Improved Method for DNA Extraction and Purification from Tetrahymena pyriformis
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- SDS 20% solution (Thermo Fisher; Waltham, MA, United States; Cat.no.:AM9820);
- Chelex 100 matrix (BIO-RAD; Hercules, CA, United States; Cat.no.:1421253);
- Tris, 1 M, pH 8.0 (Thermo Fisher; Waltham, MA, United States; Cat. no.: AM9855G);
- Ethylene diamine tetra acetic acid (EDTA, Thermo Fisher Scientific; Waltham, MA, United States; Cat. no.: 17892);
- Ethanol 100% (VWR chemicals, VWR, Radnor, PA, United States; Cat. no.: 64-17-5);
- Proteinase K (Thermo Fisher Scientific; Waltham, MA, United States; Cat. No.: EO0491);
- Agarose molecular grade (Roche, Burgess Hill, United Kingdom; Cat. no.: 11388983001);
- Tris- Ethylenediaminetetraacetic acid (EDTA), (TE, St. Louis, MI, United States; Cat. No.: 99302);
- Restriction endonuclease HindIII (Thermo Scientific, Waltham, MA, United States; Cat.no.:ER0501);
- GeneRuler 1 kb DNA Ladder (Thermo Scientific, Roskilde, Denmark; Cat.no.:SM0311);
- Primers MT (see Table 1);
- Ethidium Bromide (Thermo Fisher; Wilmington, DE, United States; Cat.no.: 10342020);
- Deoxynucleotide (dTNP) mixture (25 mM) (Takara bio; Shiga, Japan; Cat.no.: 4030);
- Taq Polymerase; (Thermo Fisher; Naerum, Denmark; Cat.no.: 10342020);
- Manganese (II) chloride (Thermo Scientific; Karlsruhe, Allemagne, Cat.no.: 7773-01-5);
2.2. Equipment
- Microcentrifuge tubes 1.5 mL (Eppendorf, Hamburg, Germany; Cat. no.: T9661-500EA);
- Pipette-tips, 10 µL, 200 µL, 1 mL (Axygen, Union City, CA, United States; Cat.no.: 14-222-690, 14-222-812 and14-222-737);
- Shaking heat block (Thermomixer, Eppendorf; Hercules, CA, United States, Cat.no.: 363000233);
- Water bath (JULABO TW9; JULABO, Seelbach, Germany; CAT.no.: 9550323);
- Vortex (Genie 2; Scientific Industries, Bohemia, NY, United States; Cat.no.: SI-T236);
- Laboratory scales (Sartorius, Göttingen, Germany; Cat.no.: ENTRIS623i-1S);
- pH meter (HANNA Edge HI2020-02; HANNA, Woonsocket, RI, United States; Cat.no.: HI2020-20);
- Homogenizer (Bullet Blender; Next Advance; Bohemia, NY United States; Cat.no.: 344-0014);
- Autoclave (HIRAYAMA, Saitama, Japan; Cat.no.: 344-0014);
- Microcentrifuge (Eppendorf; Cat. No.: 5424R);
- −20 °C freezer (Thermo scientific™; Goteborg, Sweden; Cat.no.: TSX2320FD);
- 4 °C refrigerator (Nor-Lake™, Goteborg, Sweden; Cat.no.: 22-650-528);
- MilliQ water purification system (Merck Millipore, Burlington, MA, United States; Cat.no.: ZIQ7003T0);
- Gel electrophoresis system (Bio-Rad, Hercules, CA, United States; Cat.no.: 150-0739);
- NanoDrop One Microvolume UV-Vis Spectrophotometers (Thermo Fisher Scientific; Wilmington, DE, United States; Cat.no.: 13-400-519);
- BD Accuri C6 Plus Flow Cytometer (BD Biosciences; Hercules, CA, United States; Cat.no.: 170-3612);
- Qubit fluorometer (Thermo Fisher; Hercules, CA, United States; Cat. no.: Q33226);
- Molecular Imager Gel Doc XR System (BIO-RAD; Hercules, CA, United States; Cat.no.: 1708195EDU);
- Chef-DR II Gel Electrophoresis System (BIO-RAD; Hercules, CA, United States; Cat.no.: 170-3612);
- Polymerase chain reaction (PCR) tubes (Axygen; Hercules, CA, United States; Cat. no.: 14-222-262);
- Rotaphor (Biometra; Gottingen, Germany; Cat.no.: 846-021-101);
- Thermocycler (Super Cycler SC300G, Kyratec, Mansfield, Australia; Cat.no.: SC-200);
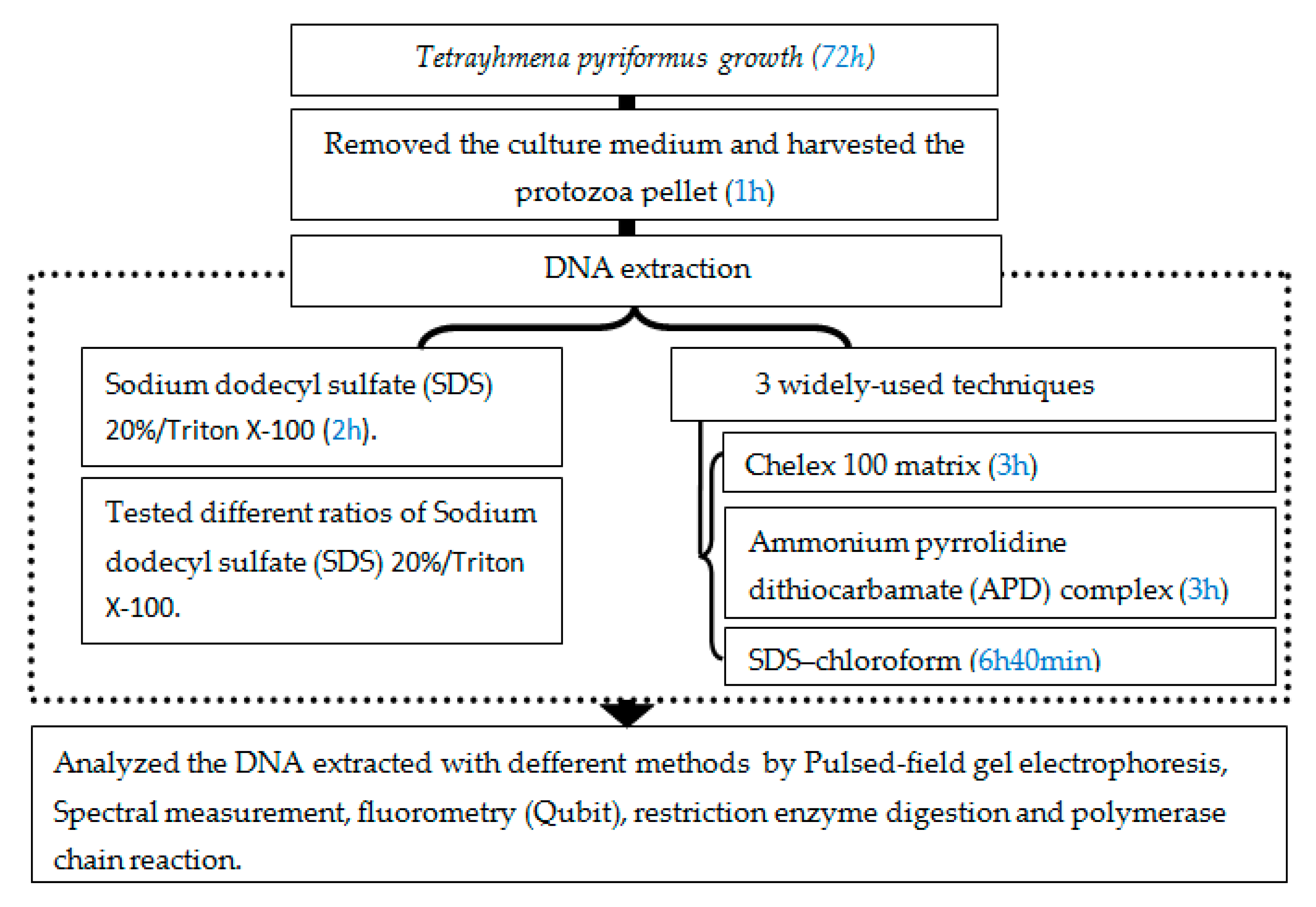
3. Procedure
3.1. Strains and Culture Conditions
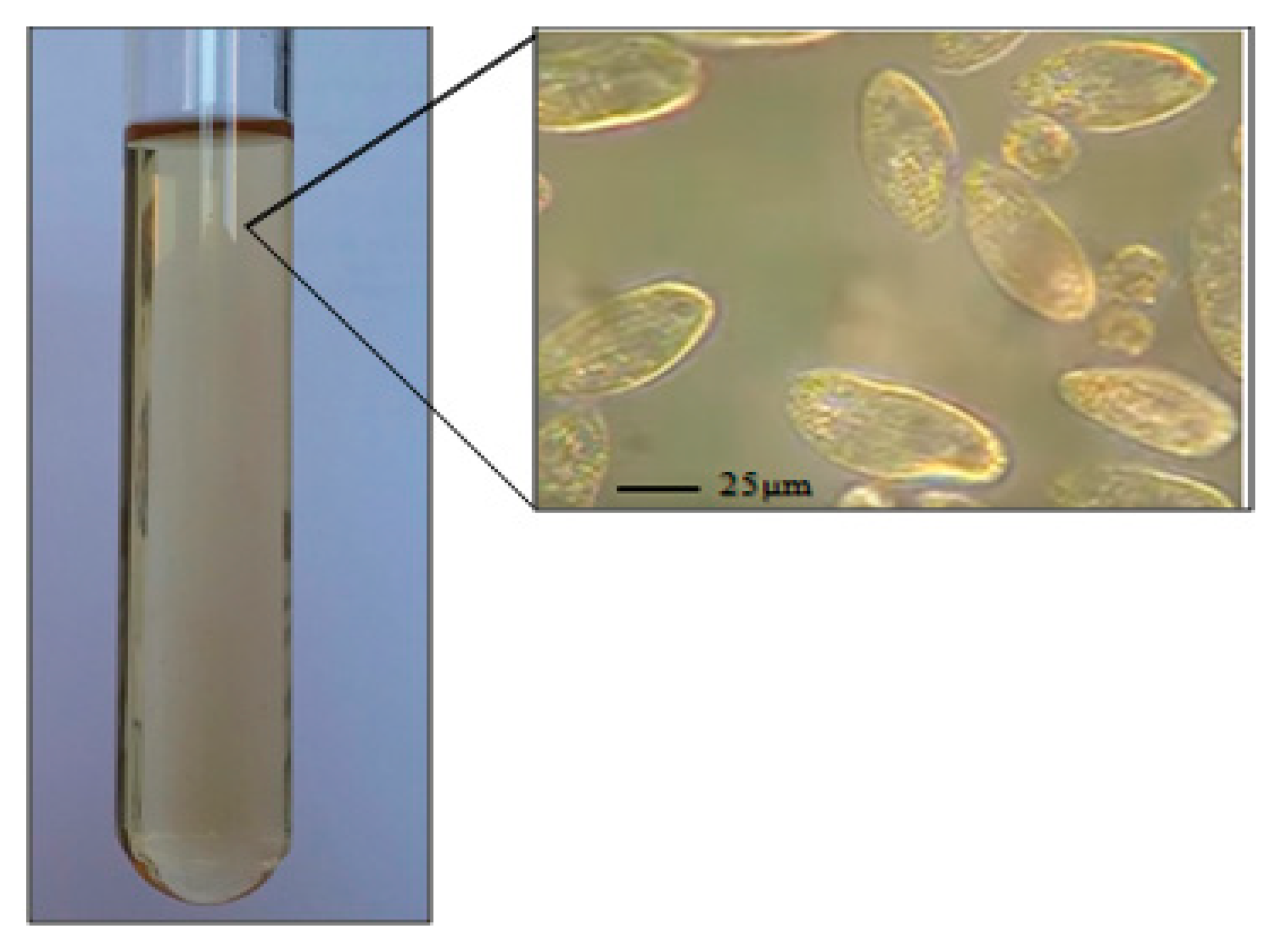
- Tetrahymena pyriformis (strain GL, L 1630/1, from The Culture Collection of Algae and Protozoa, The Botany School, Downing Street, Cambridge) was cultured axenically in 5 mL of the standard medium (PPYE), containing proteose peptone (1.5%, w/v) and yeast extract (0.25%, w/v) (Figure 2).
- The culture was then inoculated with 1% (v/v) Tetrahymena pyriformis (GL, L 1630/1) preculture in the same medium and incubated at 28 °C without shaking for 72 h [7].
- At the end of the incubation time, the culture (6 × 106 cells/mL) was then harvested at the exponential phase by centrifugation at 5000 rpm for 5 min at 4 °C.
CRITICAL STEP To remove the medium completely, decant the medium from the centrifuge tube after centrifugation, invert the centrifuge tube on a paper towel to remove any residual liquid, then tap the tube gently on the paper towel to remove any liquid stuck to the sides of the tube.
CRITICAL STEP To get more culture for DNA extraction, repeat the above process by adding more culture to the same centrifuge tube.
- After removing the medium, wash the protozoa cells with 500 μL ice cold Tris–EDTA (TE) solution (Tris–EDTA: 100 mM Tris, 10 mM EDTA, pH 8.0), resuspend the protozoa pellet by vortexing or slow rounds of pipetting, and centrifuge at 4 °C for 10 min at 5000 rpm.
- Afterwards, completely remove the supernatant from the tube, add 3.5 mL of TE solution, and resuspend the protozoa pellet by vortexing or slow rounds of pipetting; then, divide it into aliquots of 1 mL and submit to DNA extraction, as reported below.OPTIONAL STEP We follow the proliferation of a cell population with regards to their DNA content using flow cytometry (BD Accuri C6 Plus).
3.2. DNA Extraction Methods
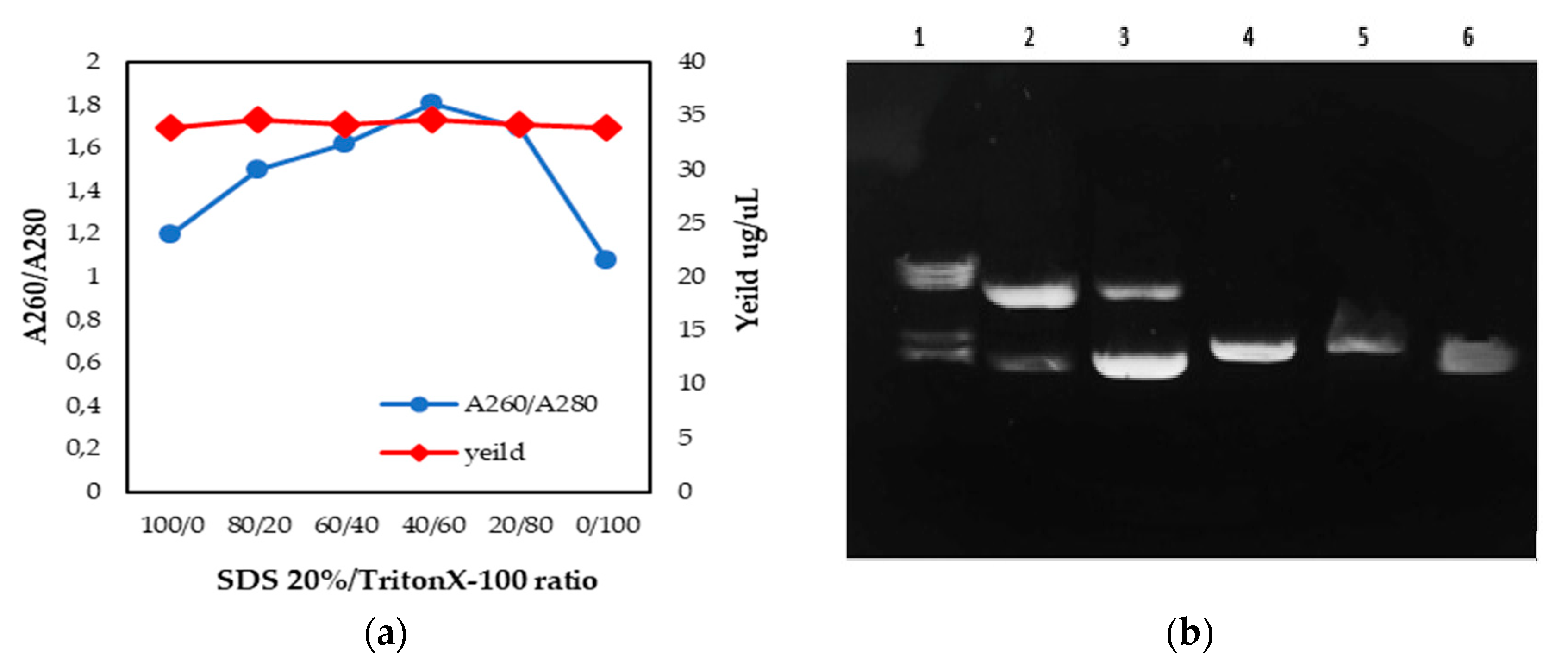
3.2.1. Sodium Dodecyl Sulphate 20%/Triton X-100 (the Addition of Mixed Surfactant)
- Preparation of the 20% SDS solution/Triton X-100 complexes (time for fulfillment: 1 h, Figure 3)
- To perform the SDS/Triton X-100 extraction method, the extraction complex 20% SDS solution/Triton X-100 was prepared beforehand.OPTIONAL STEP If sodium dodecyl sulfate (20% solution) is not available, it can be prepared with SDS solid. To prepare 100 mL of SDS (20% solution), weigh 20 g of SDS in a 250 mL conical flask/beaker. Add 80 mL deionised/Milli-Q water and mix, heat to 68 °C. Adjust the volume to 100 mL with deionised/Milli-Q water and mix again.
- SDS (20% solution) and Triton X-100 (mass ratios of 0, 20, 40, 60, 80, and 100 wt %) were mixed intensively in a vortex mixer, and placed in a water bath at 25 °C for 45 min.
CRITICAL STEP If there SDS in the solvent precipitate, re-dissolve it by warming the mix at 60 °C for 10 min.
-
- PAUSE STEP The mix can be stored at room temperature for several months. Do not store the SDS (20% solution) at 4 °C, because SDS will precipitate at temperatures below 15 °C.
- Cell breaking using thermal shock methods (time for fulfillment: 30 min, Figure 3):
- The tubes (1 mL protozoa pellet) were incubated in a water bath at 95 °C for 20 min (intermittently vortexing every 5 min).
- The tubes were put on ice for 10 min.
- Purification of DNA (time for fulfillment: 10 min, Figure 3):
- A total of 2.5 mL of the SDS/Triton X-100/H2O complex was added to the tube.
- The tubes were incubated in a water bath at 45 °C for 5 min.
- After that, 14 µl of proteinase K solution (20 mg/mL) was added to the suspension, and the tubes were mixed well.
- DNA recovery (time for fulfillment: 20 min, Figure 3)
- First, 500 μL of 70% ethanol was added to the tube.
- The tube was closed and inverted several times
- The tubes were centrifuged at 14000 rpm (maximum speed) for 10 min at 25 °C.
- The supernatant was completely removed.OPTIONAL STEP to remove the supernatant, one can decant the supernatant after the first centrifugation.
-
- CRITICAL STEP Take care with this step, as the pellet sometimes does not adhere tightly to the tube and can be lost while removing the supernatant.
- Liquid remains will be stuck on the wall of microcentrifuge tube.
- A second flash spin is sufficient to collect all the liquid at the bottom, which can be then removed by pipetting.
- The pellet was air dried for 5 min.
- The traces of ethanol must then be removed, as they may inhibit some enzyme reactions and dissolve the pellet in 25 μL sterile double distilled water or TE (pH 8.0).
-
- CRITICAL STEP Do not overdry the pellet; overdried pellets are difficult to dissolve.OPTIONAL STEP To dissolve the pellet, one can vortex the solution gently for a brief period and also incubate it at 37 °C for ∼20 min.
-
- PAUSE STEP The solution can be stored at 4 °C for a few days or stored at −20 °C for years
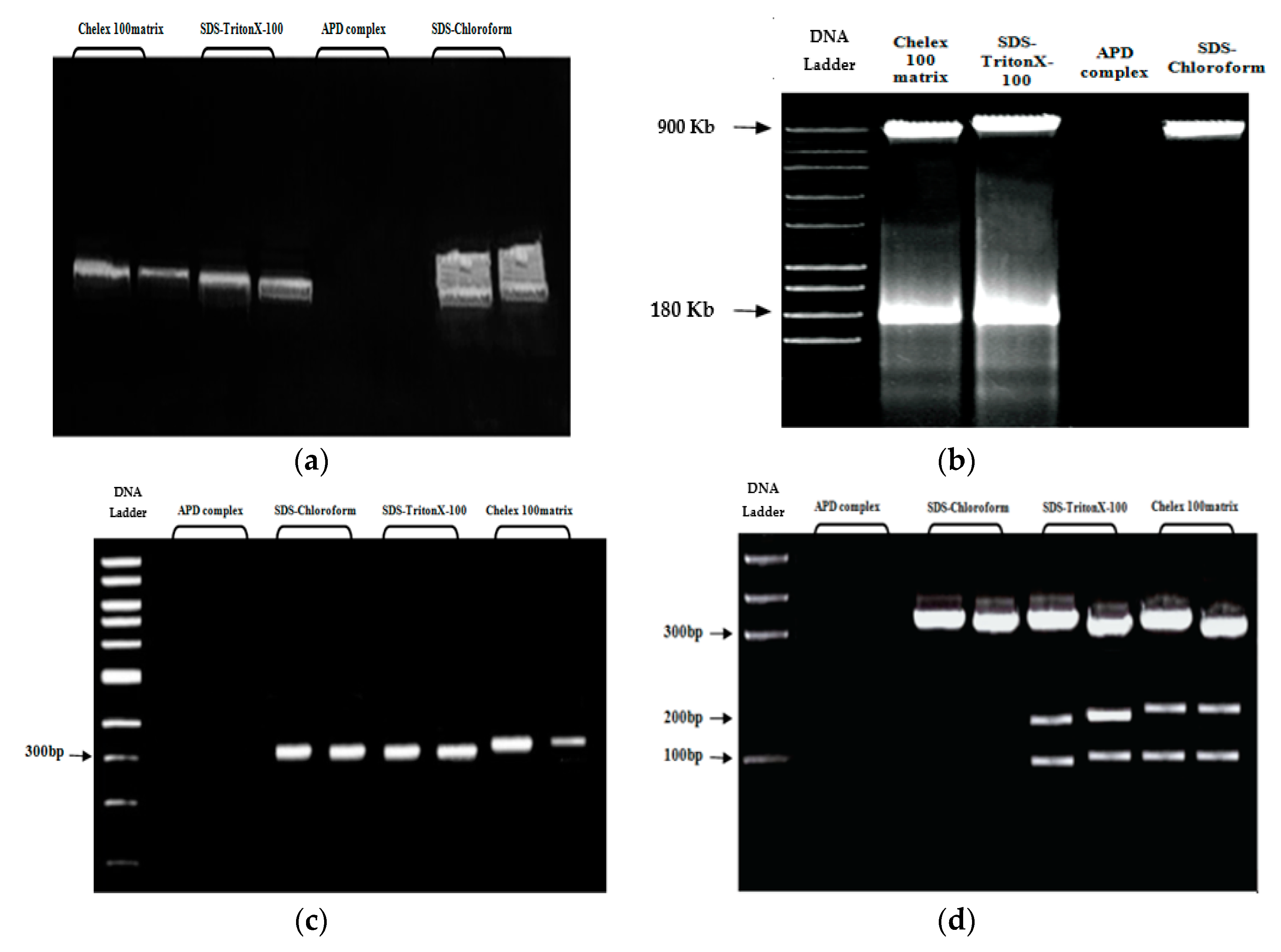
3.2.2. DNA Extraction by Chelex 100 Resin APD Complex and SDS–Chloroform
3.3. Cost and Time Consumed
3.4. Evaluation of the Genomic DNA Quality, Quantity, and Integrity
3.5. Pulsed-Field Gel Electrophoresis (PFGE)
3.6. Polymerase Chain Reaction Amplification of the Metallothionein (MT) Gene
3.7. Restriction Enzyme Digestion Analysis
4. Expected Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darcy, P.; Kelly, J.P.; Leonard, B.E.; Henry, A.B. The effect of lofepramine and other related agents on the motility of Tetrahymena pyriformis. Toxicol. Lett. 2002, 128, 207–214. [Google Scholar] [CrossRef]
- Leick, V.; Bog-Hansen, T.C.; Christensen, S.T.; Kaufman, S.J. Concanavalin A Receptors and the Chemosensory Behaviour of Tetrahymena thermophila. Exp. Biol. Online 1996, 1, 1–12. [Google Scholar] [CrossRef]
- Yu, S.; Geng, J.; Zhou, P.; Wang, J.; Chen, X.; Hu, J. New hydroxyapatite monolithic column for DNA extraction and its application in the purification of Bacillus subtilis crude lysate. J. Chromatogr. 2008, 1183, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.W.; Fredlake, C.P.; Doherty, E.A.S.; Barron, A.E. DNA sequencing and genotyping in miniaturized electrophoresis systems. Electrophoresis 2004, 25, 3564–3588. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Nogueira, S.; Gadanho, M.; Chaves, S. DNA extraction: Finding the most suitable method. In Molecular Microbial Diagnostic Methods; Academic Press: Amesterdam, The Netherlands, 2016; pp. 135–154. [Google Scholar]
- Fenicia, L.; Anniballi, F.; De Medici, D.; Delibato, E.; Aureli, P. SYBR Green real-time PCR method to detect Clostridium botulinum type A. Appl. Environ. Microbiol. 2007, 73, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Pousada, R.C.; Cyrne, M.L.; Hayes, D. Characterization of Preribosomal Ribonucleoprotein Particles from Tetrahymena pyriformis. Eur. J. Biochem. 1979, 102, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a Medium for Simple Extraction of DNA for PCR-Based Typing from Forensic Material. BioTechniques 2013, 54, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Manaffar, R.; Maleki, R.; Zare, S.; Agh, N.; Soltanian, S.; Sehatnia, B.; Sorgeloos, P.; Bossier, P.; Van, G. A New Method for Rapid DNA Extraction from Artemia (Branchiopoda, Crustacea). Int. J. Bioeng. Life Sci. 2010, 6, 123–127. [Google Scholar]
- Green, M.R.; Sambrook, J. Molecular cloning. In A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Carle, G.F.; Frank, F.; Olson, M.V. Electrophoretic separations of large DNA molecule by periodic inversion of electric field. Science 1986, 232, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Pok, L.; Vincent, K.L.L.; King, M.C. Tilapia metallothionein genes: PCR-cloning and gene expression studies. Biochim. Biophys. Acta 2005, 1731, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Conover, R.K.; Brunk, C.F. Macronuclear DNA Molecules of Tetrahymena thermophila. Mol. Cell. Biol. 1986, 6, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.C.; Gorovsky, M.A. Comparison of the DNA sequences of Tetrahymena macro- and micronuclei. Chromosoma 1974, 48, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.C.; Yao, C.H. Repeated hexanucleotide C-C-C-C-A-A is present near free ends of macronuclear DNA of Tetrahymena. Proc. Natl. Acad. Sci. USA 1981, 78, 7436–7439. [Google Scholar] [CrossRef] [PubMed]
| Primer Set (Reference) | Sequence (5′–3′), (3′–5′) | Denaturing (°C; min) | Annealing (°C; min) | Elongation (°C; min) | N° cycles |
|---|---|---|---|---|---|
| Metallothionein (MT) primers [12] | CGTGAATAAAATGGATAAGGTTAATAA, CATTTGCAACATTCACAAGTCTTAC | 94; 3.5 | 55; 0.4 | 72;0.3 | 30 |
| DNA Extraction Methods | DNA Extraction Protocol Stage | Time Required (min) | Toxic Compound | Cost Per Sample (US$) | A260/280 1 | NanoDrop µg/µL | Qubit µg/µL | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell Lysis | Denaturation of Nucleoproteins | Removal of Contaminants | DNA Precipitation | |||||||
| SDS-TritonX100 | Thermal shock | Proteinase K | SDS/Triton X-100 | Ethanol | 2 h | None | ˂ 3 | 1.81 | 34.75 | 33.77 |
| Chelex100matrix | Heat | Chelex/Proteinase K | Chelex | Isopropanol | 3 h | Isopropanol | ˂ 3 | 1.84 | 2.62 | 2.08 |
| APD complex | SDS | Proteinase K | Ammonium Pyrrolidine Dithiocarbamate | Ethanol | 3 h | None | ˃ 3 | ND | ND | ND |
| SDS-chloroform | SDS | Phenol/Proteinase K | Phenol-chlorofom isoamyl alcohol | Ethanol | 6 h 40 min | Phenol, chloroform | ˂ 3 | 1.08 | 30.87 | 28.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Maaiden, E.; El Kharrassi, Y.; Essamadi, A.K.; Moustaid, K.; Nasser, B. Improved Method for DNA Extraction and Purification from Tetrahymena pyriformis. Methods Protoc. 2019, 2, 40. https://doi.org/10.3390/mps2020040
El Maaiden E, El Kharrassi Y, Essamadi AK, Moustaid K, Nasser B. Improved Method for DNA Extraction and Purification from Tetrahymena pyriformis. Methods and Protocols. 2019; 2(2):40. https://doi.org/10.3390/mps2020040
Chicago/Turabian StyleEl Maaiden, Ezzouhra, Youssef El Kharrassi, Abdel Khalid Essamadi, Khadija Moustaid, and Boubker Nasser. 2019. "Improved Method for DNA Extraction and Purification from Tetrahymena pyriformis" Methods and Protocols 2, no. 2: 40. https://doi.org/10.3390/mps2020040
APA StyleEl Maaiden, E., El Kharrassi, Y., Essamadi, A. K., Moustaid, K., & Nasser, B. (2019). Improved Method for DNA Extraction and Purification from Tetrahymena pyriformis. Methods and Protocols, 2(2), 40. https://doi.org/10.3390/mps2020040