Impedance Measures and a Mounting Technique for Drosophila: Larval Movements, Heart Rate, Imaging, and Electrophysiology
Abstract
1. Introduction
2. Methods
2.1. Larvae Expressing Light-Sensitive Channels
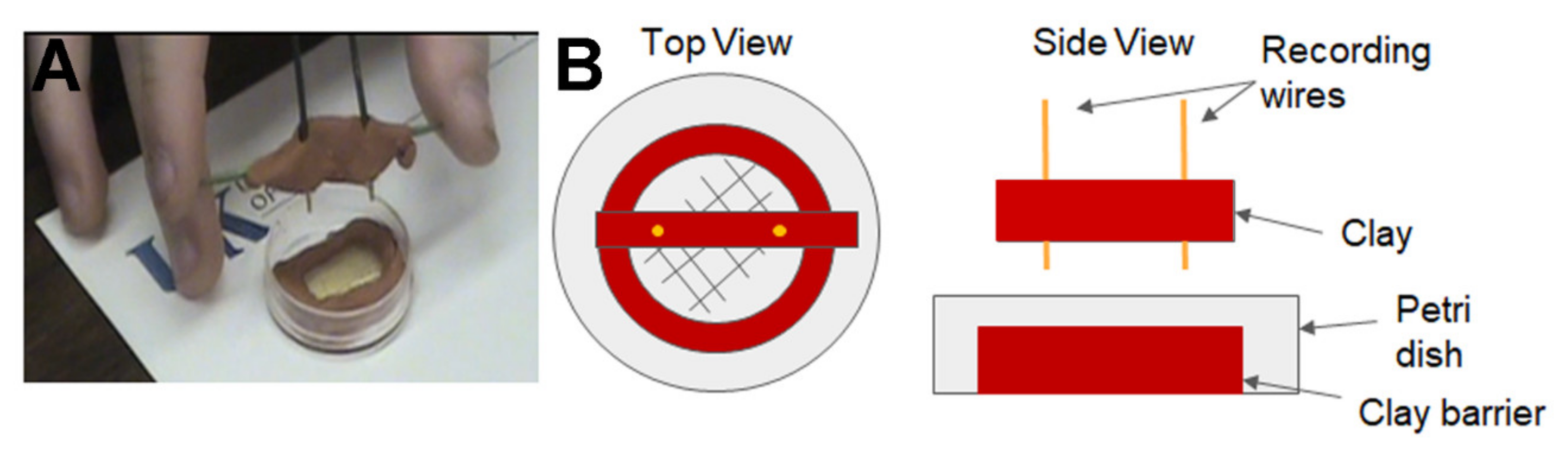
2.2. Impedance Measures of Larval Movement
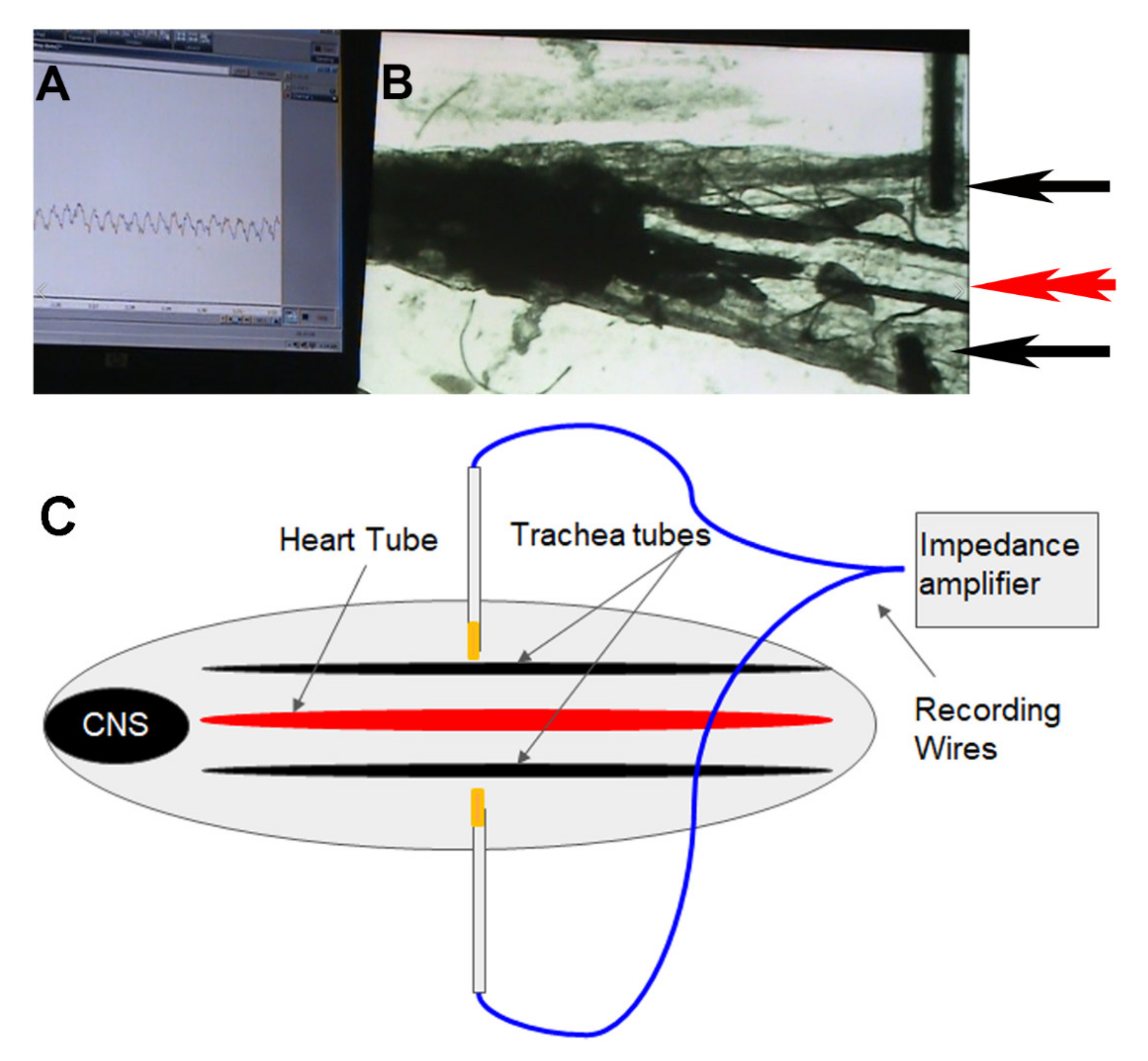
2.3. Impedance Measures of Larval Heart Beats
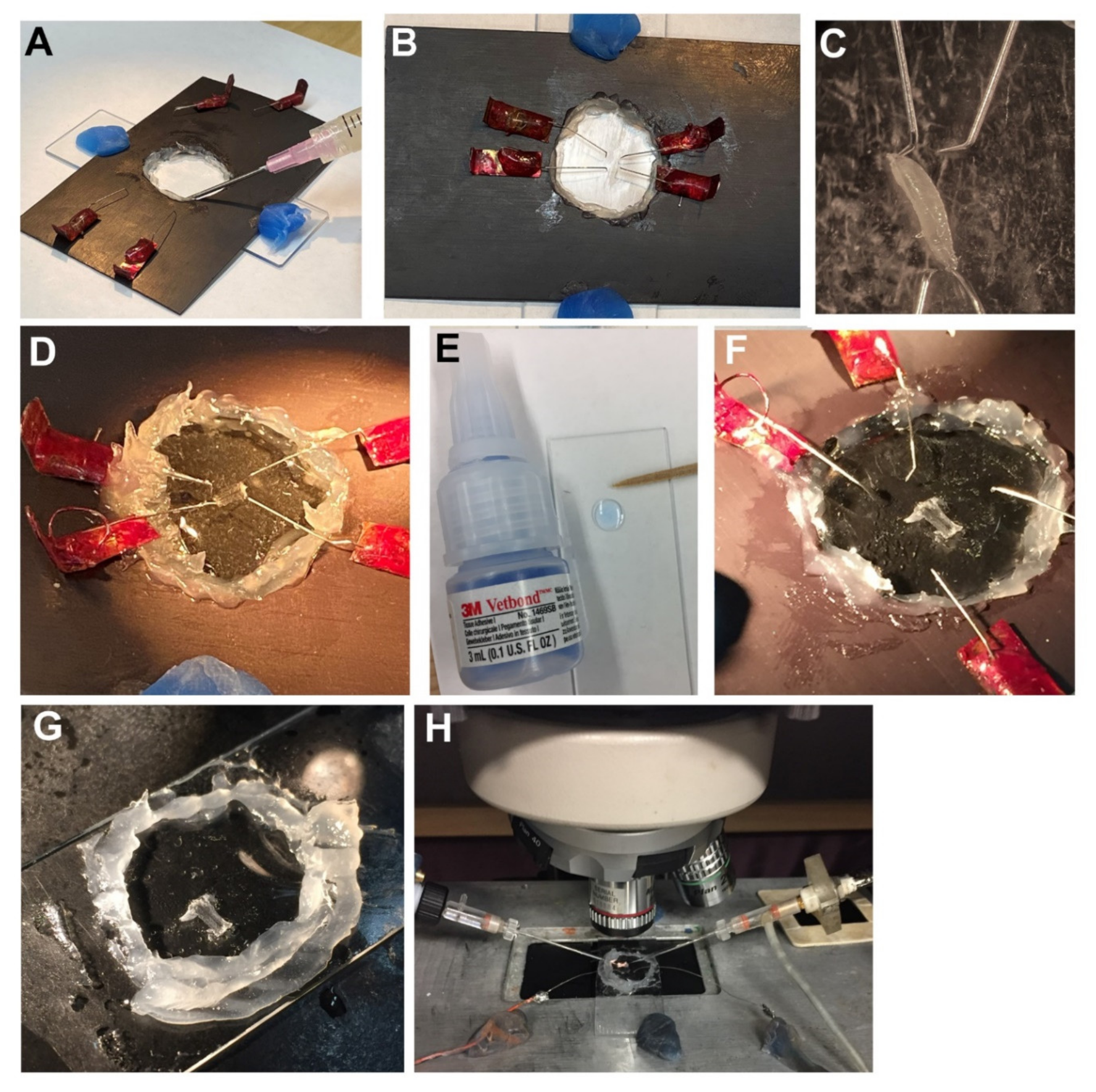
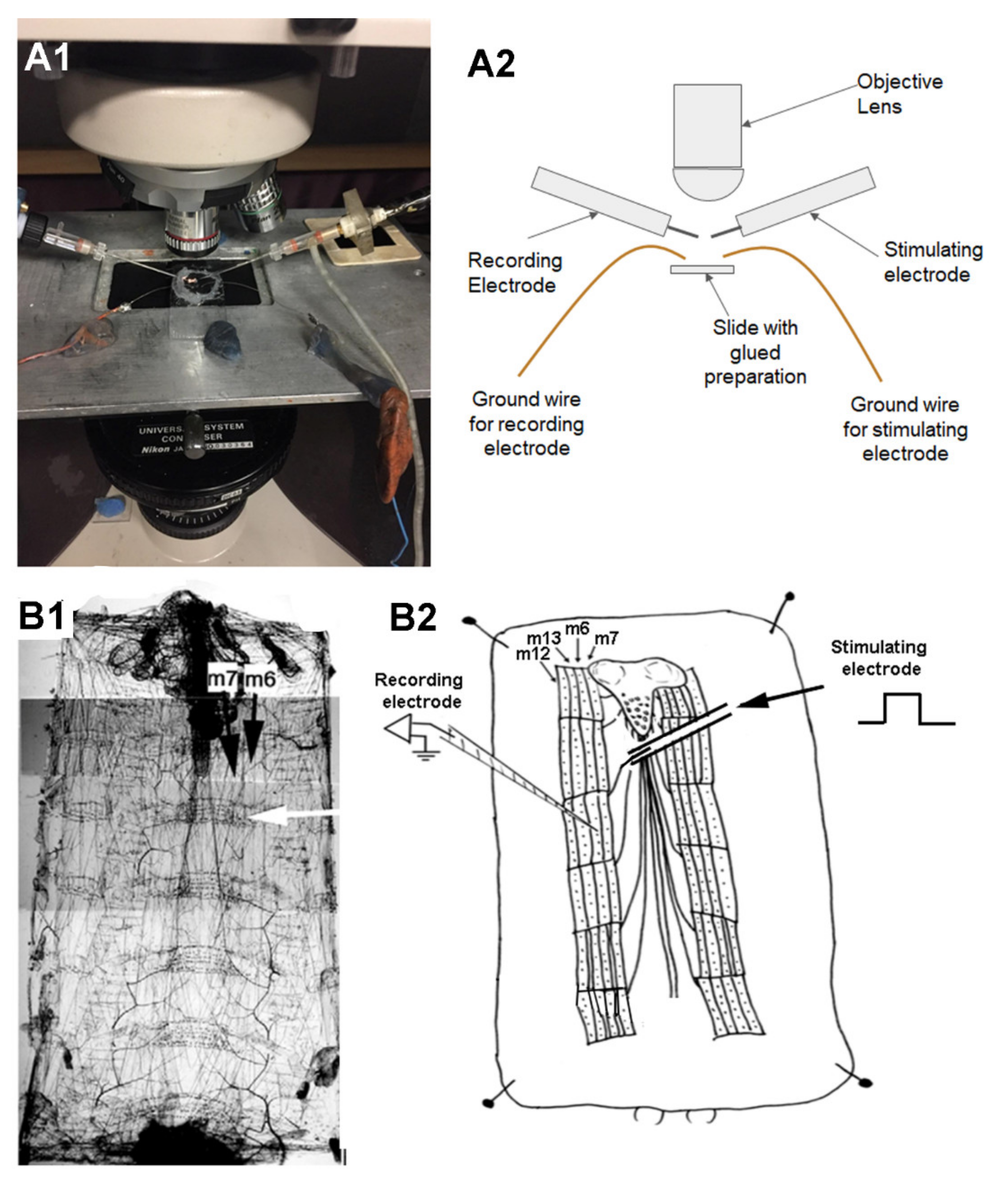
2.4. Recording Synaptic Responses in a Larva Glued to a Glass Slide
3. Results
3.1. Larval Movements with Impedance Detection
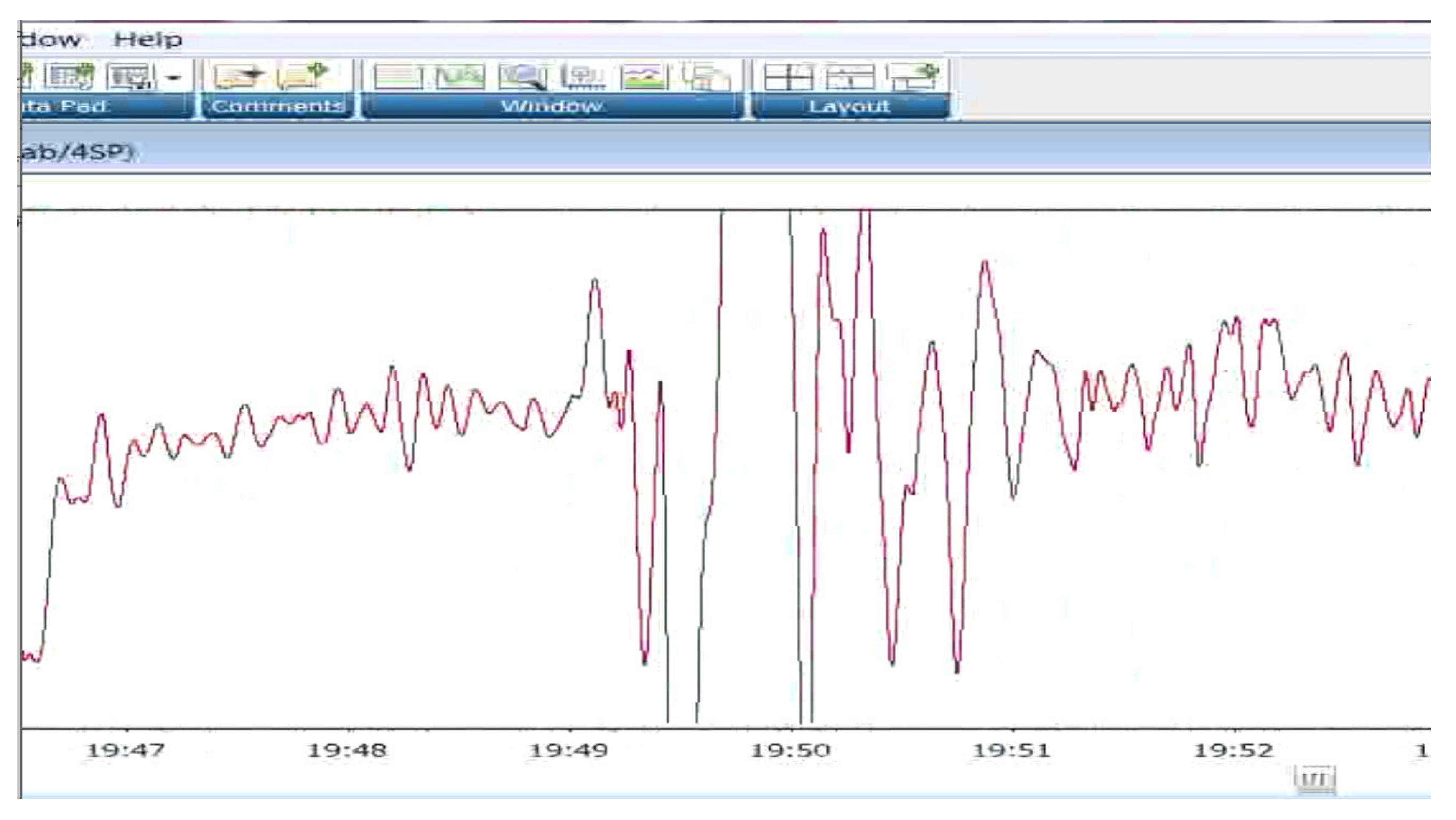
3.2. Impedance Measures of Heart Rate
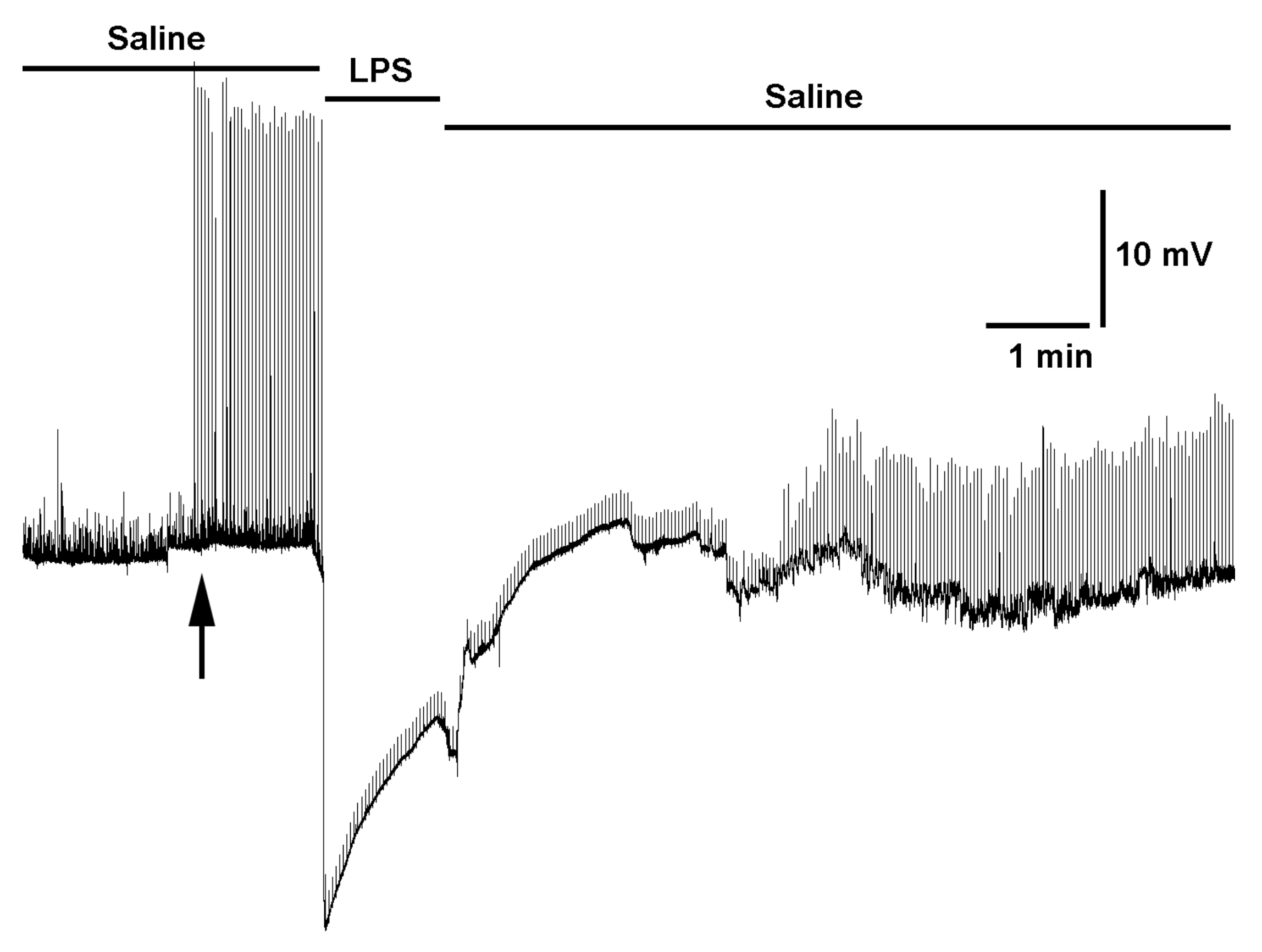
3.3. Synaptic Measures at NMJs of a Glued Larva
4. Discussion
- Need to place wires in moist media for detecting body wall movements.
- Animals crawl out of dish. Maybe use “ant farm” technique with glass slides and moist food.
- Small distance between wires easier to detect signals. How small of a movement can be detected has not been determined.
- No light is required during the measures. Good for optogenetic studies for light activated channels.
- Can be used for body movements as well as heart rate monitoring.
- Can be used in cold or incubator at different temperatures (i.e., no fogging of camera lens).
- Need to use small amounts of glue.
- Can glue mouth closed or position larvae in wrong position and cannot reposition.
- Sometimes glue stick is attached to larvae if touch larva by mistake.
- Glue dries out prior to getting larvae stuck and taking too long to glue preparation down while reducing the saline around preparation.
- Insect pin removal allows freedom to place electrophysiological electrodes in various positions without pins interfering.
- Much easier for focal macro-patching over nerve terminal varicosities.
- Close positioning of objective lens for imaging of motor nerve terminals in live preparations.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef]
- Potter, S.; Sifers, J.; Yocom, E.; Blümich, S.L.E.; Potter, R.; Nadolski, J.; Harrison, D.A.; Cooper, R.L. Effects of inhibiting mTOR with rapamycin on behavior, development, neuromuscular physiology and cardiac function in larval Drosophila. Biol. Open 2019, 8, bio046508. [Google Scholar] [CrossRef]
- Tolwinski, N.S. Introduction: Drosophila-A Model System for Developmental Biology. J. Dev. Biol. 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Meinertzhagen, I.A.; Takemura, S.Y.; Lu, Z.; Huang, S.; Gao, S.; Ting, C.Y.; Lee, C.H. From form to function: The ways to know a neuron. J. Neurogenet. 2009, 23, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Thum, A.S.; Gerber, B. Connectomics and function of a memory network: The mushroom body of larval Drosophila. Curr. Opin. Neurobiol. 2019, 54, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 28–33. [Google Scholar] [CrossRef]
- Morgan, T.H. Sex limited inheritance in Drosophila. Science 1910, 32, 120–122. Available online: http://www.jstor.org/stable/pdf/1635471.pdf (accessed on 13 October 2019). [CrossRef]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef]
- Harnish, J.M.; Deal, S.L.; Chao, H.T.; Wangler, M.F.; Yamamoto, S. In vivo functional study of disease-associated rare human variants using Drosophila. J. Vis. Exp. 2019, 20, e59658. [Google Scholar] [CrossRef]
- Ingham, P.W. From Drosophila segmentation to human cancer therapy. Development 2018, 145, dev168898. [Google Scholar] [CrossRef] [PubMed]
- Tue, N.T.; Dat, T.Q.; Ly, L.L.; Anh, V.D.; Yoshida, H. Insights from Drosophila melanogaster model of Alzheimer’s disease. Front. Biosci. (Landmark Ed.) 2020, 25, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, L. Can the Drosophila model help in paving the way for translational medicine in heart failure? Biochem. Soc. Trans. 2016, 44, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Röder, L. Cardiac pathologies and aging: Lessons from a tiny heart. Med. Sci. 2016, 32, 470–477. [Google Scholar]
- Caldwell, J.C.; Miller, M.M.; Wing, S.; Soll, D.R.; Eberl, D.F. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc. Natl. Acad. Sci. USA 2003, 100, 16053–16058. [Google Scholar] [CrossRef]
- McParland, A.L.; Follansbee, T.L.; Ganter, G.K. Measurement of larval activity in the Drosophila activity monitor. J. Vis. Exp. 2015, 98, e52684. [Google Scholar] [CrossRef]
- University of Illinois at Chicago College of Pharmacy, Drug Information Group. Light-sensitive injectable prescription drugs. Hosp. Pharm. 2014, 49, 136–163. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Shmihel, H.V.; Lylyk, M.P.; Vytvytska, O.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Alpha-ketoglutarate attenuates toxic effects of sodium nitroprusside and hydrogen peroxide in Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2015, 40, 650–659. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef]
- Arrenberg, A.B.; Stainier, D.Y.; Baier, H.; Huisken, J. Optogenetic control of cardiac function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Camporeze, B.; Manica, B.A.; Bonafé, G.A.; Ferreira, J.J.C.; Diniz, A.L.; de Oliveira, C.T.P.; Mathias, L.R., Jr.; de Aguiar, P.H.P.; Ortega, M.M. Optogenetics: The new molecular approach to control functions of neural cells in epilepsy, depression and tumors of the central nervous system. Am. J. Cancer Res. 2018, 8, 1900–1918. [Google Scholar] [PubMed]
- Govorunova, E.G.; Cunha, S.R.; Sineshchekov, O.A.; Spudich, J.L. Anion channelrhodopsins for inhibitory cardiac optogenetics. Sci. Rep. 2016, 6, 33530. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2007, 2, e299. [Google Scholar] [CrossRef] [PubMed]
- Kopton, R.A.; Baillie, J.S.; Rafferty, S.A.; Moss, R.; Zgierski-Johnston, C.M.; Prykhozhij, S.V.; Schneider-Warme, F. Cardiac electrophysiological effects of light-activated chloride channels. Front. Physiol. 2018, 9, 1806. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.C.; Morais, C.; Bugedo, G.; Bruhn, A.; Morales, A.; Borges, J.B.; Costa, E.; Retamal, J. Electrical impedance tomography in acute respiratory distress syndrome. Crit. Care 2018, 22, 263. [Google Scholar] [CrossRef]
- Nagy, J.A.; DiDonato, C.J.; Rutkove, S.B.; Sanchez, B. Permittivity of ex vivo healthy and diseased murine skeletal muscle from 10 kHz to 1 MHz. Sci. Data 2019, 6, 37. [Google Scholar] [CrossRef]
- Ball, R.; Xing, B.; Bonner, P.; Shearer, J.; Cooper, R.L. Long-term maintenance of neuromuscular junction activity in cultured Drosophila larvae. Comp. Biochem. Physiol. A 2003, 134, 247–255. [Google Scholar] [CrossRef]
- Harrison, D.A.; Cooper, R.L. Characterization of development, behavior, and neuromuscular physiology in the phorid fly: Megaselia scalaris. Comp. Biochem. Physiol. A 2003, 136, 427–439. [Google Scholar] [CrossRef]
- Richmond, J. Dissecting and recording from the C. elegans neuromuscular junction. J. Vis. Exp. 2009, 24, e1165. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Uradu, H.; Majeed, Z.R.; Cooper, R.L. Optogenetic drive of Drosophila heart rate at different temperatures and Ca2+ concentrations. Physiol. Rep. 2016, 4, e12695. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.E.; Mauss, A.; Borst, A.; Cooper, R.L. Effects of chloride flux on Drosophila heart rate. Methods Protoc. 2019, 2, 73. Available online: https://www.mdpi.com/2409-9279/2/3/73 (accessed on 1 January 2020). [CrossRef] [PubMed]
- Dawydow, A.; Gueta, R.; Ljaschenko, D.; Ullrich, S.; Hermann, M.; Ehmann, N.; Gao, S.; Fiala, A.; Langenhan, T.; Nagel, G.; et al. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA 2014, 111, 13972–13977. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.; Sifers, J.; Yocom, E.; Blümich, S.L.E.; Potter, R.; Nadolski, J.; Harrison, D.A.; Cooper, R.L. Acute and chronic effects of inhibiting dTOR by rapamycin on development, behavior, and physiology in Drosophila. Biol. Open 2019, 8, bio046508. [Google Scholar] [CrossRef]
- Cooper, A.S.; Rymond, K.E.; Ward, M.A.; Bocook, E.L.; Cooper, R.L. Monitoring heart function in larval Drosophila melanogaster for physiological studies. J. Vis. Exp. 2009, 32, e1596. Available online: http://www.jove.com/index/details.stp?id=1596 (accessed on 1 January 2020).
- Desai-Shah, M.; Papoy, A.R.; Ward, M.; Cooper, R.L. Roles of the Sarcoplasmic/Endoplasmic reticulum Ca2+-ATPase, plasma membrane Ca2+-ATPase and Na+/Ca2+ exchanger in regulation of heart rate in larval Drosophila. Open Physiol. J. 2010, 3, 16–36. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Stacy, A.; Cooper, R.L. Pharmacological identification of serotonin receptor subtypes on the Drosophila larval heart. J. Comp. Physiol. B 2014, 184, 205–219. [Google Scholar] [CrossRef]
- Malloy, C.; Ritter, K.; Robinson, J.; Cooper, R.L. Pharmacological identification of cholinergic receptor subtypes on Drosophila melanogaster larval heart. J. Comp. Physiol. B 2016, 186, 45–57. [Google Scholar] [CrossRef]
- Kurdyak, P.; Atwood, H.L.; Stewart, B.A.; Wu, C.-F. Differential physiology and morphology of motor axons to ventral longitudinal muscles in larval Drosophila. J. Comp. Neurol. 1994, 350, 463–472. [Google Scholar] [CrossRef]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.-F. Improved stability of Drosophila larval neuromuscular preparation in haemolymph-like physiological solutions. J. Comp. Physiol. A 1994, 175, 179–191. [Google Scholar] [CrossRef]
- Muller, K.J.; Nicholls, J.G.; Stent, G.S. Neurobiology of the Leech; Cold Spring Harbor Laboratory: New York, NY, USA, 1981. [Google Scholar]
- Cooper, R.L.; McNabb, M.; Nadolski, J. The effects of a bacterial endotoxin LPS on synaptic transmission at the neuromuscular junction. Heliyon 2019, 5, e01430. Available online: https://www.heliyon.com/article/e01430 (accessed on 1 January 2020). [CrossRef] [PubMed]
- De Castro, C.; Titlow, J.; Majeed, Z.R.; Cooper, R.L. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A 2014, 200, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Istas, O.; Greenhalgh, A.; Cooper, R.L. The Effects of a Bacterial Endotoxin on Behavior and Sensory-CNS-Motor Circuits in Drosophila Melanogaster. 2019. Available online: https://www.mdpi.com/2075-4450/10/4/115 (accessed on 1 January 2020).
- Ballinger-Boone, C.; Anyagaligbo, O.; Bernard, J.; Bierbower, S.M.; Dupont-Versteegden, E.E.; Ghoweri, A.; Greenhalgh, A.; Harrison, D.; Istas, O.; McNabb, M.; et al. The effects of bacterial endotoxin (LPS) on cardiac and synaptic function in various animal models: Larval Drosophila, crayfish, crab, and rodent. Int. J. Zool. Res. 2020, in press. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Castro, N.; Cooper, R.L. Impedance Measures and a Mounting Technique for Drosophila: Larval Movements, Heart Rate, Imaging, and Electrophysiology. Methods Protoc. 2020, 3, 12. https://doi.org/10.3390/mps3010012
de Castro N, Cooper RL. Impedance Measures and a Mounting Technique for Drosophila: Larval Movements, Heart Rate, Imaging, and Electrophysiology. Methods and Protocols. 2020; 3(1):12. https://doi.org/10.3390/mps3010012
Chicago/Turabian Stylede Castro, Noah, and Robin Lewis Cooper. 2020. "Impedance Measures and a Mounting Technique for Drosophila: Larval Movements, Heart Rate, Imaging, and Electrophysiology" Methods and Protocols 3, no. 1: 12. https://doi.org/10.3390/mps3010012
APA Stylede Castro, N., & Cooper, R. L. (2020). Impedance Measures and a Mounting Technique for Drosophila: Larval Movements, Heart Rate, Imaging, and Electrophysiology. Methods and Protocols, 3(1), 12. https://doi.org/10.3390/mps3010012





