thermal Stress on PAE/BP-A Levels. Methods and Protocols. 2020; 3(1):23. https://doi.org/10.3390/mps3010023
Chicago/Turabian StyleNotardonato, Ivan, Sergio Passarella, Giuseppe Ianiri, Cristina Di Fiore, Mario Vincenzo Russo, and Pasquale Avino. 2020. "Analytical Scheme for Simultaneous Determination of Phthalates and Bisphenol A in Honey Samples Based on Dispersive Liquid–Liquid Microextraction Followed by GC-IT/MS. Effect of the Thermal Stress on PAE/BP-A Levels" Methods and Protocols 3, no. 1: 23. https://doi.org/10.3390/mps3010023
APA StyleNotardonato, I., Passarella, S., Ianiri, G., Di Fiore, C., Russo, M. V., & Avino, P. (2020). Analytical Scheme for Simultaneous Determination of Phthalates and Bisphenol A in Honey Samples Based on Dispersive Liquid–Liquid Microextraction Followed by GC-IT/MS. Effect of the Thermal Stress on PAE/BP-A Levels. Methods and Protocols, 3(1), 23. https://doi.org/10.3390/mps3010023








