An Inexpensive Staining Alternative for Gelatin Zymography Gels
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
2.1.1. Reagents
- Heat-inactivated fetal bovine serum (Gibco Life Technologies, Paisley, UK; # 10500)
- Prestained protein standard (New England BioLabs, Frankfurt am Main, Germany; # P7719G)
- Coomassie brilliant blue R-250 (Sigma-Aldrich, Vienna, Austria; # 27816-25G)
- Acetic acid (Sigma-Aldrich, Vienna, Austria; # 33209-1L-M)
- Ethanol (Sigma-Aldrich, Vienna, Austria; # 32221-1L-M)
- Water (Sigma-Aldrich, Vienna, Austria; # 38796-1L)
- Glycerol (Sigma-Aldrich, Vienna, Austria; # G7757-1L-M)
- Ponceau S (Sigma-Aldrich, Vienna, Austria; # P3504-10G)
- Gelatin from bovine skin, type B (Sigma-Aldrich, Vienna, Austria; # G9391)
- Triton X-100 (Sigma-Aldrich, Vienna, Austria; # T8787-100ML)
- 1 M Trizma hydrochloride buffer solution (Sigma-Aldrich, Vienna, Austria; # 93313-1L)
- 2 M zinc sulfate solution (Sigma-Aldrich, Vienna, Austria; # 83265-250ML-F)
- Sodium dodecyl sulfate solution (Sigma-Aldrich, Vienna, Austria; # 05030-500ML-F)
- 1.5 M Tris pH 8.8 (Bio-Rad Laboratories, Vienna, Austria; # 161-0798)
- 0.5 M Tris pH 6.8 (Bio-Rad Laboratories, Vienna, Austria; # 161-0799)
- 30% acrylamide/bis solution, 29:1 (Bio-Rad Laboratories, Vienna, Austria; # 161-0157)
- 4× Laemmli sample buffer (Bio-Rad Laboratories, Vienna, Austria; # 161-0747)
- 10× Tris glycine SDS electrophoresis buffer (Bio-Rad Laboratories, Vienna, Austria; # 161-0772)
- Ammonium persulfate (Bio-Rad Laboratories, Vienna, Austria; # 161-0700)
- TEMED (Bio-Rad Laboratories, Vienna, Austria; # 161-0800)
- Calcium chloride (Carl Roth GmbH, Karlsruhe, Germany; # CN93.1)
2.1.2. Solutions
| 1.5 M Tris pH 8.8 | 2 mL |
| 30% Acrylamide/Bis Solution, 29:1 | 2 mL |
| Deionized water | 3 mL |
| Gelatin (10 mg/mL) | 1 mL |
| 10% SDS | 0.08 mL |
| 10% APS | 0.08 mL |
| TEMED | 0.01 mL |
| 0.5 M Tris pH 6.8 | 0.625 mL |
| 30% Acrylamide/Bis Solution, 29:1 | 0.335 mL |
| Deionized water | 1.5 mL |
| 10% SDS | 0.025 mL |
| 10% APS | 0.025 mL |
| TEMED | 0.005 mL |
| Triton X-100 | 25 mL |
| 1 M Tris-HCl, pH 7.5 | 50 mL |
| 1 M CaCl2 | 5 mL |
| 2 M ZnSO4 | 1 μL |
| Deionized water | 920 mL |
| Triton X-100 | 0.5 mL |
| 1 M Tris-HCl, pH 7.5 | 50 mL |
| 1 M CaCl2 | 5 mL |
| 2 M ZnSO4 | 1 μL |
| Deionized water | 944.5 mL |
| Coomassie brilliant blue R | 0.3 g |
| Acetic acid | 2 mL |
| Ethanol | 90 mL |
| Deionized water | 108 mL |
| Acetic acid | 50 mL |
| Ethanol | 125 mL |
| Deionized water | 325 mL |
| Ponceau S | 0.25 g |
| Acetic acid | 10 mL |
| Deionized water | 190 mL |
| Glycerol | 6 mL |
| Ethanol | 60 mL |
| Deionized water | 134 mL |
| Glycerol | 6 mL |
| Deionized water | 194 mL |
2.2. Equipment
- Centrifuge 5424R (Eppendorf Austria GmbH, Vienna, Austria; # 5404000410)
- Water bath (GFL Gesellschaft für Labortechnik mbH, Burgwedel, Germany; # 1013)
- Eppendorf Research plus Micropipettes (VWR International GmbH, Vienna, Austria; # 613-1143)
- Orbital shaker (GFL Gesellschaft für Labortechnik mbH, Burgwedel, Germany; # 3017)
- PowerPac™ HC High-Current Power Supply (Bio-Rad Laboratories, Vienna, Austria; # 164-5052)
- Mini-PROTEAN® Tetra Cell (Bio-Rad Laboratories, Vienna, Austria; # 1658000)
- Gel drying frames (Sigma-Aldrich, Vienna, Austria; # Z377597)
- Vilber Lourmat E-Box gel documentation imaging system (VWR International GmbH, Vienna, Austria; # 730-1486)
3. Procedure
3.1. Sample Preparation
- Take sample and clear by centrifugation (10,000 g/5 min at room temperature).
- Transfer supernatant into new tube and prepare samples with loading buffer (w/o β-mercaptoethanol) according to manufacturer’s instructions.
- Incubate samples at room temperature for 10 min; do not heat denature the samples as this would inactivate enzymatic activities of the proteases.
- Load samples onto SDS-gelatin-polyacrylamide gel.
3.2. Perform Gel Electrophoresis
3.3. Gelatinase Activation/Incubation
- After electrophoreses, carefully remove the gel from the electrophoretic plates.
- Place the gel in a container and wash 3 times with ~50 mL gelatinase renaturation buffer for 20 min each while agitating the container.
- Incubate the gel with ~50 mL gelatinase reaction buffer in a container at 37 °C for 18–24 h.
3.4. Gel Staining
3.4.1. Option A Coomassie Gel Staining and Destaining
- Stain the gel with 20 mL Coomassie gel staining solution for 1 h at room temperature; use a shaker for even distribution of the staining solution.
- Destain the gel with Coomassie gel destaining solution for at least 30 min until clear bands are visible; change the destaining solution at least 4 times with 25 mL each.
3.4.2. Option B Ponceau Gel Staining and Destaining
- Stain the gel with 20 mL Ponceau gel staining solution for 1 h at room temperature; use a shaker for even distribution of the staining solution.
- Destain the gel with water for at least 30 min until clear bands are visible; change the water at least 4 times with 25 mL each.
3.5. Gel Scan and Quantification
- Seal the gel in a plastic wrap.
- Immediately scan the gel with a digital scanner (include gelatinase standards for normalization purposes if necessary).
- Quantify the integrated density values of the gelatinase bands using NIH ImageJ or a similar software.
3.6. Archiving
- Equilibrate gels with ~50 mL of the appropriate gel preservation solution for 15 min.
- Pre-wet cellophane sheets with deionized water and assemble the gel drying frames according to manufacturer’s instructions.
- Dry the gels overnight.
4. Expected Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conlon, G.A.; Murray, G.I. Recent Advances in Understanding the Roles of Matrix Metalloproteinases in Tumour Invasion and Metastasis. J. Pathol. 2019, 247, 629–640. [Google Scholar] [PubMed]
- Gargiulo, S.; Gamba, P.; Poli, G.; Leonarduzzi, G. Metalloproteinases and Metalloproteinase Inhibitors in Age-Related Diseases. Curr. Pharm. Des. 2014, 20, 2993–3018. [Google Scholar] [PubMed] [Green Version]
- Malemud, C.J. Matrix Metalloproteinases (Mmps) in Health and Disease: An Overview. Front. Biosci. A J. Virtual Libr. 2006, 11, 1696–1701. [Google Scholar]
- Bencsik, P.; Bartekova, M.; Görbe, A.; Kiss, K.; Pálóczi, J.; Radosinska, J.; Szűcs, G.; Ferdinandy, P. Mmp Activity Detection in Zymograms. Methods Mol. Biol. 2017, 1626, 53–70. [Google Scholar] [PubMed]
- Kupai, K.; Szucs, G.; Cseh, S.; Hajdu, I.; Csonka, C.; Csont, T.; Ferdinandy, P. Matrix Metalloproteinase Activity Assays: Importance of Zymography. J. Pharmacol. Toxicol. Methods 2010, 61, 205–209. [Google Scholar] [PubMed]
- Miura, R.O.; Yamagata, S.; Miura, Y.; Harada, T.; Yamagata, T. Analysis of Glycosaminoglycan-Degrading Enzymes by Substrate Gel Electrophoresis (Zymography). Anal. Biochem. 1995, 225, 333–340. [Google Scholar] [PubMed]
- Vandooren, J.; Geurts, N.; Martens, E.; Van Den Steen, P.E.; Opdenakker, G. Zymography Methods for Visualizing Hydrolytic Enzymes. Nat. Methods 2013, 10, 211–220. [Google Scholar] [PubMed]
- Wilkesman, J.; Kurz, L. Zymography Principles. Methods Mol. Biol. 2017, 1626, 3–10. [Google Scholar] [PubMed]
- Leber, T.M.; Balkwill, F.R. Zymography: A Single-Step Staining Method for Quantitation of Proteolytic Activity on Substrate Gels. Anal. Biochem. 1997, 249, 24–28. [Google Scholar] [PubMed]
- Harper, S.; Speicher, D.W. Detection of Proteins on Blot Membranes. Curr. Protoc. Protein Sci. 1995, 10.8.1–10.8.7. [Google Scholar] [CrossRef]
- Blakesley, R.W.; Boezi, J.A. A New Staining Technique for Proteins in Polyacrylamide Gels Using Coomassie Brilliant Blue G250. Anal. Biochem. 1977, 82, 580–582. [Google Scholar] [PubMed]
- Yan, S.J.; Blomme, E.A.G. In Situ Zymography: A Molecular Pathology Technique to Localize Endogenous Protease Activity in Tissue Sections. Vet. Pathol. 2003, 40, 227–236. [Google Scholar] [PubMed]
- Sander, H.; Wallace, S.; Plouse, R.; Tiwari, S.; Gomes, A.V. Ponceau S Waste: Ponceau S Staining for Total Protein Normalization. Anal. Biochem. 2019, 575, 44–53. [Google Scholar] [PubMed]
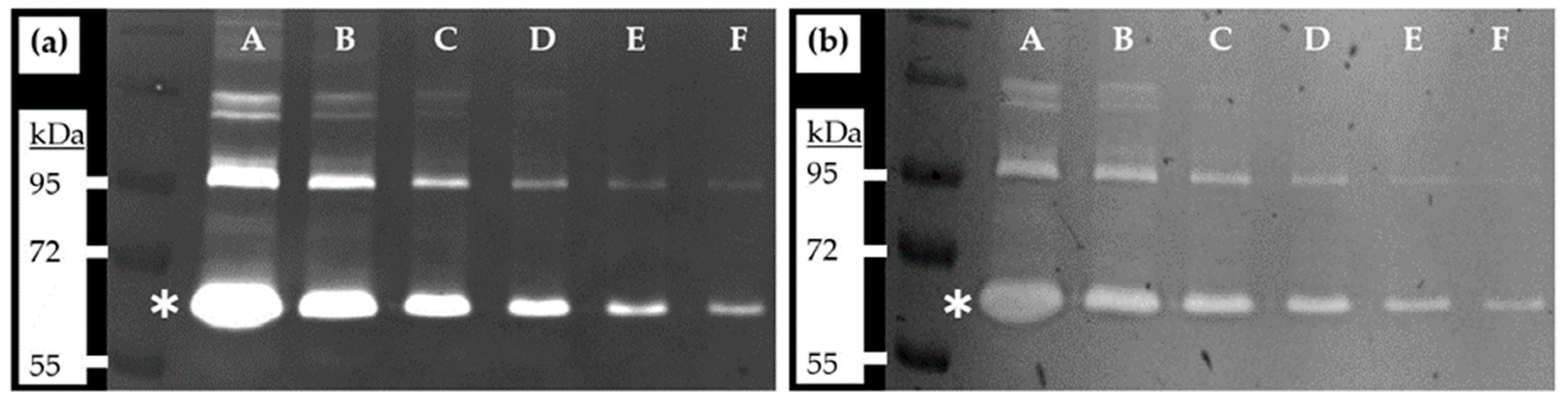
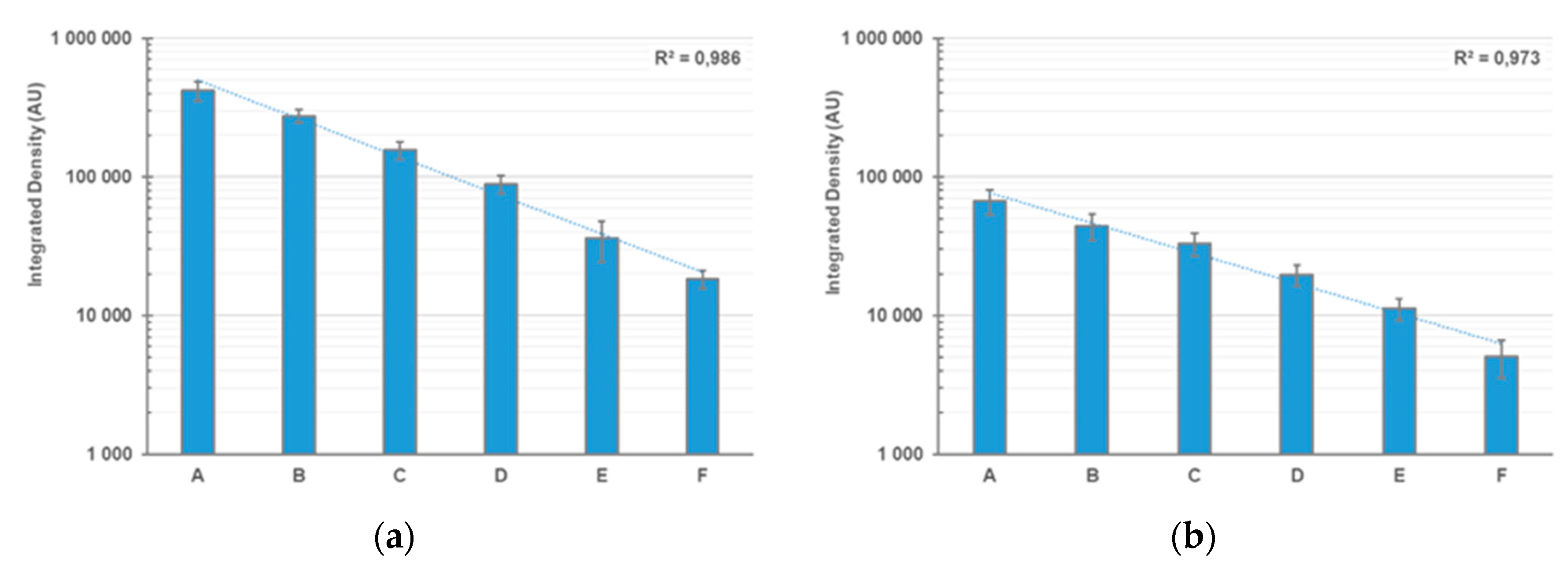
| Sample | FBS Equivalent |
|---|---|
| A | 1 µL |
| B | 0.5 µL |
| C | 0.25 µL |
| D | 0.125 µl |
| E | 0.0625 µL |
| F | 0.03125 µL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wechselberger, C.; Doppler, C.; Bernhard, D. An Inexpensive Staining Alternative for Gelatin Zymography Gels. Methods Protoc. 2019, 2, 61. https://doi.org/10.3390/mps2030061
Wechselberger C, Doppler C, Bernhard D. An Inexpensive Staining Alternative for Gelatin Zymography Gels. Methods and Protocols. 2019; 2(3):61. https://doi.org/10.3390/mps2030061
Chicago/Turabian StyleWechselberger, Christian, Christian Doppler, and David Bernhard. 2019. "An Inexpensive Staining Alternative for Gelatin Zymography Gels" Methods and Protocols 2, no. 3: 61. https://doi.org/10.3390/mps2030061
APA StyleWechselberger, C., Doppler, C., & Bernhard, D. (2019). An Inexpensive Staining Alternative for Gelatin Zymography Gels. Methods and Protocols, 2(3), 61. https://doi.org/10.3390/mps2030061





