1. Introduction
In immunohistochemistry, several types of tissue immunostains are utilized to analyze morphological features, cellular structures, cell type, and the presence or absence of microorganisms. The most popular of the staining methods for diagnostic potential is the utilization of hematoxylin and eosin (H&E) staining [
1]. H&E stains reveal structural information, with specific functional implications. H&E staining of tissue is used to assess cellular and morphological structures, identify the type of tissue, morphological variability, cell type, and pathological changes. The use of H&E staining has been the most effective and utilized procedure for pathological diagnosis of patient neoplasia for over a century [
2,
3]. It has allowed pathologists to pinpoint focal areas of a specimen-containing aggressive tissue and foster a proper diagnosis [
4]. Therefore, developing procedures to re-utilize these archived samples to determine individual biomarker expression levels (and potential protein–protein association) could assist in determining disease progression and directions for appropriate treatments.
H&E staining is used in conjunction with a variety of tissue fixatives and allows the display of various cellular and tissue components, including the extracellular matrix, the cellular cytoplasm, and the nuclear structures [
3]. The hematoxylin is converted into its oxidization product hematein, which is a basic dye that stains acidic (basophilic) tissue components (ribosomes, nuclei, and rough endoplasmic reticulum) a darker purple color and the acidic eosin dye stains other protein structures of the tissue (stroma, cytoplasm, muscle fibers) a pink color [
2,
4,
5]. They are also valuable in distinguishing normal structural components from neoplastic regions. However, with the current procedures, H&E staining is utilized along with sequential sections stained with antibody. Serial sectioning may cut through the region of intent and may result in the loss of regions necessary for critical diagnosis. This is particularly an issue with smaller core needle biopsies (CNBs) that are of a limited size and number. These samples are considered “precious” in regard to availability and require the utmost accuracy in testing procedures to result in proper diagnoses.
A major advantage of a method that allows the reuse of the H&E-stained slide is that it will alleviate the need for additional sequentially sectioned slides, particularly with the diminutive CNBs. Due to the of size of CNBs, they are also subject to tissue sample exhaustion with the loss of the diagnostic lesion. This method would present a major practicality when a particular region of interest is no longer available in the sample block due to sequential cuts. The ability to reuse the initial H&E containing the lesion could be critical. De-staining these H&E-stained tissue slides could also potentially reduce the need for re-biopsy.
Another advantage of re-staining archived H&E-stained slides is due the rapidly expanding use of whole-slide imaging (WSI), also known as digital pathology (DP) or virtual pathology. DP is a technology that involves the high-speed and high-resolution digital acquisition of images representing entire stained tissue sections from glass slides in a format that allows them to be viewed by a pathologist on a computer monitor [
6]. This streamlines the ability of a surgical pathologist to make a primary diagnosis utilizing digitized images of the H&E-stained slide, allowing digital preservation while the H&E and other stains are fresh [
7]. As the validation of this technology becomes widespread, the method reported here could be used for analysis of stored H&E-stained slides for subsequent diagnosis of tumor subtypes within a patient sample or future discovery of novel target proteins.
2. Experimental Design
This procedure details steps to de-stain and reutilize archived H&E stained slides for antibody immunostaining modalities. For our research, prostate cancer was initially chosen due to frequent limitations of tissue in sample biopsies and the requirement for biomarker study. Prostate cancer (PCa) is also known to express variable levels of several markers associated with disease progression, such as phosphatase tensin homolog (PTEN) and ETS-Related Gene (ERG), making it a viable target for testing this procedure. A link between the PTEN pathway and ERG protein expression has previously been evaluated in various prostate cancer studies [
8,
9,
10,
11,
12,
13]. In studies investigating the trend of PTEN loss in tumors of prostatectomies and locally recurring castrate-resistant prostate cancers (CRCPs) with ERG overexpression, the data showed that the loss of PTEN was significantly associated with ERG positivity [
11]. Another study indicated that the combination of ERG overexpression and PTEN deletion is common in aggressive capsular penetrating lesions [
14]. Therefore, we decided that using antibodies targeting PTEN and ERG would be the validated markers in this study.
We first used archived H&E-stained slides from PCa resections or biopsies stored for at least one year (with film coverslips) to demonstrate proof that the H&Es could be reutilized for biomarker stain using an H&E de-staining procedure with standard laboratory equipment and reagents. De-identified patient tissue samples were provided with no link to information that can be used to identify patients. Oversight for tissue acquisition was managed by the UA Cancer Center, an NCI designated Comprehensive Cancer Center. De-identified FFPE prostate tissue multi-Array (TMA), adenocarcinoma, lung, colon, and skin tissue slide samples (
Table 1) were acquired from Roche Tissue Diagnostics (RTD)/Ventana Medical Systems Inc. (VMSI or Ventana), Tucson, Arizona. De-identified PCa CNBs (
Table 1) used for initial feasibility testing of these procedures, were provided and serial sectioned by the University of Arizona Cancer Center Tissue Acquisition and Cellular/Molecular Analysis Resource (TACMASR) core support service with the approval of the University of Arizona Institutional Review Board (IRB). Initial H&E analysis (
Table 1), was provided by Dr. Ray Nagle. Several of the additional sample tissue specimens used within this study (liver, lung, normal colon and PCa TMA) were exhausted and only the H&E stained slide remained available for testing. For the samples in the cohort that retained tissue availability, sample slides for each tissue were H&E-stained and cover-slipped using the reagents and staining procedures on the Sakura Tissue-Tek Prisma
Plus & Film Automated Slide Stainer & Coverslipper at VMSI and allowed to air dry in a fume hood for approximately 10 min.
2.1. Reagents and Materials
Acetone (VWR, Visalia, CA, USA; BDH1101-4LP)
Xylene (Millipore, Billerica, MA, USA; 58235)
100%–95% Ethanol
Distilled water (DI)
Reaction Buffer (Tris based, 7.6 ± 0.2 pH) (Proprietary reagent, Ventana/RTD, Tucson, AZ, USA)
H&E stained tissue slides
Paraffin embedded sample tissues
Microscope slides (Matsunami TOMO®, Bellingham, WA or Superfrost™, Thermo Fischer Scientific, Waltham, MA, USA)
Slide coverslips (VWR, Visalia, CA, USA; 48393 251)
Mounting medium (coverslip sealant) (Thermo Scientific, Waltham, MA, USA; 8312-4)
2.2. Equipment
Slide Baskets (Sakura Finetek, Torrence, CA, USA; 4768)
Plastic staining dish (container) [with lids preferably] (Sakura Finetek, Torrence, CA, USA)
Forceps
Kimwipes™
Brightfield microscope (Olympus BX40)
Parafilm (optional) ((Parafilm M® Laboratory film, VWR, Visalia, CA, USA)
Slide Scanner (Leica Aperio AT2; Leica Biosystems, Buffalo Grove, IL, USA, DP200 Slide Scanner RTD, Tucson, AZ, USA)
Incubator (60 °C ± 5 °C) (VWR, Visalia, CA, USA)
BenchMark ULTRA (optional)
Slide Coverslipper (Sakura Tissue -Tek Prisma Plus & Film Automated Slide stainer and Coverslipper; Sakura Finetek, Torrence, CA, USA)
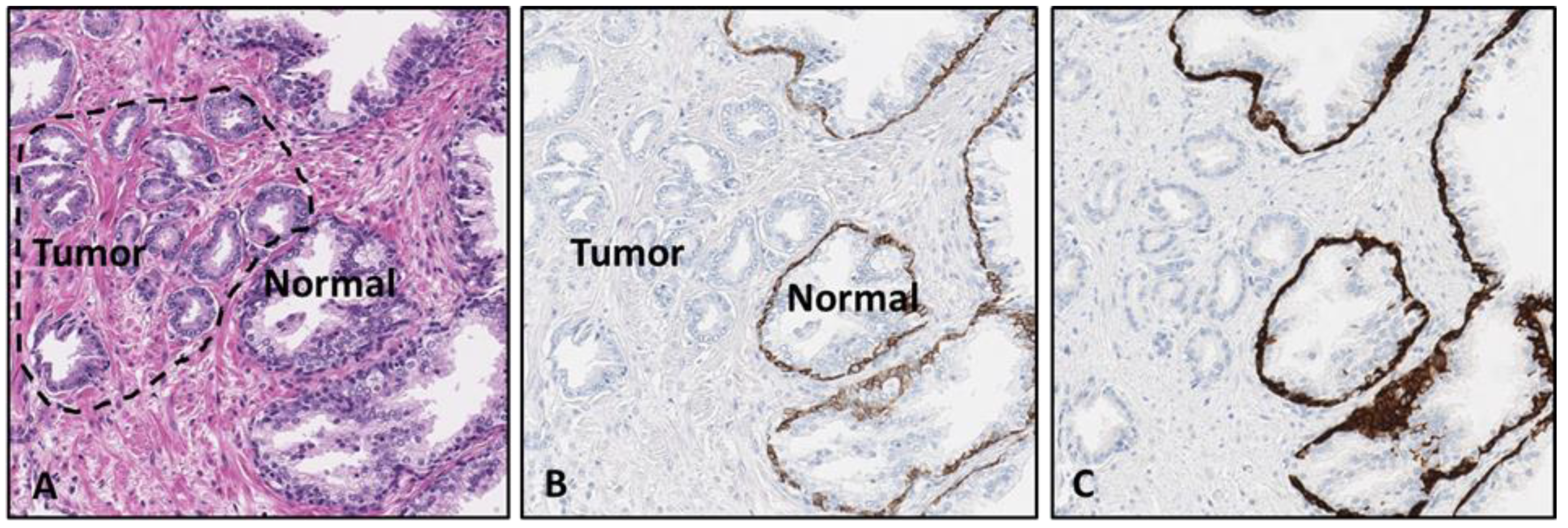
The initial antibodies chosen for the proof-of-concept testing were the on-market products VENTANA anti-p40 (B28) mouse monoclonal antibody (data not shown), anti-cytokeratin 5/14 (CK5/14) (EP1601Y/LL002) rabbit and mouse monoclonal antibody cocktail from Cell Marque (
Figure 1) and a rabbit polyclonal antibody (Ab) against the laminin-binding extracellular domain of integrin alpha 6 (CD49f) from the lab of Dr. Anne Cress at the University of Arizona, Department of Cellular and Molecular Medicine. The CD49f antibody was formulated and optimized from a 1 mg/mL stock concentrate to a 1:800 dilution in a pH = 7.3 Phosphate Avidin antibody diluent containing a proprietary B5 blocker, goat globulins, and 55 mM NaCl concentration with Proline preservative.
The antibodies utilized for additional immunostaining of five selected de-stained archived H&E stained slides to analyze and compare immunostaining intensities with corresponding sequential slides were the VENTANA anti-High Molecular Weight Cytokeratin (HMWCK) and p63 (34βE12 + 4A4, respectively) mouse basal cell cocktail, Cell Marque anti-CK 8&18 (B22.1 &23.1) rabbit monoclonal, VENTANA anti-E-cadherin (36) mouse monoclonal, anti-CD49f and VENTANA anti-ERG (EPR3864) rabbit monoclonal. Each reused (re-stained) H&E stained slide was de-stained according to the procedure listed in this report and immunostained with the various antibodies along with the corresponding sequential slide for each sample (see Results section).
IHC DAB detection was accomplished by utilizing a VENTANA OptiView DAB IHC Detection kit and VENTANA ultraView DAB IHC Detection Kits. Chromogen detection was accomplished by utilizing a VENTANA Discovery Chromomap Detection Kit to target and detect the anti-HMWCK + p63 mouse monoclonal antibody cocktail with the secondary antibody linker conjugated with hydroxyquinazoline and anti-hydroxyquinazoline (HQ) horseradish peroxidase (HRP) enzymes for detection with a VENTANA Discovery Purple Chromogen. The detection for the anti-CD49f rabbit polyclonal antibody included an anti-rabbit nitrolpirazole (NP) conjugated secondary and anti-NP HRP for detection of the VENTANA Discovery Teal Chromogen. The detection of the sample slides was accomplished utilizing the LEICA AT2 slide scanner and the VENTANA Digital Pathology 200 (DP200) slide scanner.
3. Procedure
De-Staining the H&E Slides. Time to Completion: ~2 h
Perform the de-staining procedures in a fume hood using manual wash stations (baths) containing the reagent solvents listed in reagents and materials section. These procedures negate the need for heat to remove sealed coverslips and result in safe removal without tissue damage.
- 1.
Place the H&E archived index slides into slide baskets to allow manual rinsing.
- 2.
Manually soak slides in Acetone for 10 min to remove coverslip.
![Mps 02 00086 i001 Mps 02 00086 i001]() CRITICAL STEP
CRITICAL STEP Cover the container of acetone with either container lid or parafilm to reduce acetone evaporation (evaporation reduce effectiveness of coverslip removal).
- 3.
Remove the coverslip with forceps slowly to reduce damage to tissue (longer intervals allow coverslip to slide off independently). Discard coverslip.
- 4.
Moderately rinse slides 3 times in xylene bath to remove any remaining adhesive, allow slides to sit in the bath to remove all sealant (Approximately 1-min hold times should suffice between rinses). [Rinse = 30+ Dips in reagent] [Hold = allowing slides to sit in reagent container between rinses].
- 5.
Rinse slides (5–6 times) with 3 min hold intervals between rinses in 95% EtOH for ~30 min to remove eosin stain. [Total time for 95% EtOH procedure is approx. 30 min, however intervals may be increased for removal of eosin from larger tissue sections.]
![Mps 02 00086 i002 Mps 02 00086 i002]() CRITICAL STEP
CRITICAL STEP Cover with caps or paraffin between rinses to reduce reagent evaporation. Moderately rinse slides during hold times to expedite eosin removal.
- 6.
Rinse slides in distilled (DI) H2O and lightly tap on Kimwipes™ to remove excess.
- 7.
Rinse slides in Reaction Buffer (3–4 rinses with 2 min hold intervals) to remove Hematoxylin (cap or paraffin cover to reduce evaporation).
- 8.
OPTIONAL STEP Apply extra rinses in reaction buffer to expedite removal of hematoxylin.
- 9.
Allow slides to dry in hood in fume hood for 5 min (do not allow tissue to dry completely this will impact immunostaining intensity).
- 10.
Run antibody IHC assay detection protocols on instruments or manual procedures (will be variable, depending on biomarker).
- 11.
Wash completed slides in water and dawn dish detergent mix to remove any residual reagents
- 12.
Dehydrate slides and coverslip on Sakura coverslipper or manual dehydrate rinse with xylene > acetone > 80% EtOH > 90% EtOH > 100% EtOH > xylene and coverslip using mounting medium.
![Mps 02 00086 i003 Mps 02 00086 i003]() PAUSE STEP
PAUSE STEP Slides may remain in xylene and reaction buffer steps for extended periods without damaging the tissue.
4. Expected Results
The method described in this study utilized forty-nine sample H&E-stained resection and CNB slides that were analyzed and commented on by a board-certified pathologist for normal or neoplastic status, Gleason grade, preservation status, and any distinguishing features for the categorization of potential aggressiveness. The initial testing was accomplished using DAB IHC detection kits to determine retention of marker stain intensity. During the initial stages of this study, multiple test samples demonstrated lower intensities as a result of utilizing an un-optimized protocol (data not shown). However, continued editing and updates to the initial procedure on re-utilized H&E index slides resulted in viable stain intensity, demonstrating the feasibility of the procedure and potential for optimization to culminate in stain intensity comparable to that of sequential slides utilizing standard procedures (
Figure 2).
The initial testing procedures resulted in moderate but visible stain intensity providing proof-of-concept. At this stage, further optimization and repeat testing was warranted to increase the stain intensity to comparable levels of those occurring using the standard antibody staining methods and to ensure reproducibility. The procedure methods were improved by four steps: 1. Applying timed reagent rinse procedures at the xylene, ethanol (EtOH), and Ventana/RTD proprietary reaction buffer steps (1-min rinse times between each hold); 2. Increasing EtOH and reaction buffer reagent rinses from 1 rinse to 5–6 and 3–4 manual rinses respectively for optimal efficiency; 3. Including an approximate 5-min drying step after the reaction buffer rinse to limit residual excess reagent interference in the online application of biomarkers; and 4. Editing online cell conditioning steps (for heat induced antigen retrieval) to reduce potential epitope destruction.
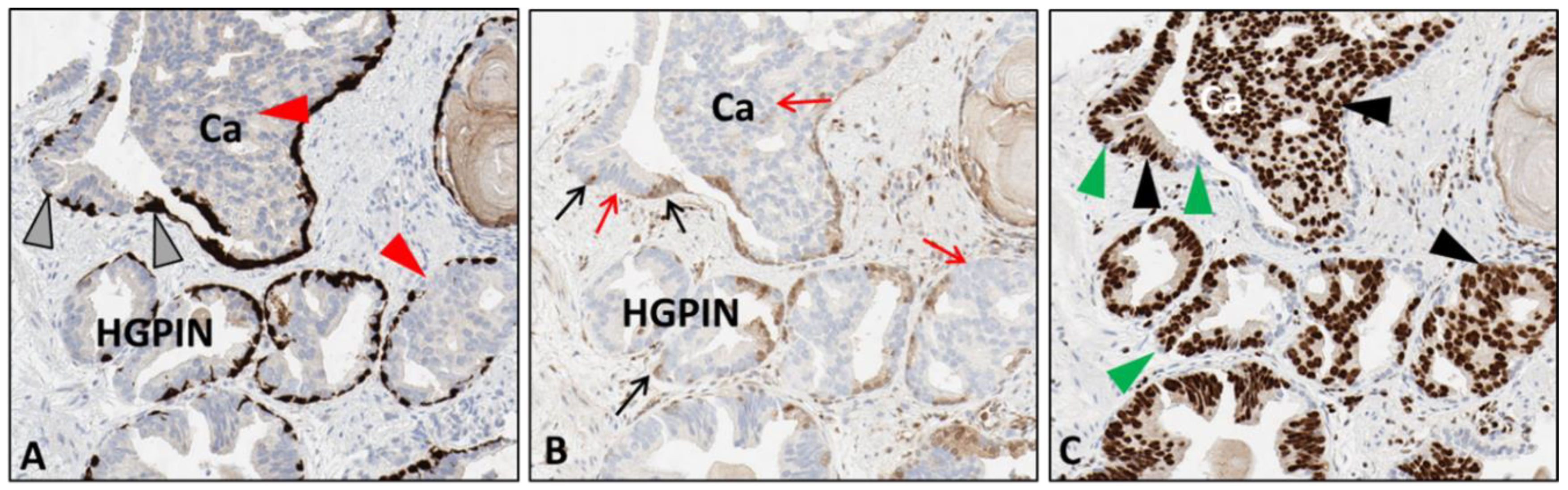
This procedure optimization was considered the standard when applied to any H&E-stained slide stored for up to 2 years but needed further optimization for tissues stored for periods 2 years or longer. The subsequent experimentation steps employed the use of antibodies targeting PTEN and ERG biomarkers, VENTANA anti-PTEN (SP218) mouse monoclonal antibody, and anti-ERG (EPR3864) rabbit monoclonal antibody. These validated markers were used since they demonstrate 1. the heterogeneous variability of aggressive prostate cancer and 2. the comparative expression of PTEN loss and ERG expression in aggressive tumors. In this procedure, steps (1–8) represent the optimized H&E de-staining procedures. However, during the testing, unforeseen scheduling resulted in slight deviations (extended reagent HOLD times) in steps 4 and 7 that lead to the determination that certain steps, which were the xylene and reaction buffer HOLD times, could be amended without incurring damage to samples. The updated procedure, which only involved an extended xylene hold time and is essentially the same optimized procedure, resulted in comparable stain intensity to the standard protocol and allowed the ability of distinct determination of aggressive tumor areas (
Figure 3). After the successful completion of a sequential round of experimentation using IHC DAB, we tested whether antibodies targeting multiple biomarkers could be applied for detection with the use of chromogenic detection reagents. Again, prostate adenocarcinoma CNBs were used as experimental specimens for the de-stain and re-stain procedure. The antibodies chosen were specific for the integrin α6 (CD49f) laminin-binding domain and HMWCK + p63. These markers were chosen due to known membranous expression levels (CD49f) and cytoplasmic and nuclear (HMWCK + p63 respectively) positive expression levels in non-neoplastic basal cells of prostate tissue. In PCa, CD49f expression is membranous and aggressive and invasive disease exhibits an intracellular expression pattern [
14,
15,
16,
17,
18]. It is also associated with poor patient prognosis, reduced survival, and increased metastasis [
19,
20,
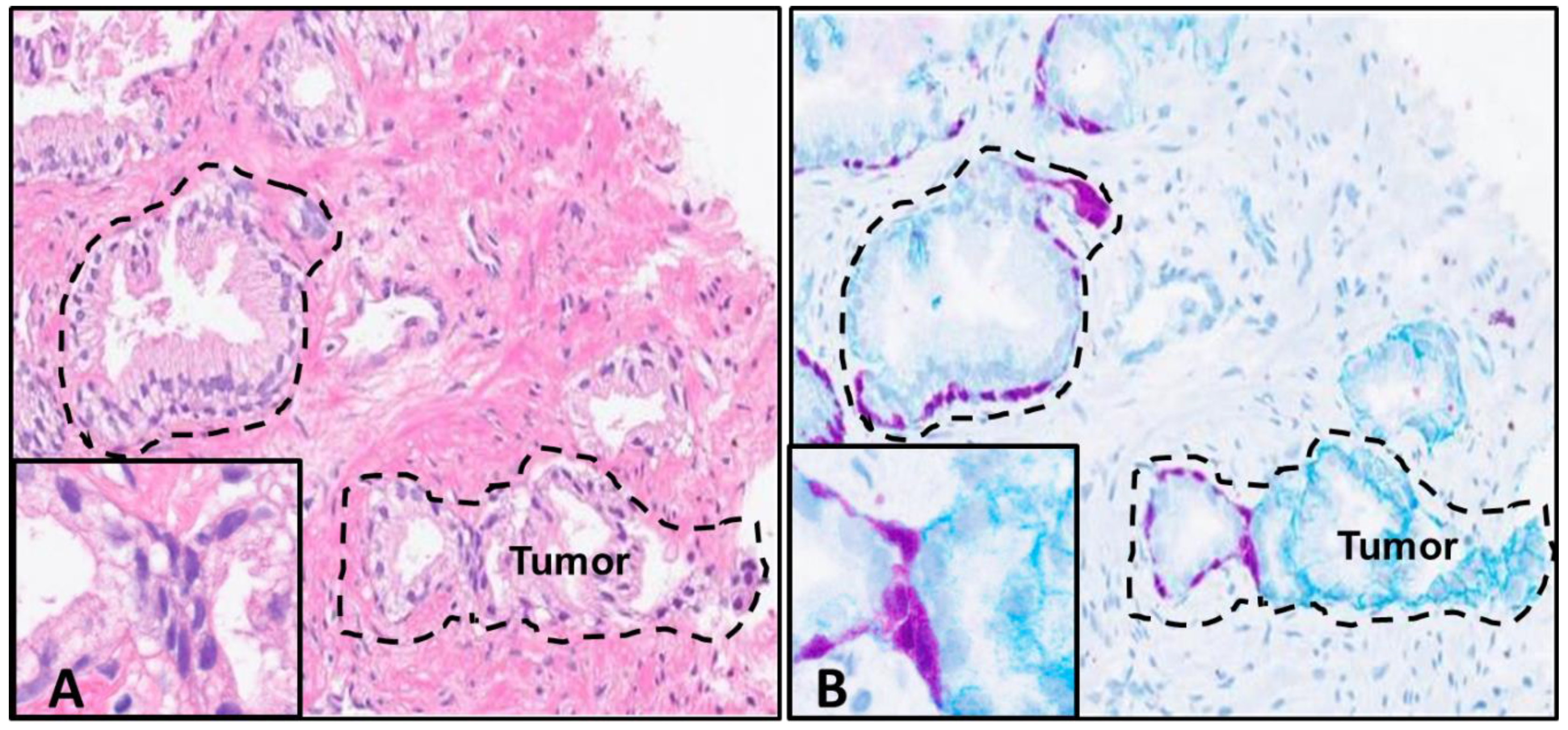
21]. These markers were not expected to colocalize but to demonstrate the expression pattern of both non-neoplastic and neoplastic structures and focal areas in tissue after marker application, following the de-stain procedure.
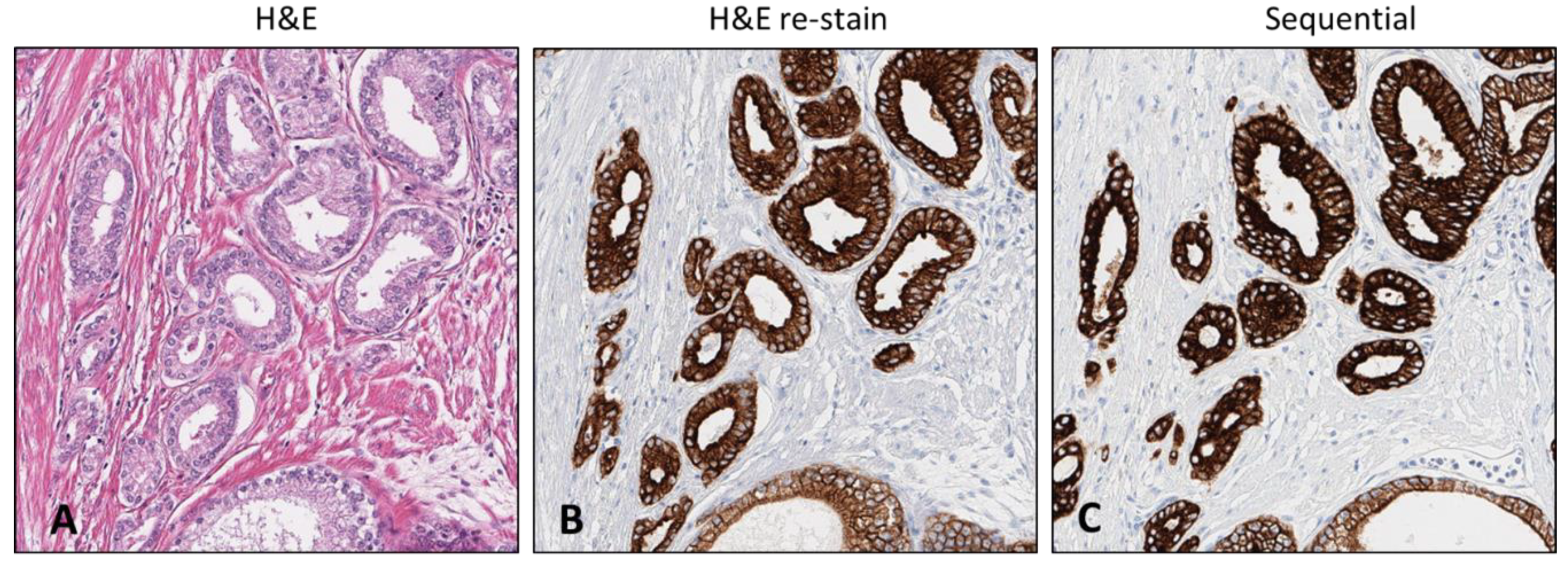
The expected outcome was to demonstrate definitive areas of non-neoplastic vs. tumor regions with the application of antibodies and chromogen detection. This would allow the simultaneous detection of normal and aggressive structures in one tissue sample after pathologist analysis of the H&E-stained slide, allowing for the potential utilization of one slide. The results demonstrate strong stain intensities for both targets and well-defined areas of demarcation of non-neoplastic vs. tumor structures. As expected, both markers are visible in normal basal cells of normal prostate glands (although the HMWCK + p63 stain intensity primarily masks the CD49f signal in those areas), but CD49f antibody clearly displays an intracellular and cytoplasmic expression in the areas of budding tumor (
Figure 4).
These positive results from testing samples archived up to 2 years warranted the evaluation of the potential ability of this procedure to be utilized with other tissue types, for H&E stained slides archived 2 years or more, and samples archived utilizing glass coverslips. Therefore, five archived PCa CNBs (2 years 11 months), a normal colon (2 years 1 month), liver and lung samples (4 years) along with 4-plus year (4+) PCa resection (4 years 11 months) H&E stained sample slides sealed with thin film coverslip were tested. For the testing of H&E stained slides sealed with glass coverslips, archived PCa CNBs (2 years 11 months), skin samples (5 years) and a PCa TMA sample (12 years) were tested. During the execution of this procedure, the removal of the coverslip was determined to be a limiting factor. Therefore, the parameters involved with the removal was tracked and recorded in this report (
Table 2). After the removal of the H&E stain, the sample slides were re-stained with selected antibodies utilizing optimized protocols adapted for IHC on de-stained H&E slides (
Table 3).
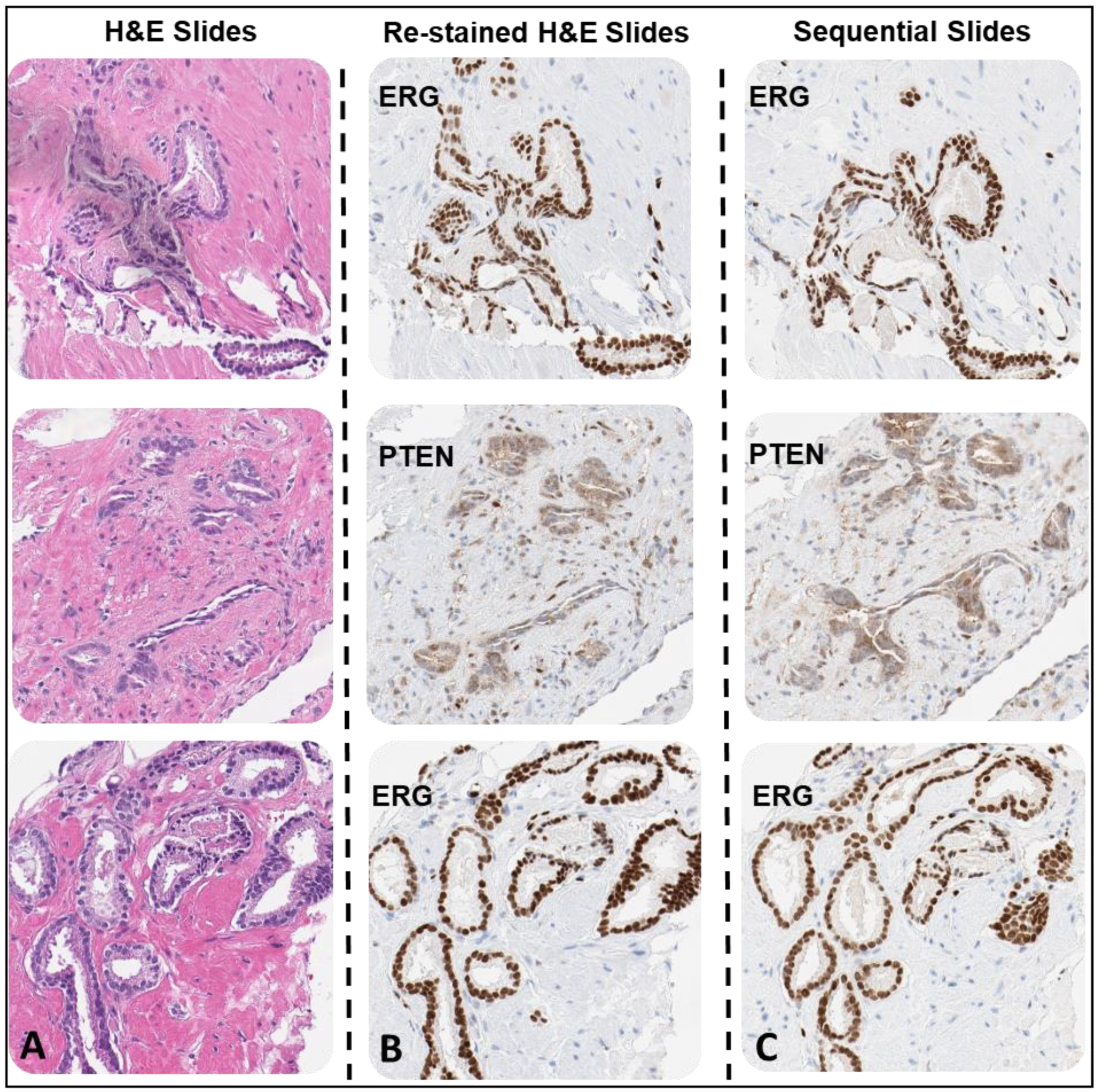
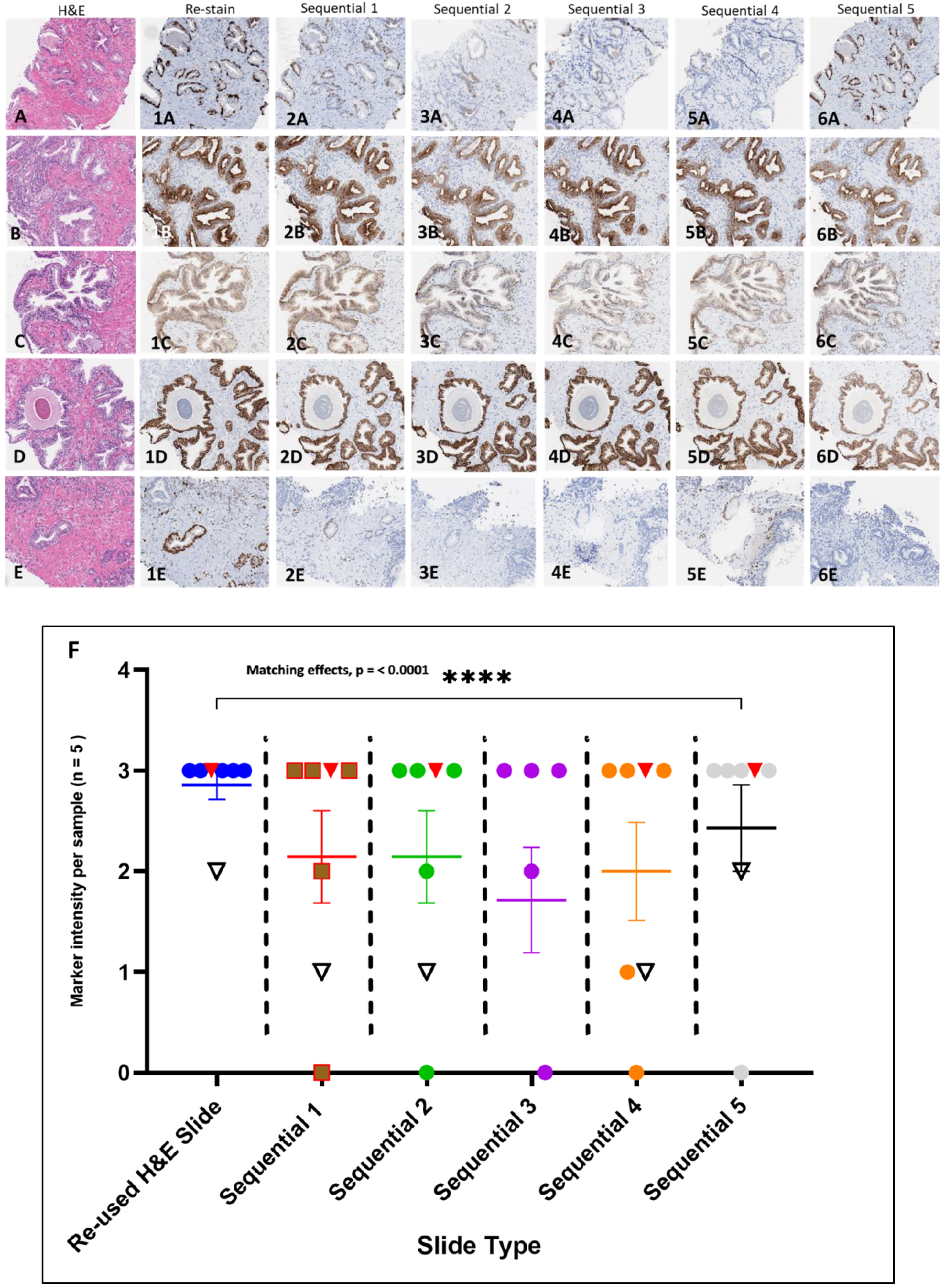
The testing of the H&E stained slide samples archived 2 years or more involved extended coverslip removal and reagent rinse times (2+, 4, 4+, 5, and 12-year archived samples) which indicated that archival time, storage condition and coverslip type may play a factor in slide processing with the procedure. The processing of 2-year archived H&E stained slides sealed with thin film (all PCa CNBs) only required minimal extension time of coverslip removal (to ~60 min) but resulted in H&E stain removal and comparable antibody immunostaining intensities compared to the corresponding sequential slides (
Figure 5A–6E). The reused H&E and corresponding sequential slides were evaluated by a board-certified pathologist in a side by side comparison for immunostaining intensity (
Table 4). The histopathologic analysis focused on any present tumor or normal regions for intensity. The data analysis indicates that there was a significant matching in the immunostaining intensities between the reused H&E stained slides and sequential comparator slides immunostained with the various antibodies (
Figure 5F).
The processing of the 4 and 4+ year archived H&E stained slides (PCa resections) sealed with thin film coverslip required extended time of coverslip removal (~38 and 47 h) but resulted in H&E stain removal. The archived H&E stained slides sealed with glass coverslip for 2+, 5 and 12-year (PCa resection, two skin and PCa TMA) required 1–2 days and 4–5 days for coverslip removal. The 4+ year archived sample resulted in comparable intensity (CK 8 &18) to the sequential comparator slide (
Figure 6). The reused 12-year archived PCa TMA H&E stained slide immunostained with Ventana anti-ERG resulted in immunostaining but intensity was variable across different cores but demonstrated feasibility (data not shown). The reused 5-year archived H&E stained slides (skin resections) sealed with glass coverslips also required extended time for coverslip removal and reagent rinses. The resulting H&E stain removal exhibited residual H&E stain on the slides resulting in incomplete immunostaining (ERG) (data not shown). The resulting retention of the hematoxylin and eosin on the slides may have potentially impacted the results and will need further inquiry on storage conditions to ascertain steps to mitigate any issue. We found that the storage conditions of older H&E stained slides (particularly with glass coverslips) caused extensive adhesion of the coverslip to the tissue slide due to the extended time in storage, requiring a slight extension of extraction procedures. In addition, we observed that pre-analytics impacted H&E removal, resulting in some residual retention. Unfortunately, due to age of the slide, pre-analytical data was not available.
5. Discussion
When patients are suspected of having PCa, a tissue sample is required for diagnosis. The sampling of the potentially neoplastic area may be assisted through means of ultrasound (US) or multiparametric magnetic resonance imaging (mpMRI) guided techniques. Sample resections and needle biopsies are routinely formalin-fixed and processed and embedded for histological sampling then stained for H&E and IHC, allowing pathologists to analyze an excised patient tissue sample from the affected area after diagnosis to differentiate between cancer and non-neoplastic events, such as benign prostatic hyperplasia. The H&E-stained slide plays a critical role in assisting the diagnosis of the pathologist in corroborating the initial findings with MRI and US procedures. Traditionally, after pathologist analysis and diagnosis, the samples can then be processed with biomarkers targeting detection of epitopes that are overexpressed in aggressive tumors. Currently this is the standard procedure deployed in companion diagnostics that allows for the stratification of patients who may benefit from a specific therapeutic intervention.
The accurate evaluation of biomarkers with these samples is critical for patient diagnosis, particularly with smaller samples, such as CNBs, fine needle aspirates, and potentially transurethral resection of prostate samples (TURPS). The smaller size of these tissue samples limits tissue availability and requires precise testing for important results. Loss of available tissue slides is a risk that could be mitigated with the use of H&E slide de-stain and re-stain procedures. The potential to detect multiple markers using chromogenic multiplexing on a single indexed tissue slide that had been analyzed and diagnosed by a pathologist to definitively contain aggressive tumor, leaves open the possibility of predictive companion diagnostics with minimal sampling. This may provide the opportunity for a one sample/one result diagnosis limiting the invasive nature of tissue specimen collection, which benefits the patient greatly.
There are few reports that provide instructions for removal of the H&E staining that leaves the target epitopes intact for potential reuse of the slide for selective biomarkers. Current existing protocols (and forums) only discuss de-stain procedures for slides that have stained inadequately, or have been stained with excessive hematoxylin and have lengthy protocol steps that may extend the procedure hours to days. Others may require the use of more corrosive reagents (% HCL solutions). Procedures utilizing either beta-mercaptoethanol/sodium dodecyl sulfate (2ME/SDS), 6 guanidinium hydrochloride (GnHCL) or 6 M Urea have been demonstrated to elute antibodies from immunostained tissues on positively charged glass slides (or glass coverslips) for sequential antibody re-stain [
22,
23]. However, these methods focused on the removal of the bound primary antibody and the reagents used were not intended to remove the H&E stain. For this report, an innovative method utilizing non-corrosive reagents was created and applied in a particular procedure using these reagents in sequence that optimized the H&E slide de-stain. This procedure removed the majority of the visible stain while retaining tissue integrity and morphology and allowed the preparation of specified IHC protocol to re-stain the sample sides. The primary tissue sample used for initial testing was prostate adenocarcinoma, however, this will translate to other tissues.
This study utilized liver, colon, skin and PCa resections and CNB samples for procedure testing. The study included the addition of antibodies detecting clinically relevant biomarkers such as PTEN, ERG, E-cadherin, Racemase (p504s), cytokeratin 8 and 18 and the CD49f protein for potential indication of aggressiveness and antibodies against HMWCK cocktailed with a p63 marker (a p53 homologue containing the N-terminal transactivation domain) as well as the variant p40 marker (lacking the N-terminal domain), that will detect the presence of normal basal cells of prostatic glands. These antibodies were critical in detection of differentiating prostatic adenocarcinomas vs. the detection of non-neoplastic prostatic tissue, as well as determination of intracellular marker activity and basal cell attenuation, respectively. Moreover, during this study the positive outcome from testing various tissue samples archived beyond 4 years utilizing thin film and 12 years with glass coverslips yielded promising results indicating tissue epitopes remain stable on H&E stained slides archived at a minimum of 4 years. This indicates that the procedure may be useful for the interrogation of other clinically relevant proteins in tissues other than prostate and for H&E-stained slides stored for longer periods of time. However, the conditions of the slide storage and the type of adhesives applied to seal the slide may have an impact on results. Another factor may be the specific antibody selected for each specific study. The antibodies used for this study yielded promising results but each antibody demonstrates various qualities, therefore, continued optimization may be warranted for this procedure.
Further experimentation will be repeated involving archived specimen slides utilizing film coverslips that have been stored for 4 years and more, as well as continued interrogation of samples sealed with glass coverslips. This will determine the robustness of the procedure to encompass reproducible testing of samples from decades past to incorporate newly discovered targets to test protein expression that may offer answers to questions that may have remained unsolved. Moreover, with the development of newer chromogen dyes, the possibility of utilizing one slide for multiple markers may now become a distinct possibility saving valuable time and resources. The results demonstrated in this report can be considered the first step towards a more extensive study incorporating much larger cohorts that may ultimately utilize this procedure as a viable tool in cancer diagnosis and treatments.
Author Contributions
Conceptualization, design, J.P.H., A.E.C., and R.B.N.; development of methodology, J.P.H.; experiments, J.P.H.; acquisition of sample tissue, J.P.H., R.B.N., H.A.T.; data analysis and interpretation, J.P.H., J.C.G., R.B.N.; writing-original draft preparation, J.P.H.; writing-review and/or manuscript editing, J.P.H., K.D., E.R., R.B.N.; administrative, technical, or material support, W.J.F., J.C.G., A.E.C., R.B.N.; study supervision, A.E.C.; R.B.N.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA023074 and supported by Institutional Research Grant number IRG-16-124-37- IRG from the American Cancer Society.
Acknowledgments
We thank the TACMASR core service of the University of Arizona Cancer Center for providing core needle biopsies samples. We would like to acknowledge the Ventana/RTD Integrated Core (iCore) services and Scott Gill for providing tissue sample resections for testing as well as Angela Schumacher and Adrian Murillo for providing reagents and chromogen detection kits. We would also like to thank William Harryman for editorial assistance.
Conflicts of Interest
J.P.H., K.D., E.R., W.J.F. and J.C.G. are paid employees of Ventana/Roche Tissue Diagnostics. A.E.C, H.A.T. and R.B.N. have no conflicts to declare.
References
- Bancroft, J.D.; Layton, C. The Hematoxylin and Eosin. In Theory Practice of Histological Techniques, 7th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Oxford, UK, 2013; pp. 173–186. [Google Scholar] [CrossRef]
- Chan, J.K.C. The Wonderful Colors of the Hematoxylin–Eosin Stain in Diagnostic Surgical Pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008. [Google Scholar] [CrossRef]
- Titford, M. The Long History of Hematoxylin. Biotech. Histochem. 2005, 80, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology: Methods and Protocols; Day, C.E., Ed.; Springer: New York, NY, USA, 2014; Volume 1180, pp. 31–43. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Feldman, M.D.; Abels, E.; Ashfaq, R.; Beltaifa, S.; Cacciabeve, N.G.; Cathro, H.P.; Cheng, L.; Cooper, K.; Dickey, G.E.; et al. Whole Slide Imaging Versus Microscopy for Primary Diagnosis in Surgical Pathology: A Multicenter Blinded Randomized Noninferiority Study of 1992 Cases (Pivotal Study). Am. J. Surg. Pathol. 2018, 42. [Google Scholar] [CrossRef] [PubMed]
- Zarella, M.D.; Bowman, D.; Aeffner, F.; Farahani, N.; Xthona, A.; Absar, S.F.; Parwani, A.; Bui, M.; Hartman, D.J. A Practical Guide to Whole Slide Imaging: A White Paper from the Digital Pathology Association. Arch. Pathol. Lab. Med. 2018, 143, 222–234. [Google Scholar] [CrossRef]
- Ayala, G.; Frolov, A.; Chatterjee, D.; He, D.; Hilsenbeck, S.; Ittmann, M. Expression of ERG Protein in Prostate Cancer: Variability and Biological Correlates. Endocr. Relat. Cancer 2015, 22, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Mehra, R.; Lonigro, R.J.; Wang, L.; Suleman, K.; Menon, A.; Palanisamy, N.; Tomlins, S.A.; Chinnaiyan, A.M.; Shah, R.B. Fluorescence in Situ Hybridization Study Shows Association of PTEN Deletion with ERG Rearrangement during Prostate Cancer Progression. Mod. Pathol. 2009, 22, 1083. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical Implications of PTEN Loss in prostate Cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Leinonen, K.A.; Saramaki, O.R.; Furusato, B.; Kimura, T.; Takahashi, H.; Egawa, S.; Suzuki, H.; Keiger, K.; Ho Hahm, S.; Isaacs, W.B.; et al. Loss of PTEN is associated with aggressive behavior in ERG-Positive Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2333–2344. [Google Scholar] [CrossRef]
- Nagle, R.B.; Algotar, A.M.; Cortez, C.C.; Smith, K.; Jones, C.; Sathyanarayana, U.G.; Yun, S.; Riley, J.; Nagy, D.; Dittamore, R.; et al. ERG overexpression and PTEN status predict capsular penetration in prostate carcinoma. Prostate 2013, 73, 1233–1240. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Joshua, A.M.; Cunha, I.W.; Coudry, R.A.; Fonseca, F.P.; Ludkovski, O.; Zielenska, M.; Soares, F.A.; Squire, J.A. Absence of TMPRSS2: ERG Fusions and PTEN Losses in Prostate Cancer Is Associated with a Favorable Outcome. Mod. Pathol. 2008, 21, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Ports, M.O.; Nagle, R.B.; Pond, G.D.; Cress, A.E. Extracellular Engagement of 6 Integrin Inhibited Urokinase-Type Plasminogen Activator-Mediated Cleavage and Delayed Human Prostate Bone Metastasis. Cancer Res. 2009, 69, 5007–5014. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Anderson, T.A.; Gard, J.M.C.; Sroka, I.C.; Strautman, S.R.; Nagle, R.B.; Morrissey, C.; Knudsen, B.S.; Cress, A.E. Characterization of Laminin Binding Integrin Internalization in Prostate Cancer Cells. J. Cell. Biochem. 2017, 118, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Harryman, W.L.; Hinton, J.P.; Rubenstein, C.P.; Singh, P.; Nagle, R.B.; Parker, S.J.; Knudsen, B.S.; Cress, A.E. The Cohesive Metastasis Phenotype in Human Prostate Cancer. Biochim. Biophys. Acta 2016, 1866, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Sroka, I.C.; Anderson, T.A.; McDaniel, K.M.; Nagle, R.B.; Gretzer, M.B.; Cress, A.E. The laminin binding integrin alpha6beta1 in prostate cancer perineural invasion. J. Cell. Physiol. 2010, 224, 283–288. [Google Scholar] [CrossRef]
- Sroka, I.C.; Chopra, H.; Das, L.; Gard, J.M.; Nagle, R.B.; Cress, A.E. Schwann Cells Increase Prostate and Pancreatic Tumor Cell Invasion Using Laminin Binding A6 Integrin. J. Cell. Biochem. 2016, 117, 491–499. [Google Scholar] [CrossRef]
- Friedrichs, K.; Ruiz, P.; Franke, F.; Gille, I.; Terpe, H.-J.; Imhof, B.A. High Expression Level of α6 Integrin in Human Breast Carcinoma Is Correlated with Reduced Survival. Cancer Res. 1995, 55, 901. [Google Scholar]
- Landowski, T.H.; Gard, J.; Pond, E.; Pond, G.D.; Nagle, R.B.; Geffre, C.P.; Cress, A.E. Targeting Integrin 6 Stimulates Curative-Type Bone Metastasis Lesions in a Xenograft Model. Mol. Cancer Ther. 2014, 13, 1558–1566. [Google Scholar] [CrossRef]
- Stewart, R.L.; West, D.; Wang, C.; Weiss, H.L.; Gal, T.; Durbin, E.B.; O’Connor, W.; Chen, M.; O’Connor, K.L. Elevated integrin α6β4 expression is associated with venous invasion and decreased overall survival in non-small cell lung cancer. Hum. Pathol. 2016, 54, 174–183. [Google Scholar] [CrossRef]
- Bolognesi, M.M.; Manzoni, M.; Scalia, C.R.; Zannella, S.; Bosisio, F.M.; Faretta, M.; Cattoretti, G. Multiplex Staining by Sequential Immunostaining and Antibody Removal on Routine Tissue Sections. J. Histochem. Cytochem. 2017, 65, 431–444. [Google Scholar] [CrossRef]
- Gendusa, R.; Scalia, C.R.; Buscone, S.; Cattoretti, G. Elution of High-affinity (>10−9 KD) Antibodies from Tissue Sections: Clues to the Molecular Mechanism and Use in Sequential Immunostaining. J. Histochem. Cytochem. 2014, 62, 519–531. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 CRITICAL STEP Cover the container of acetone with either container lid or parafilm to reduce acetone evaporation (evaporation reduce effectiveness of coverslip removal).
CRITICAL STEP Cover the container of acetone with either container lid or parafilm to reduce acetone evaporation (evaporation reduce effectiveness of coverslip removal). CRITICAL STEP Cover with caps or paraffin between rinses to reduce reagent evaporation. Moderately rinse slides during hold times to expedite eosin removal.
CRITICAL STEP Cover with caps or paraffin between rinses to reduce reagent evaporation. Moderately rinse slides during hold times to expedite eosin removal. PAUSE STEP Slides may remain in xylene and reaction buffer steps for extended periods without damaging the tissue.
PAUSE STEP Slides may remain in xylene and reaction buffer steps for extended periods without damaging the tissue.