The Effects of Chloride Flux on Drosophila Heart Rate
Abstract
1. Introduction
2. Methods
2.1. Drosophila Lines
2.2. Preparation of Fly Food Supplemented with ATR
2.3. LEDs
2.4. Intact and Dissected Larvae
2.5. Measures of Membrane Potential in Cardiac and Body Wall Muscles
2.6. Statistical Analysis
3. Results
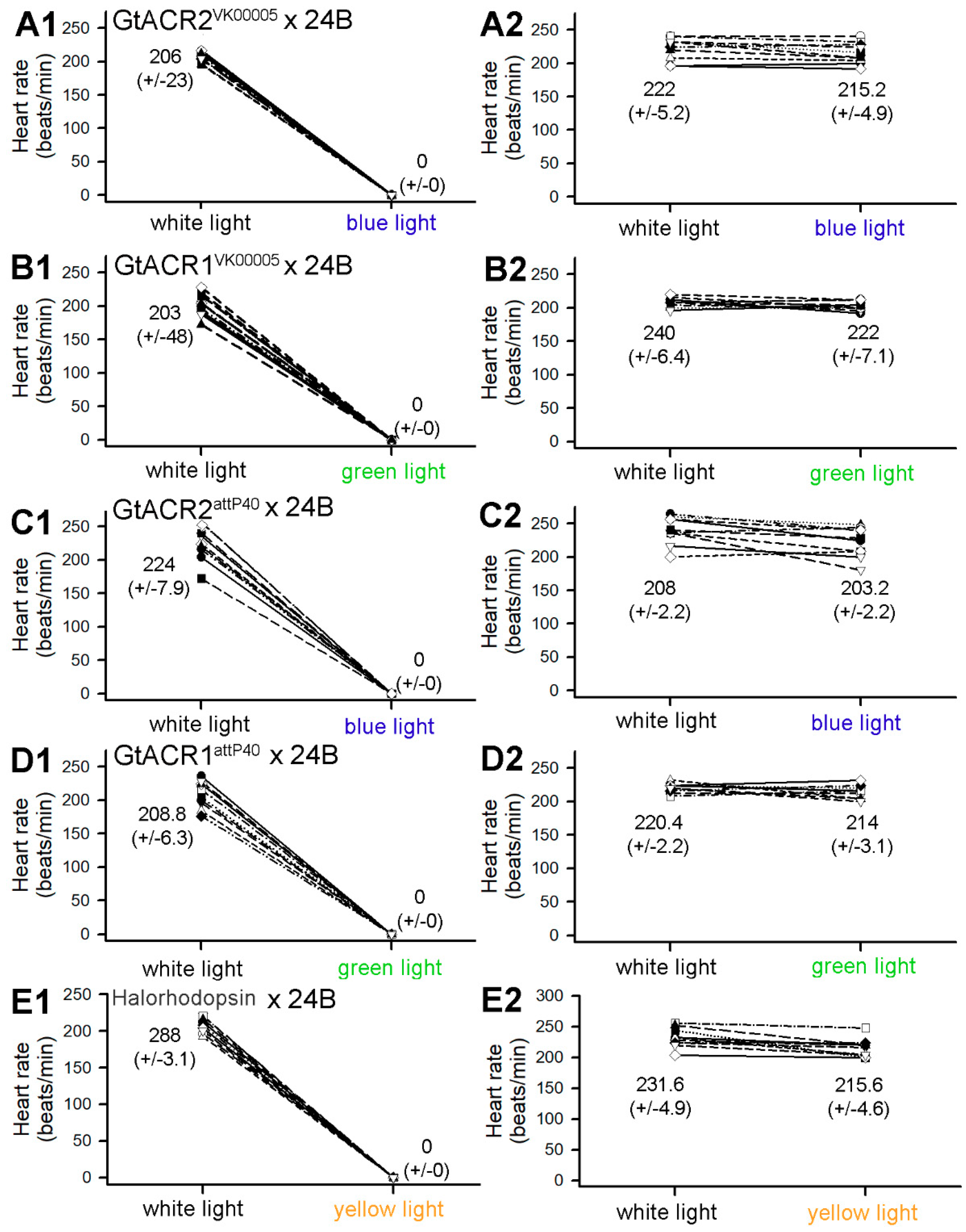
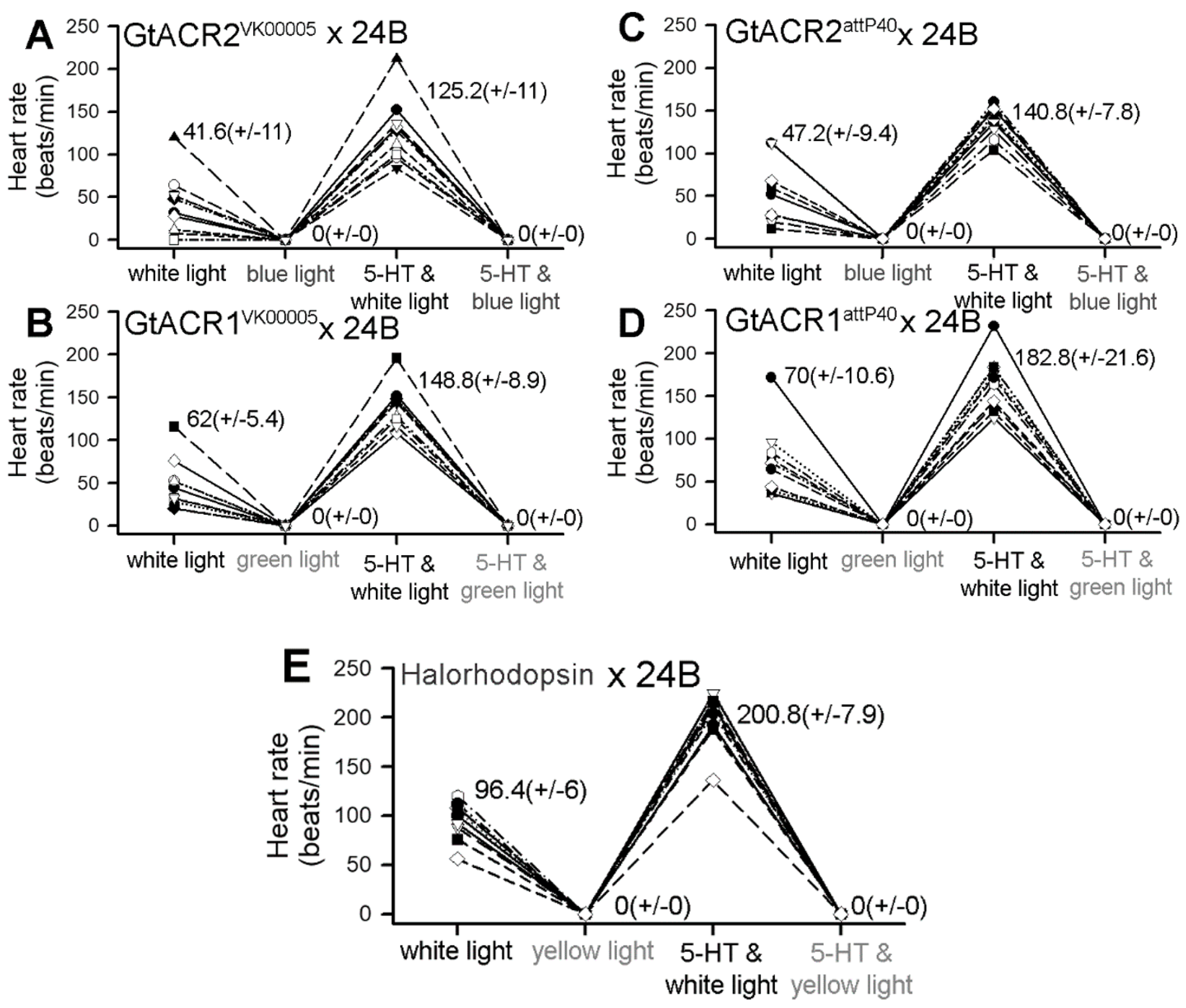
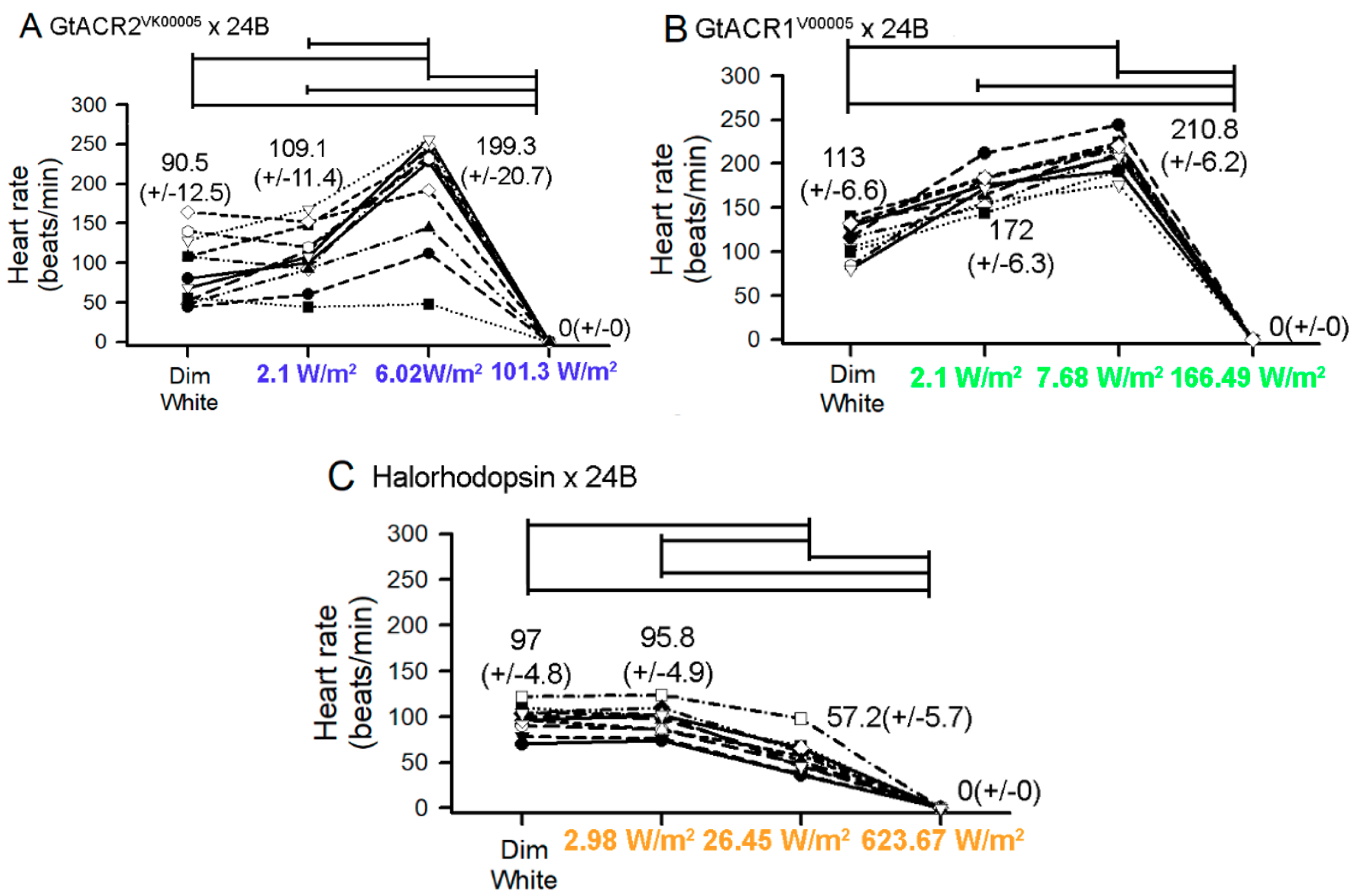
3.1. Heart Rates within Intact Larvae
3.2. Heart Rates within Dissected Larvae
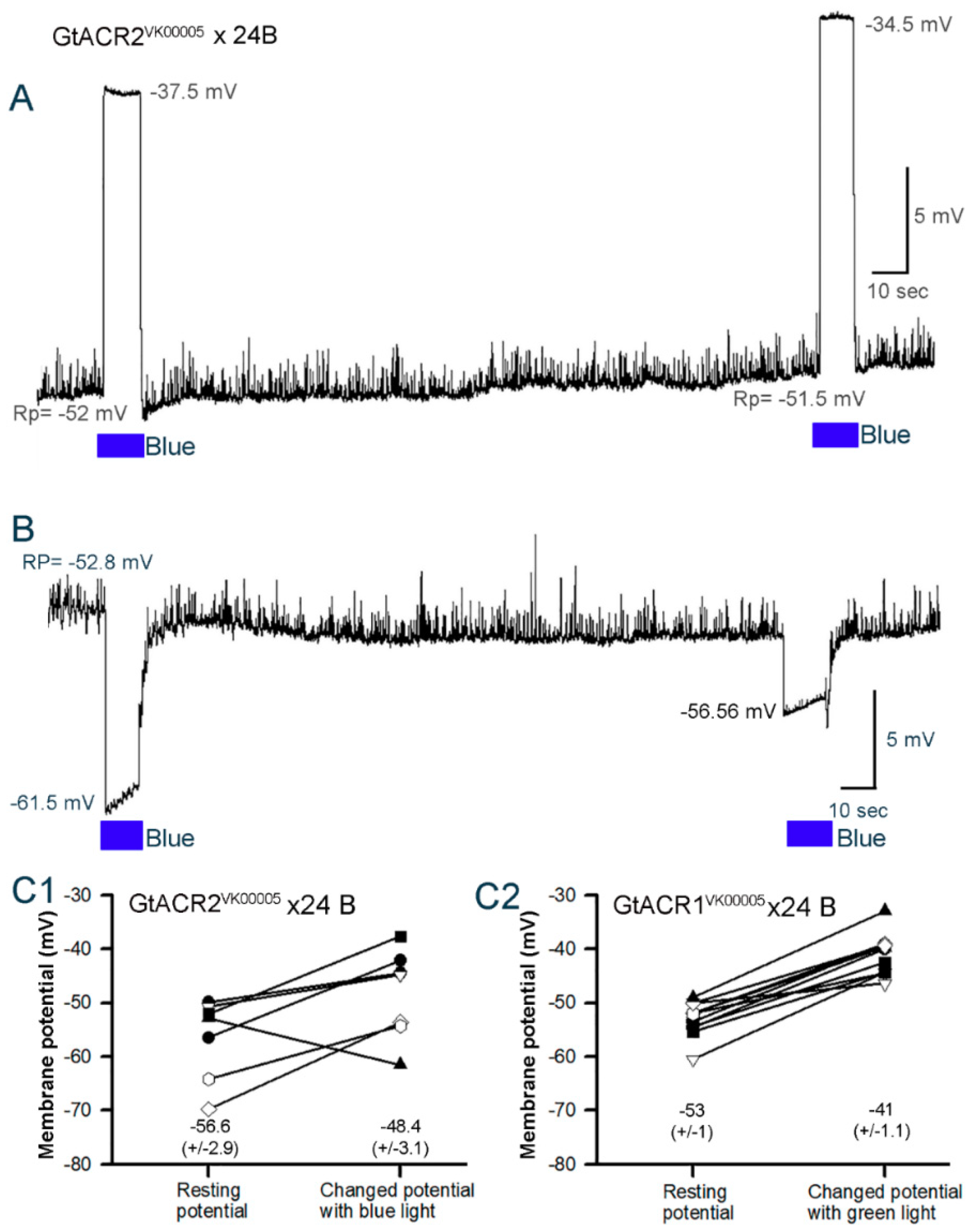
3.3. Membrane Potential in Cardiac and Body Wall Muscles
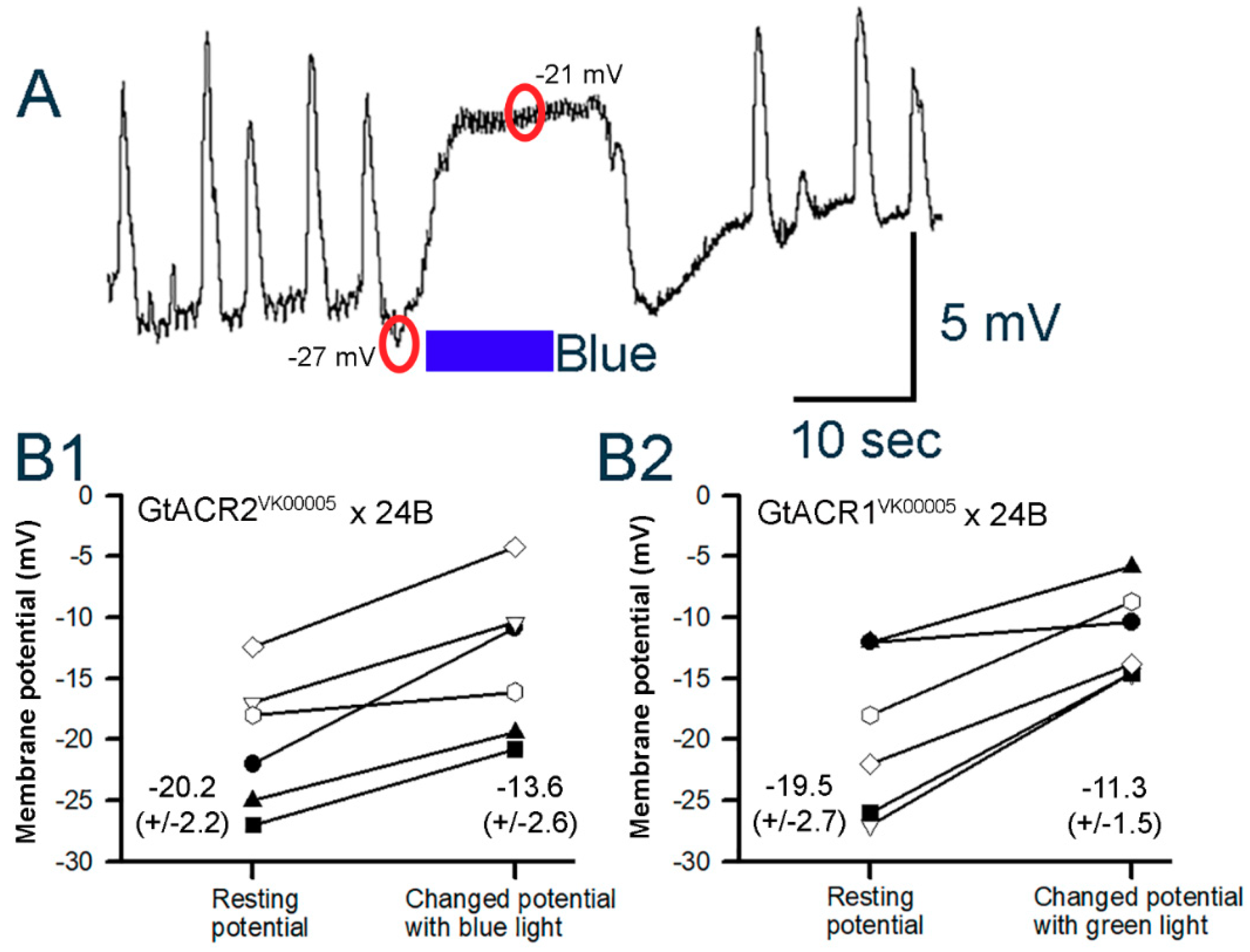
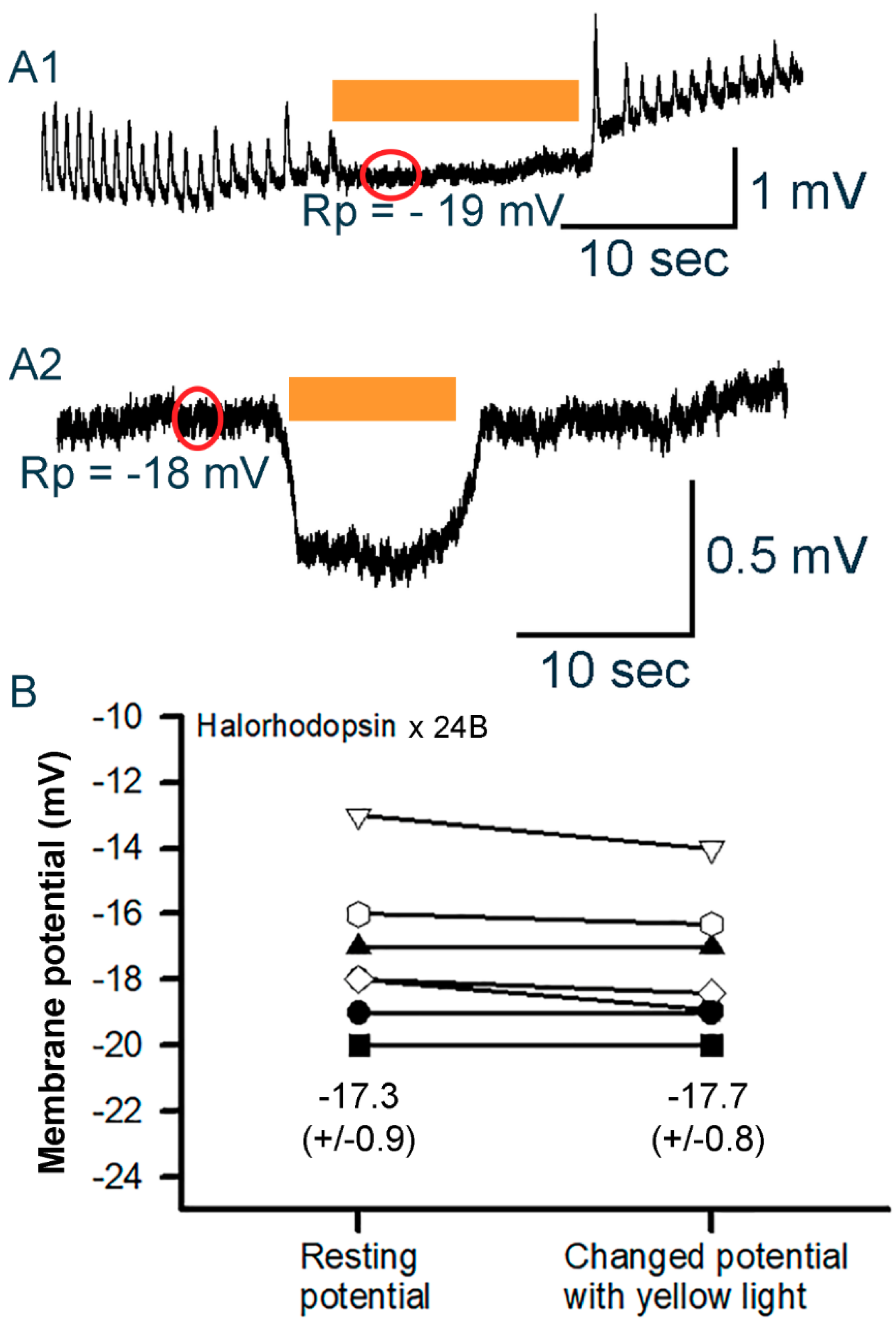
3.3.1. Body Wall Muscles
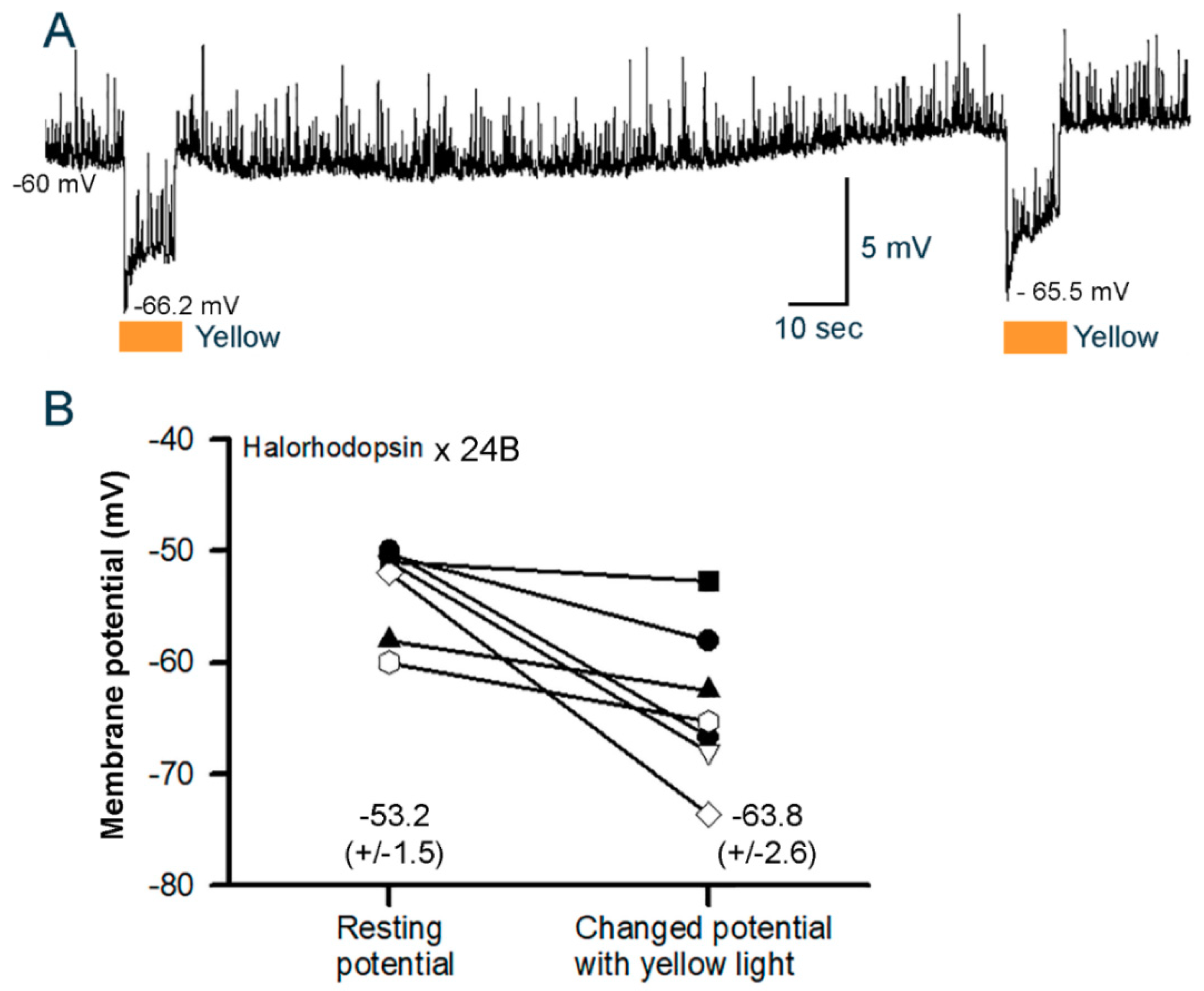
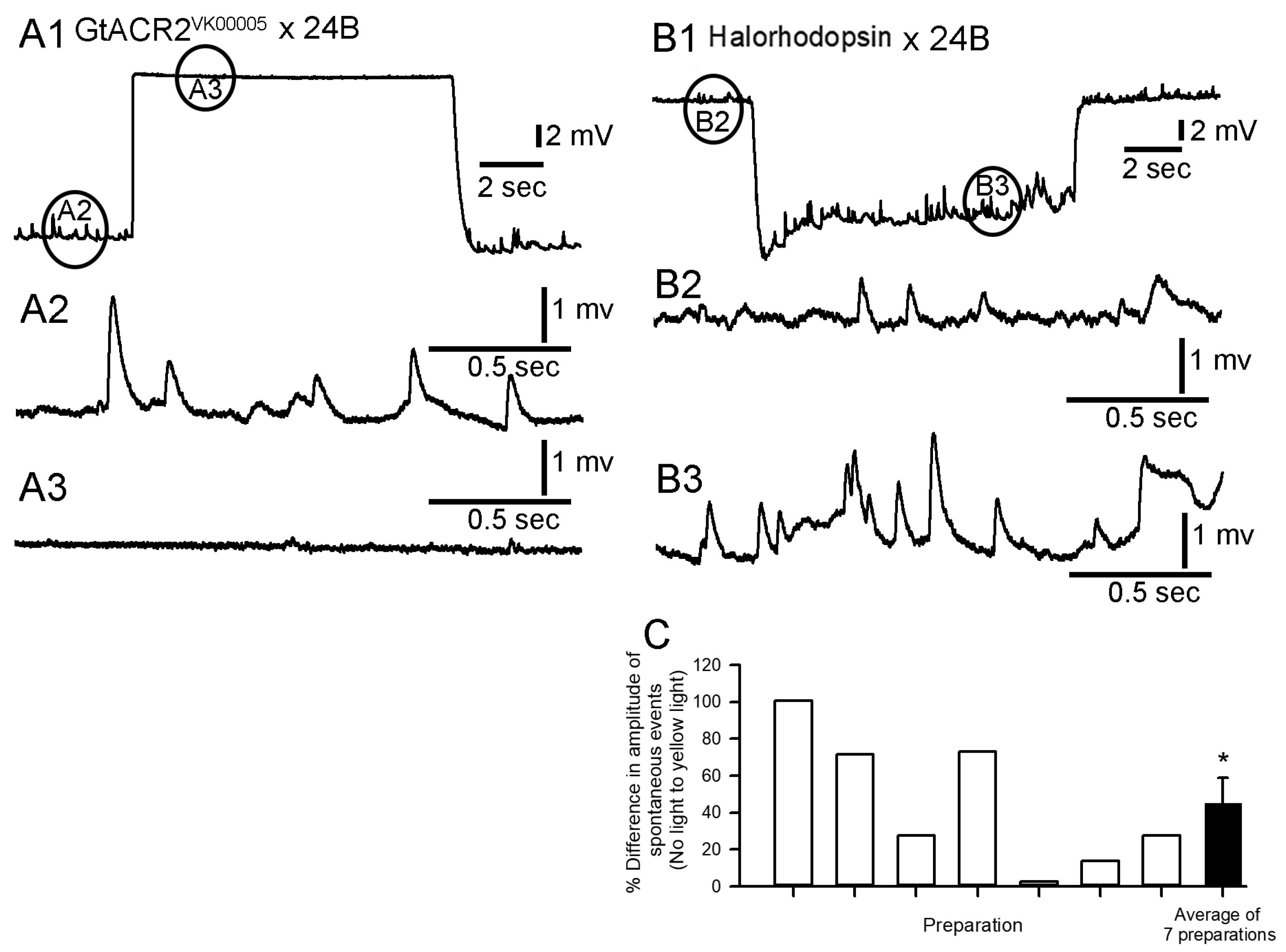
3.3.2. Cardiac Muscle
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Blacher, J.; Levy, B.I.; Mourad, J.J.; Safar, M.E.; Bakris, G. From epidemiological transition to modern cardiovascular epidemiology: Hypertension in the 21st century. Lancet 2016, 388, 530–532. [Google Scholar] [CrossRef]
- Qu, Y.S.; Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; El Sherif, N.; Boutjdir, M. Autoimmune calcium channelopathies and cardiac electrical abnormalities. Front. Cardiovasc. Med. 2019, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; Qin, Y.; Wang, J.; Zhou, L. Mutations in voltage-gated L-type calcium channel: Implications in cardiac arrhythmia. Channels 2018, 12, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, U.; Gepstein, L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol. 2015, 33, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.; Frank, L.W.; Arditi, L.I. Probable postcardiotomy syndrome following implantation of a transvenous pacemaker: Report of the first case. Heart J. 1975, 90, 627–630. [Google Scholar] [CrossRef]
- Stefanidis, A.S.; Margos, P.N.; Kotsakis, A.A.; Papasteriadis, E.G. Three-dimensional echocardiographic documentation of pacemaker lead perforation presenting as acute pericarditis. Hell. J. Cardiol. 2009, 50, 335–337. [Google Scholar]
- Wolk, B.; Dandes, E.; Martinez, F.; Helguera, M.; Pinski, S.L.; Kirsch, J. Postcardiac injury syndrome following transvenous pacer or defibrillator insertion: CT imaging and review of the literature. Curr. Probl. Diagn. Radiol. 2013, 42, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tillman, F.; Kim, J.; Makhlouf, T.; Osae, L. A comprehensive review of chronic heart failure pharmacotherapy treatment approaches in African Americans. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719840192. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.P.; Heitor Costa, L.; Alves da Costa, F.; Leufkens, H.G.; Martins, A.P. Identification of potentially inappropriate medications with risk of major adverse cardiac and cerebrovascular events among elderly patients in ambulatory setting and long-term care facilities. Clin. Interv. Aging 2019, 14, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Arrenberg, A.B.; Stainier, D.Y.; Baier, H.; Huisken, J. Optogenetic control of cardiac function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Govorunova, E.G.; Cunha, S.R.; Sineshchekov, O.A.; Spudich, J.L. Anion channelrhodopsins for inhibitory cardiac optogenetics. Sci. Rep. 2016, 6, 33530. [Google Scholar] [CrossRef] [PubMed]
- Kopton, R.A.; Baillie, J.S.; Rafferty, S.A.; Moss, R.; Zgierski-Johnston, C.M.; Prykhozhij, S.V.; Stoyek, M.R.; Smith, F.M.; Kohl, P.; Quinn, T.A.; et al. Cardiac electrophysiological effects of light-activated chloride channels. Front. Physiol. 2018, 9, 1806. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, C.; Holmes, P.; Winter, J.; Correia, J.; Ou, X.; Dong, R.; He, S.; Kirchhof, P.; Fabritz, L.; Rajpoot, K.; et al. Cardiac optogenetics and optical mapping–overcoming spectral congestion in all-optical cardiac electrophysiology. Front. Physiol. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2007, 2, e299. [Google Scholar] [CrossRef] [PubMed]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Camporeze, B.; Manica, B.A.; Bonafé, G.A.; Ferreira, J.J.C.; Diniz, A.L.; de Oliveira, C.T.P.; Mathias, L.R., Jr.; de Aguiar, P.H.P.; Ortega, M.M. Optogenetics: The new molecular approach to control functions of neural cells in epilepsy, depression and tumors of the central nervous system. Am. J. Cancer Res. 2018, 8, 1900–1918. [Google Scholar] [PubMed]
- Bruegmann, T.; Malan, D.; Hesse, M.; Beiert, T.; Fuegemann, C.J.; Fleischmann, B.K.; Sasse, P. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 2010, 7, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Musti, A.M.; Bamberg, E.; Hegemann, P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science 2002, 296, 2395–2398. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13950. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Wietek, J.; Wiegert, J.S.; Adeishvili, N.; Schneider, F.; Watanabe, H.; Tsunoda, S.P.; Vogt, A.; Elstner, M.; Oertner, T.G.; Hegemann, P. Conversion of channelrhodopsin into a light-gated chloride channel. Science 2014, 344, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Lee, S.Y.; Ramakrishnan, C.; Deisseroth, K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science 2014, 344, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Govorunova, E.G.; Sineshchekov, O.A.; Janz, R.; Liu, X.; Spudich, J.L. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 2015, 349, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Mauss, A.S.; Busch, C.; Borst, A. Optogenetic neuronal silencing in Drosophila during visual processing. Sci. Rep. 2017, 7, 13823. [Google Scholar] [CrossRef] [PubMed]
- Banghart, M.; Borges, K.; Isacoff, E.; Trauner, D.; Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 2004, 7, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Post, R.J.; Ho, Y.Y.; Warden, M.R. Making sense of optogenetics. Int. J. Neurophychopharmacol. 2015, 18, pyv079. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, A.Y.; Roshchin, M.V.; Smirnova, G.R.; Dolgikh, D.A.; Balaban, P.M.; Ostrovsky, M.A. Chloride conducting light activated channel GtACR2 can produce both cessation of firing and generation of action potentials in cortical neurons in response to light. Neurosci. Lett. 2017, 640, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Cheng, R.K.; Ho, J.; Krishnan, S.; Mohammad, F.; Claridge-Chang, A.; Jesuthasan, S. Optical inhibition of larval zebrafish behaviour with anion channelrhodopsins. BMC Biol. 2017, 15, 103. [Google Scholar] [CrossRef]
- Forli, A.; Vecchia, D.; Binini, N.; Succol, F.; Bovetti, S.; Moretti, C.; Nespoli, F.; Mahn, M.; Baker, C.A.; Bolton, M.M.; et al. Two-Photon bidirectional control and imaging of neuronal excitability with high spatial resolution in vivo. Cell Rep. 2018, 22, 3087–3098. [Google Scholar] [CrossRef]
- Mohammad, F.; Stewart, J.C.; Ott, S.; Chlebikova, K.; Chua, J.Y.; Koh, T.W.; Ho, J.; Claridge-Chang, A. Optogenetic inhibition of behavior with anion channelrhodopsins. Nat. Methods 2017, 14, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Schobert, B.; Lanyi, J.K. Halorhodopsin is a light-driven chloride pump. J. Biol. Chem. 1982, 257, 10306–10313. [Google Scholar] [PubMed]
- Akasaka, T.; Ocorr, K. Drug discovery through functional screening in the Drosophila heart. Methods Mol. Biol. 2009, 577, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Bier, E.; Bodmer, R. Drosophila, an emerging model for cardiac disease. Gene 2004, 342, 1–11. [Google Scholar] [CrossRef]
- Cripps, R.M.; Olson, E.N. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev. Biol. 2002, 246, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Ocorr, K.; Perrin, L.; Lim, H.Y.; Qian, L.; Wu, X.; Bodmer, R. Genetic control of heart function and aging in Drosophila. Trends Cardiovasc. Med. 2007, 17, 177–182. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Wessells, R.J.; Bodmer, R. Screening assays for heart function mutants in Drosophila. Biotechniques 2004, 37, 58–60. [Google Scholar] [CrossRef]
- Choma, M.A.; Suter, M.J.; Vakoc, B.J.; Bouma, B.E.; Tearney, G.J. Physiological homology between Drosophila melanogaster and vertebrate cardiovascular systems. Dis. Models Mech. 2011, 4, 411–420. [Google Scholar] [CrossRef]
- Cooper, A.S.; Rymond, K.E.; Ward, M.A.; Bocook, E.L.; Cooper, R.L. Monitoring Heart Function in Larval Drosophila melanogaster for Physiological Studies. J. Vis. Exp. 2009, 33, e1596. [Google Scholar] [CrossRef]
- Dasari, S.; Cooper, R.L. Direct influence of serotonin on the larval heart of Drosoph. Melanogaster. J. Comp. Physiol. B 2006, 176, 349–357. [Google Scholar] [CrossRef]
- de Castro, C.; Titlow, J.; Majeed, Z.R.; Cooper, R.L. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2014, 200, 83–92. [Google Scholar] [CrossRef]
- Jones, W.D. The expanding reach of the GAL4/UAS system into the behavioral neurobiology of Drosophila. BMB Rep. 2009, 42, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, M.; Ito, K. Acute genetic manipulation of neuronal activity for the functional dissection of neural circuits—A dream come true for the pioneers of behavioral genetics. J. Neurogenet. 2012, 26, 43–52. [Google Scholar] [CrossRef]
- Schroll, C.; Riemensperger, T.; Bucher, D.; Ehmer, J.; Völler, T.; Erbguth, K.; Gerber, B.; Hendel, T.; Nagel, G.; Buchner, E.; et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 2006, 16, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Uradu, H.; Majeed, Z.R.; Cooper, R.L. Optogenetic drive of Drosophila heart rate at different temperatures and Ca2+ concentrations. Physiol. Rep. 2016, 4, e12695. [Google Scholar] [CrossRef] [PubMed]
- Owald, D.; Lin, S.; Waddell, S. Light, heat, action: Neural control of fruit fly behavior. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140211. [Google Scholar] [CrossRef]
- Rose, U.; Derst, C.; Wanischeck, M.; Marinc, C.; Walther, C. Properties and possible function of a hyperpolarisation-activated chloride current in Drosophila. J. Exp. Biol. 2007, 210, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Mahn, M.; Gibor, L.; Patil, P.; Cohen-Kashi Malina, K.; Oring, S.; Printz, Y.; Levy, R.; LampL, I.; Yizhar, O. High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins. Nat. Commun. 2018, 9, 4125. [Google Scholar] [CrossRef]
- Mahn, M.; Prigge, M.; Ron, S.; Levy, R.; Yizhar, O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 2016, 19, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, M.; Weineck, K.; Costa, J.; Cooper, R.L. Hyperpolarization by activation of halorhodopsin results in enhanced synaptic transmission: Neuromuscular junction and CNS circuit. PLoS ONE 2018, 13, e0200107. [Google Scholar] [CrossRef] [PubMed]
- Messier, J.E.; Chen, H.; Cai, Z.-L.; Xue, M. Targeting light-gated chloride channels to neuronal somatodendritic domain reduces their excitatory effect in the axon. Elife 2018, 7, e38506. [Google Scholar] [CrossRef] [PubMed]
- Wiegert, J.S.; Mahn, M.; Prigge, M.; Printz, Y.; Yizhar, O. Silencing neurons: Tools, applications, and experimental constraints. Neuron 2017, 95, 504–529. [Google Scholar] [CrossRef] [PubMed]
- Dawydow, A.; Gueta, R.; Ljaschenko, D.; Ullrich, S.; Hermann, M.; Ehmann, N.; Gao, S.; Fiala, A.; Langenhan, T.; Nagel, G.; et al. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA 2014, 111, 13972–13977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cunha, C.; Zhang, F.; Liu, Q.; Gloss, B.; Deisseroth, K.; Augustine, G.J.; Feng, G. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 2008, 36, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Titlow, J.S.; Anderson, H.; Cooper, R.L. Lights and larvae: Using optogenetics to teach recombinant DNA and neurobiology. Sci. Teach. 2014, 81, 3–9. [Google Scholar]
- Majeed, Z.R.; Abdeljaber, E.; Soveland, R.; Cornwell, K.; Bankemper, A.; Koch, F.; Cooper, R.L. Modulatory action of serotonergic system in behavior and sensory-motor circuit physiology in Drosophila melanogaster. Neural Plast. 2016. [Google Scholar] [CrossRef]
- Malloy, C.; Sifers, J.; Mikos, A.; Samadi, A.; Omar, A.; Hermanns, C.; Cooper, R.L. Using optogenetics to assess neuroendocrine modulation of heart rate in Drosophila melanogaster larvae. J. Comp. Physiol. A 2017, 203, 791–806. [Google Scholar] [CrossRef]
- Majeed, Z.; Koch, F.; Morgan, J.; Anderson, H.; Wilson, J.; Cooper, R.L. A novel educational module to teach neural circuits for college and high school students: NGSS-neurons, genetics, and selective stimulations. F1000Research 2017. Available online: https://f1000research.com/articles/6-117/v1 (accessed on 10 January 2018).
- Higgins, J.; Hermanns, C.; Malloy, C.; Cooper, R.L. Considerations in repetitive activation of light sensitive ion channels for long term studies: Channel rhodopsin in the Drosophila model. Neurosci. Res. 2017, 125, 1–10. [Google Scholar] [CrossRef]
- Desai-Shah, M.; Papoy, A.R.; Ward, M.; Cooper, R.L. Roles of the Sarcoplasmic/Endoplasmic reticulum Ca2+-ATPase, plasma membrane Ca2+-ATPase and Na+/Ca2+ exchanger in regulation of heart rate in larval Drosophila. Open Physiol. J. 2010, 3, 16–36. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Stacy, A.; Cooper, R.L. Pharmacological identification of serotonin receptor subtypes on the Drosophila larval heart. J. Comp. Physiol. B 2014, 184, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Malloy, C.A.; Ritter, K.; Robinson, J.; English, C.; Cooper, R.L. Pharmacological identification of cholinergic receptor subtypes on Drosophila melanogaster larval heart. J. Comp. Physiol. B 2016, 186, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Majeed, Z.R.; Ritter, K.; Robinson, J.; Blümich, S.L.E.; Brailoiu, E.; Cooper, R.L. New insights into the acute actions from a high dosage of fluoxetine on neuronal and cardiac function: Drosophila, crayfish and rodent models. Comp. Biochem. Physiol. C 2015, 176, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Anyagaligbo, O.; Bernard, J.; Greenhalgh, A.; Cooper, R.L. The effects of bacterial endotoxin (LPS) on cardiac function in a medicinal blow fly (Phaenicia sericata) and a fruit fly (Drosophila melanogaster). Comp. Biochem. Physiol. C 2019, 217, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kurdyak, P.; Atwood, H.L.; Stewart, B.A.; Wu, C.-F. Differential physiology and morphology of motor axons to ventral longitudinal muscle in larval Drosophila. J. Comp. Neurol. 1994, 350, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Bosch, D.; Ehrlich, I. Postnatal maturation of GABAergic modulation of sensory inputs onto lateral amygdala principal neurons. J. Physiol. 2015, 593, 4387–4409. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, G.; Wiersma, C.A. Inhibition at neuromuscular junctions in Crustacea. J. Physiol. 1958, 143, 426–440. [Google Scholar] [CrossRef]
- Dulhunty, A.F. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. J. Physiol. 1978, 276, 67–82. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Risager, A.; Vicenza de Paoli, F.; Chen, T.-Y.; Nielsen, O.B. Role of physiological CLC-1 Cl ion channel regulation for the excitability and function of working skeletal muscle. J. Gen. Physiol. 2016, 147, 291–308. [Google Scholar] [CrossRef]
- Hutter, O.F.; Noble, D. The influence of anions on impulse generation and membrane conductance in Purkinje and myocardial fibres. J. Physiol. 1959, 147, 16–17P. [Google Scholar]
- Hutter, O.F.; Noble, D. Anion conductance of cardiac muscle. J. Physiol. 1961, 157, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, E.E. Chloride ions and the membrane potential of Purkinje fibres. J. Physiol. 1961, 156, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.D.; Ngo, T.T.; Hibbard, K.L.; Murphy, C.; Jenett, A.; Truman, J.W.; Rubin, G.M. Refinement of tools for targeted gene expression in Drosophila. Genetics 2010, 186, 735–755. [Google Scholar] [CrossRef] [PubMed]
- Dudel, J. Presynaptic inhibition of the excitatory nerve terminal in the neuromuscular junction of the crayfish. Pflügers Arch. Ges. Physiol. 1963, 277, 537–557. [Google Scholar] [CrossRef]
- Dudel, J. The mechanism of presynaptic inhibition at the crayfish neuromuscular junction. Pflügers Arch. 1965, 284, 66–80. [Google Scholar] [CrossRef]
- Cooper, A.S.; Cooper, R.L. Historical view and physiology demonstration at the NMJ of the crayfish opener muscle. J. Vis. Exp. 2009, 33, e1595. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Nichols, C.D.; Cooper, R.L. 5-HT stimulation of heart rate in Drosophila does not act through cAMP as revealed by pharmacogenetics. J. Appl. Physiol. 2013, 115, 1656–1665. [Google Scholar] [CrossRef]
- Titlow, J.S.; Rufer, J.M.; King, K.E.; Cooper, R.L. Pharmacological analysis of dopamine modulation in the Drosophila melanogaster larval heart. Physiol. Rep. 2013, 1, e00020. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Yocum, E.; Sifers, J.; Uradu, H.; Cooper, R.L. Modulatory effects on Drosophila larva hearts in room temperature, acute and chronic cold stress. J. Comp. Physiol. B 2016, 186, 829–841. [Google Scholar] [CrossRef]
- Giachello, C.N.; Baines, R.A. Regulation of motoneuron excitability and the setting of homeostatic limits. Curr. Opin. Neurobiol. 2017, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moonschi, F.H.; Effinger, A.K.; Zhang, X.L.; Martin, W.E.; Fox, A.M.; Heidary, D.K.; DeRouchey, J.E.; Richards, C.I. Cell-derived vesicles for single-molecule imaging of membrane proteins. Angew. Chem. Int. Ed. 2015, 54, 481–484. [Google Scholar] [CrossRef]
- Colombo, S.F.; Mazzo, F.; Pistillo, F.; Gotti, C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem. Pharmacol. 2013, 86, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, H.G.; Tafesse, T.B.; Bule, M.H. If channel as an emerging therapeutic target for cardiovascular diseases: A review of current evidence and controversies. Front. Pharmacol. 2017, 8, 874. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanley, C.E.; Mauss, A.S.; Borst, A.; Cooper, R.L. The Effects of Chloride Flux on Drosophila Heart Rate. Methods Protoc. 2019, 2, 73. https://doi.org/10.3390/mps2030073
Stanley CE, Mauss AS, Borst A, Cooper RL. The Effects of Chloride Flux on Drosophila Heart Rate. Methods and Protocols. 2019; 2(3):73. https://doi.org/10.3390/mps2030073
Chicago/Turabian StyleStanley, Catherine E., Alex S. Mauss, Alexander Borst, and Robin L. Cooper. 2019. "The Effects of Chloride Flux on Drosophila Heart Rate" Methods and Protocols 2, no. 3: 73. https://doi.org/10.3390/mps2030073
APA StyleStanley, C. E., Mauss, A. S., Borst, A., & Cooper, R. L. (2019). The Effects of Chloride Flux on Drosophila Heart Rate. Methods and Protocols, 2(3), 73. https://doi.org/10.3390/mps2030073





