New Protocol for Cell Culture to Obtain Mitotic Chromosomes in Fishes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Tissue and Isolation of Cells
2.2. Plate Treatment
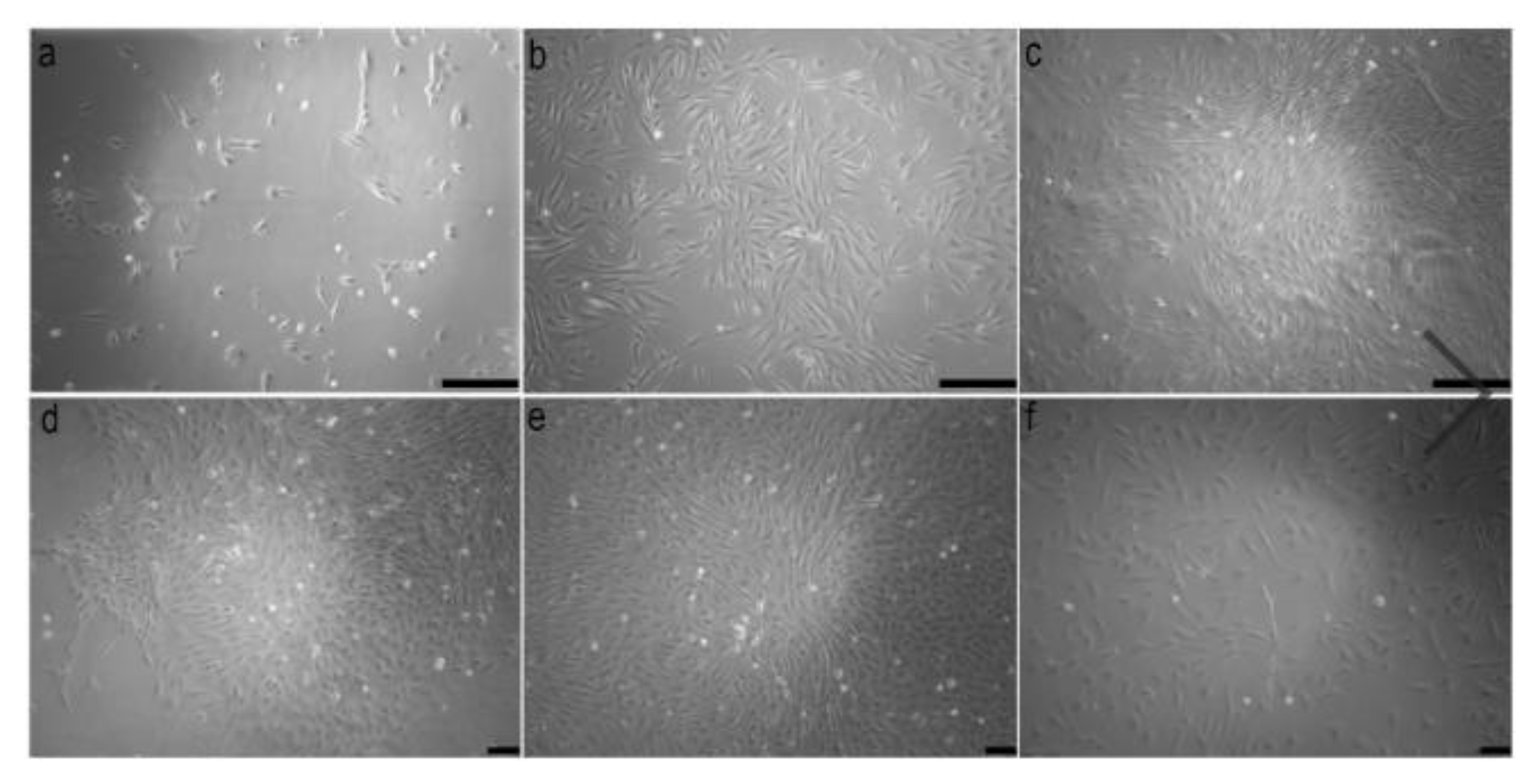
2.3. Cell Culture and Subculture
2.4. Cryopreservation and Thawing of Cells
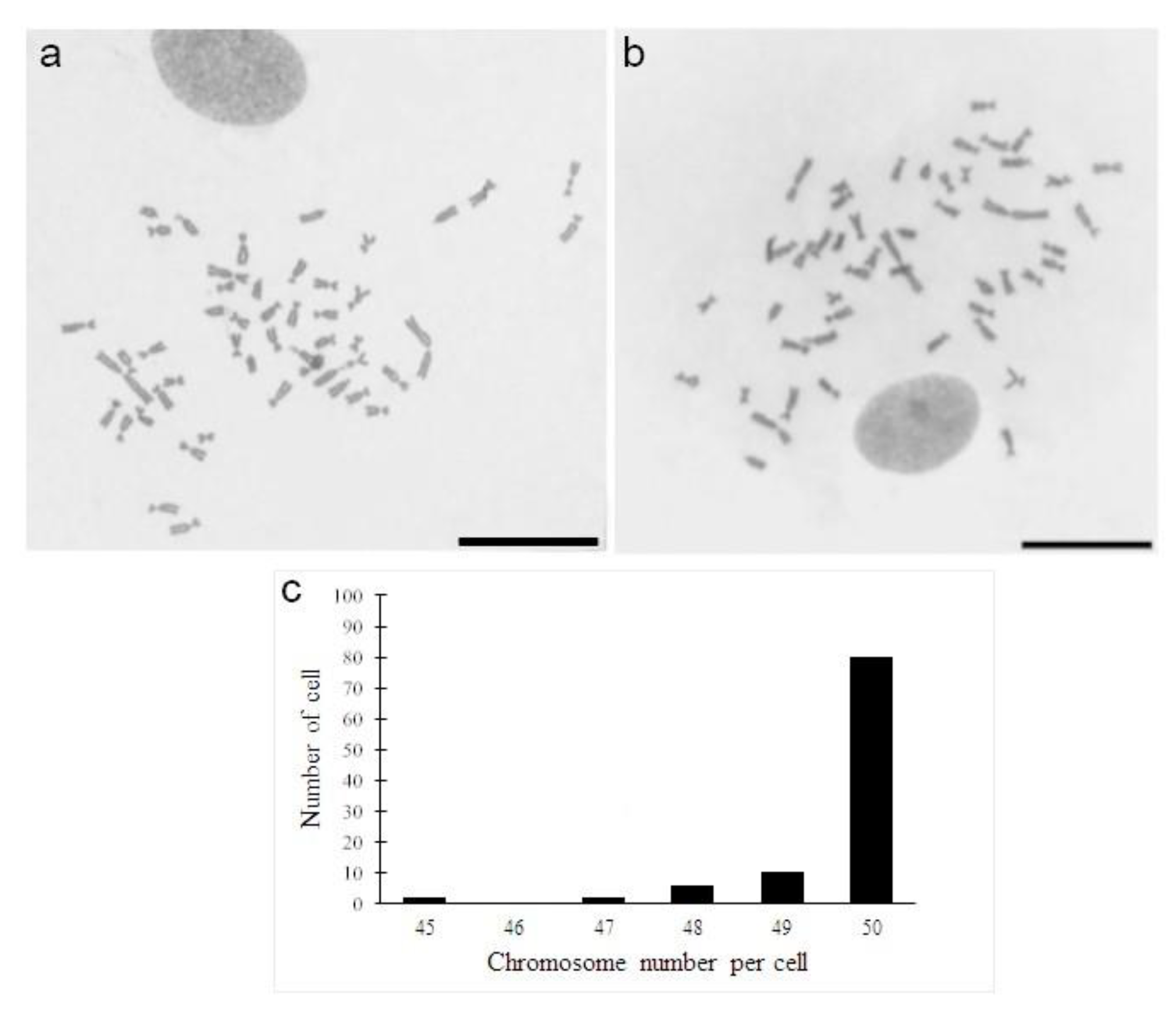
2.5. Chromosome Preparations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bols, N.C.; Lee, L.E. Technology and uses of cell cultures from the tissues and organs of bony fish. Cytotechnology 1991, 6, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cooper, R.K.; Wolters, W.R.; Tiersch, T.R. Isolation, culture and characterization of a primary fibroblast cell line from channel catfish. Cytotechnology 1998, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Villena, A.J. Applications and needs of fish and shellfish cell culture for disease control in aquaculture. Rev. Fish Biol. Fish. 2003, 13, 111–140. [Google Scholar] [CrossRef]
- Babich, H.; Borenfreund, E. Cytotoxicity and genotoxicity assays with cultured fish cells: A review. Toxicol. In Vitro 1991, 5, 91–100. [Google Scholar] [CrossRef]
- Fent, K. Fish cell lines as versatile tools in ecotoxicology: Assessment of cytotoxicity, cytochrome P4501A induction potential and estrogenic activity of chemicals and environmental samples. Toxicol. In Vitro 2001, 15, 477–488. [Google Scholar] [CrossRef]
- Nicholson, B.L.; Danner, D.J.; Wu, J. Three new continuous cell lines from marine fishes of Asia. In Vitro Cell. Dev. Biol. 1987, 23, 199–204. [Google Scholar] [CrossRef]
- Chi, S.C.; Hu, W.W.; Lo, B.J. Establishment and characterization of a continuous cell line (GF-1) derived from grouper, Epinephelus coioides (Hamilton): A cell line susceptible to grouper nervous necrosis virus (GNNV). J. Fish. Dis. 1999, 22, 173–182. [Google Scholar] [CrossRef]
- Sobhana, K.S.; George, K.C.; Venkat Ravi, G.; Ittoop, G.; Paulraj, R. Development of a Cell Culture System From Gill Explants of the Grouper, Epinephelus malabaricus (Bloch and Shneider). Asian Fish. Sci. 2009, 22, 1–6. [Google Scholar]
- Chen, S.L.; Qin, Q.W. Theory and Technology of Fishes Cell Culture; Science Press: Beijing, China, 2011. [Google Scholar]
- Wang, X.; Yang, J.; Chen, X.; Pan, X. Establishment and characterization of a fibroblast-like cell line from Anabarilius grahami (Cypriniformes: Cyprinidae). Zool. Res. 2012, 33, E89–E97. [Google Scholar] [CrossRef]
- Romanenko, S.A.; Biltueva, L.S.; Serdyukova, N.A.; Kulemzina, A.I.; Beklemisheva, V.R.; Gladkikh, O.L.; Lemskaya, N.A.; Interesova, E.A.; Korentovich, M.A.; Vorobieva, N.V.; et al. Segmental paleotetraploidy revealed in starlet (Acipenser ruthenus) genome by chromosome painting. Mol. Cytogenet. 2015, 8. [Google Scholar] [CrossRef]
- Amemiya, C.T.; John, W.B.; John, R.G. A Cell Culture Technique for Chromosome Preparation in Cyprinid Fishes. Copeia 1984, 1, 232–235. [Google Scholar] [CrossRef]
- Bejar, J.; Borrego, J.J.; Alvarez, M.C. A continuous cell line from the cultured marine fish filt-head seabream (Sparus aurata L.). Aquaculture 1997, 150, 143–153. [Google Scholar] [CrossRef]
- Bain, P.A.; Hutchinson, R.G.; Marks, A.B.; Crane, M.S.J.; Schuller, K.A. Establishment of a continuous cell line from southern bluefin tuna (Thunnus maccoyii). Aquaculture 2013, 376, 59–63. [Google Scholar] [CrossRef]
- Graf, M.; Hartmann, N.; Reichwald, K.; Englert, C. Absence of replicative senescence in cultured cells from the short-lived killifish Nothobranchius furzeri. Exp. Gerontol. 2013, 48, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Nam, Y.K.; Park, C.; Kim, H.W.; Ahn, J.; Lim, J.M.; Gong, S.P. Establishment condition and characterization of heart-derived cell culture in Siberian sturgeon (Acipenser baerii). In Vitro Cell. Dev. Biol. Anim. 2014, 50, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Lai, Y.; Luo, X.; Wang, Y.; Shi, C.; Huang, Z.; Wu, S.; Su, J. A novel fish cell line derived from the brain of Chinese perch Siniperca chuatsi: Development and characterization. J. Fish Biol. 2015, 86, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.S.; Vicari, M.R.; Abilhoa, V.; Wamser, J.P.; Cestari, M.M.; Bertollo, L.A.C.; Almeida, M.C.; Arton, R.F. Cytogenetic and comparative morphology of two allopatric populations of Astyanax altiparanae Garutti & Britski, 2000 (Teleostei: Characidae) from upper rio Paraná basin. Neotrop. Ichthyol. 2007, 5, 37–44. [Google Scholar]
- Neto, M.F.; Vicari, M.R.; Camargo, E.F.; Artoni, R.F.; Moreira-Filho, O. Comparative cytogenetics among populations of Astyanax altiparanae (Characiformes, Characidae, Incertae sedis). Genet. Mol. Biol. 2009, 32, 792–796. [Google Scholar] [CrossRef]
- Martinez, E.R.M.; Alves, A.L.; Silveira, S.M.; Foresti, F.; Oliveira, C. Cytogenetic analysis in the incertae sedis species Astyanax altiparanae Garutti and Britzki, 2000 and Hyphessobrycon eques Steindachner, 1882 (Characiformes, Characidae) from the upper Paraná river basin. Comp. Cytogenet. 2012, 6, 41–51. [Google Scholar] [CrossRef]
- Fernandes, C.A.; Martins-Santos, I.C. Cytogenetic studies in two populations of Astyanax altiparanae (Pisces, Characiformes). Hereditas 2014, 141, 328–332. [Google Scholar] [CrossRef]
- Yano, C.F.; Moreira Filho, O.; Margarido, V.P. Interpopulational comparative cytogenetics analysis among three Astyanax (Characiformes: Incertae Sedis) species of two streams of upper Paraná River basin, Brazil. Biologia 2014, 69, 790–798. [Google Scholar] [CrossRef]
- Blessing, J.J.; Marshall, J.C.; Balcombe, S.R. Humane killing of fishes for scientific research: A comparison of two methods. J. Fish Biol. 2010, 76, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.B.; Fan, T.J.; Jiang, G.J.; Xu, X.H.; Sun, A. A novel heart-cell line from brown-marbled grouper Epinephelus fuscoguttatus and its susceptibility to iridovirus. J. Fish Biol. 2010, 76, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qi, W.; Zhou, Y.; Zhang, X.; Dong, R.; Zhou, L.; Wang, D. Establishment and characterization of an ovarian cell line from Southern catfish (Silurus meridionalis). Fish Physiol. Biochem. 2014, 40, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.S.; Kawahara, G.; Kho, A.T.; Howell, M.H.; Pusack, T.J.; Myers, J.A.; Montanaro, F.; Zon, L.I.; Guyon, J.R.; Kunkel, L.M. Isolation and transcriptome analysis of adult zebrafish cells enriched for skeletal muscle progenitors. Muscle Nerve 2010, 43. [Google Scholar] [CrossRef] [PubMed]
- Keijing, A.; Haiying, L.; Shidong, G.; Kumar, D.N.T.; Qingqing, W. Preparation of fish gelatin and fish gelatin/poly(l-lactide) nanofibers by Electrospinning. Int. J. Biol. Macromol. 2010, 47, 380–388. [Google Scholar] [CrossRef]
- Merten, O.W. Advances in cell culture: Anchorage dependence. Phil. Trans. R. Soc. B 2015, 370, 20140040. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [Green Version]
- Freshney, R.I. Celture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 6th ed.; Jonh Wiley and Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Yadav, K.; Lakra, W.S.; Sharma, J.; Goswami, M.; Singh, A. Development and characterization of a cell line TTCF from endangered mahseer Tor tor (Ham.). Fish Physiol. Biochem. 2012, 38, 1035–1045. [Google Scholar] [CrossRef]
- Majeed, A.S.; Nambi, K.S.N.; Taju, G.; Hameed, S. Development, characterization and application of a new fibroblast-like cell line from kidney of a freshwater air breathing fish Channa striatus (Bloch, 1793). Acta Trop. 2013, 127, 25–32. [Google Scholar] [CrossRef]
- Gignac, S.J.; Vo, N.T.; Mikhaeil, M.S.; Alexander, J.A.N.; MacLatchy, D.L.; Schulte, P.M.; Lee, L.E. Derivation of a continuous myogenic cell culture from an embryo of common killifish, Fundulus heteroclitus. Comp. Biochem. Physiol. 2014, 175, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Foresti, F.; Oliveira, C.; Almeida-Toledo, L.F. A method for chromosome preparations from large fish specimens using in vitro short-term treatment with colchicine. Experientia 1993, 49, 810–813. [Google Scholar] [CrossRef]
- Rábová, M.; Monteiro, R.; Collares-Pereira, M.J.; Ráb, P. Rapid Fibroblast Culture for Teleost Fish Karyotyping. In Fish Cytogenetic Techniques: Ray-Fin Fishes and Chondrichthyans; Ozouf-Costaz, C., Pisano, E., Foresti, F., Toledo, L.F.A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 66–73. [Google Scholar]
| Species | Tissue | Methods for Isolate Cells | Type of Medium | % FBS 1 | Temperature (°C) | Plate Treatment | Reference |
|---|---|---|---|---|---|---|---|
| Epinephelus malabaricus | Gill | Mechanical methods (Explants) | L-15 2 | 20% | 28° ± 2 °C | No | [8] |
| Anabarilus graham | Fin | Mechanical methods (Explants) | DMEM 3/F-12 | 20% | 28 °C | No | [10] |
| Notropis ssp. | Fin and scales | Mechanical methods (Explants) | 199 | 10% | 30 °C | No | [12] |
| Sparus aurata L. | Fin | Mechanical methods (Explants) | DMEM 3/F-12 | 15% | 15% | No | [13] |
| Thunnus maccoyii | Fin, muscle, skin and spinal | Proteolytic enzymes (collagenase) | L-15 2 | 15–20% | 25 °C | No | [14] |
| Nothobranchius furzeri | Skin and fin | Proteolytic enzymes (collagenase) | DMEM3 | 10% | 28 °C | Yes (Gelatin) | [15] |
| Acipenser baerii | Heart | Proteolytic enzymes (collagenase/trysin-EDTA) | L-15 2 and DMEM 3 | 20% | 28 °C | Yes (Gelatin) | [16] |
| Siniperca chuatsi | Bain | Proteolytic enzymes (collagenase) | L-15 2 | 20% | 28 °C | No | [17] |
| Astyanax altiparanae | Muscle | Proteolytic enzymes (collagenase/trysin-ETDA) | DMEM 3 | 10% | 28 °C | Yes (Gelatin) | Present study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paim, F.G.; Maia, L.; Landim-Alvarenga, F.d.C.; Foresti, F.; Oliveira, C. New Protocol for Cell Culture to Obtain Mitotic Chromosomes in Fishes. Methods Protoc. 2018, 1, 47. https://doi.org/10.3390/mps1040047
Paim FG, Maia L, Landim-Alvarenga FdC, Foresti F, Oliveira C. New Protocol for Cell Culture to Obtain Mitotic Chromosomes in Fishes. Methods and Protocols. 2018; 1(4):47. https://doi.org/10.3390/mps1040047
Chicago/Turabian StylePaim, Fabilene G., Leandro Maia, Fernanda da Cruz Landim-Alvarenga, Fausto Foresti, and Claudio Oliveira. 2018. "New Protocol for Cell Culture to Obtain Mitotic Chromosomes in Fishes" Methods and Protocols 1, no. 4: 47. https://doi.org/10.3390/mps1040047
APA StylePaim, F. G., Maia, L., Landim-Alvarenga, F. d. C., Foresti, F., & Oliveira, C. (2018). New Protocol for Cell Culture to Obtain Mitotic Chromosomes in Fishes. Methods and Protocols, 1(4), 47. https://doi.org/10.3390/mps1040047





