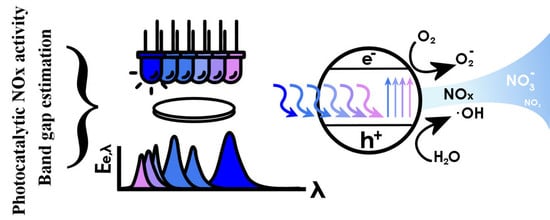
A Novel Method for the Combined Photocatalytic Activity Determination and Bandgap Estimation
Abstract
1. Introduction
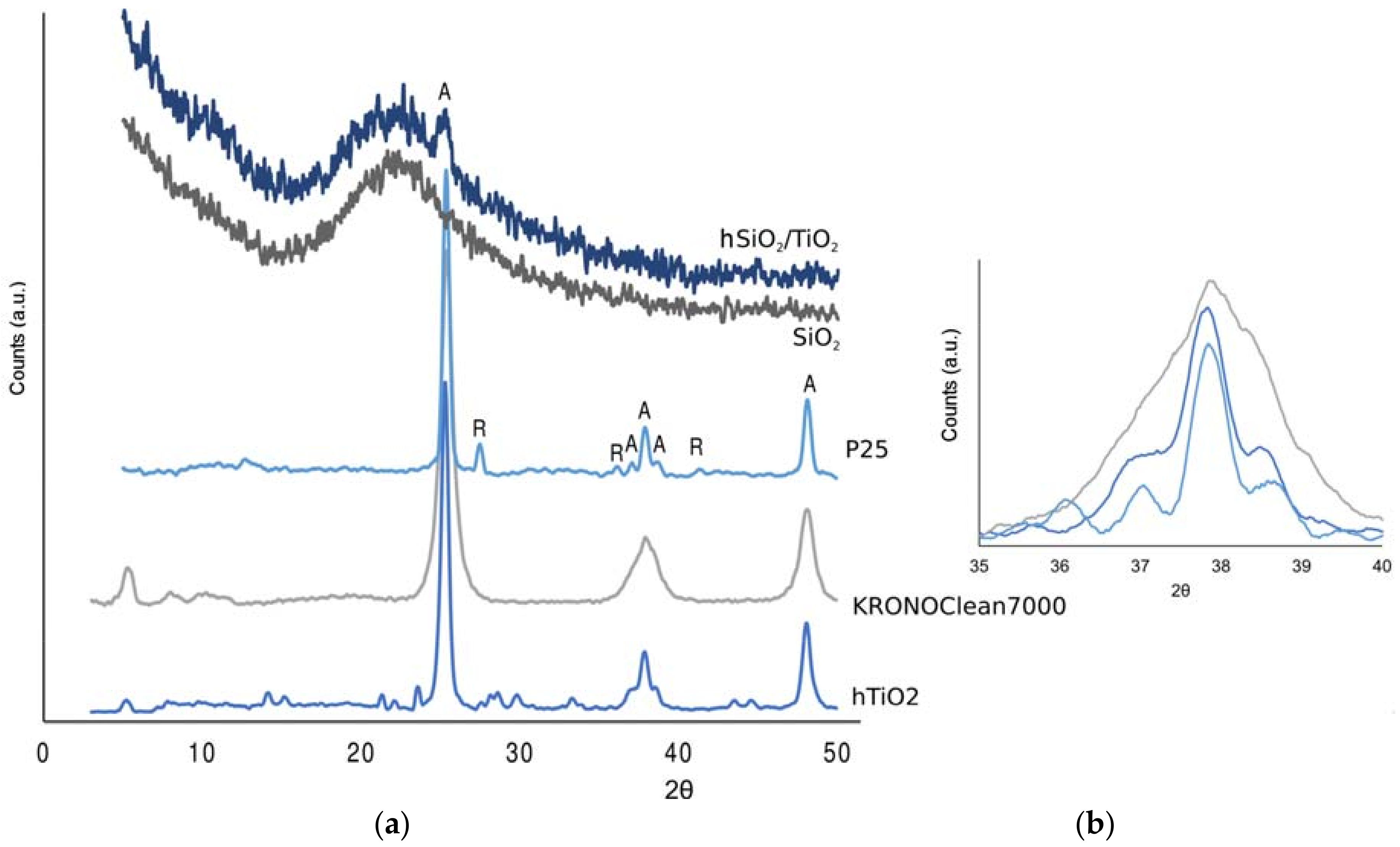
- Degussa (Evonik, Essen, Germany) P25 (from now P25) is considered a de facto standard in TiO2-photocatalysis because of its relatively high activity. It is well known that the P25 composition is made of anatase and rutile in a variable ratio, typically between 70:30 or 80:20 [1].
- KRONOClean7000 (KRONOS, Dallas, TX, USA) is a carbon-modified commercial anatase mainly addressed for indoor applications, due its visible-light response.
- A silica/titania composite (hSiO2/TiO2) was prepared, with a hydrothermal method, in our laboratory, with the aim of increasing the surface area of the photocatalyst. The titania content is 7% (w/w), in the anatase form (Section 3.1).
- The titania-only (hTiO2), synthesized under the same conditions, is tested as well for comparison.
2. Materials and Methods
2.1. Characterization of TiO2-Based Materials
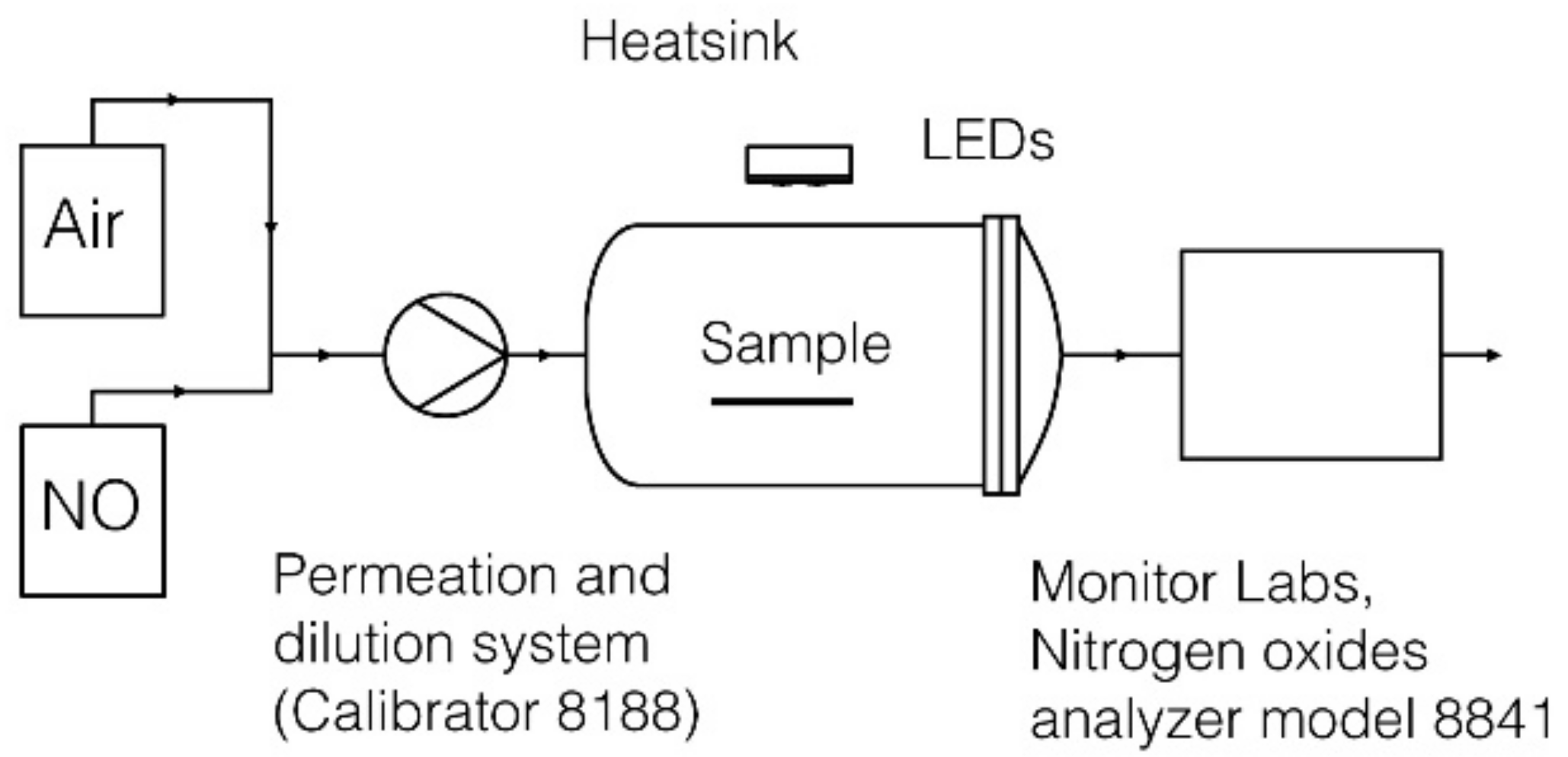
2.2. Nitrogen Oxides Apparatus
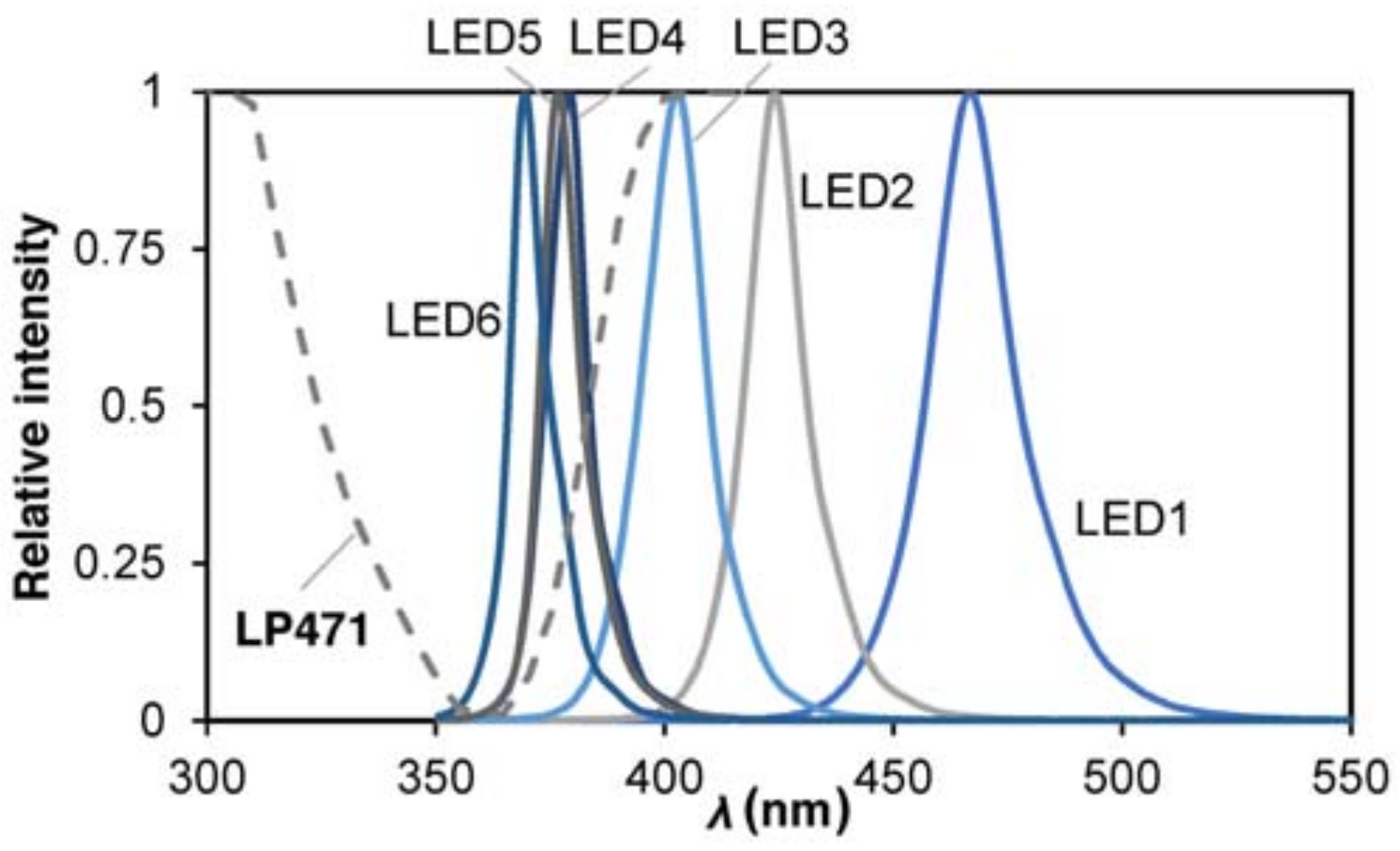
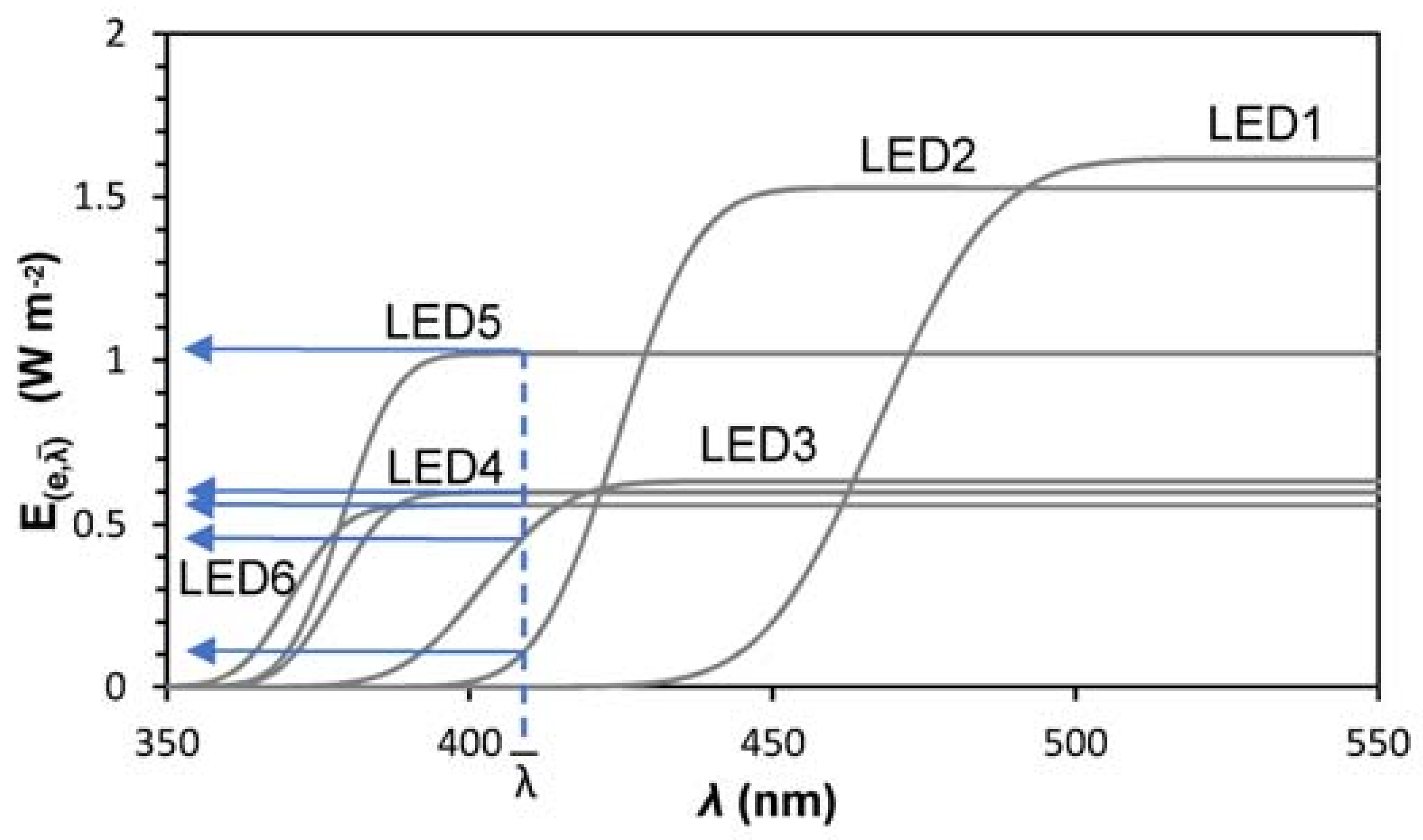
2.3. LED Characterization
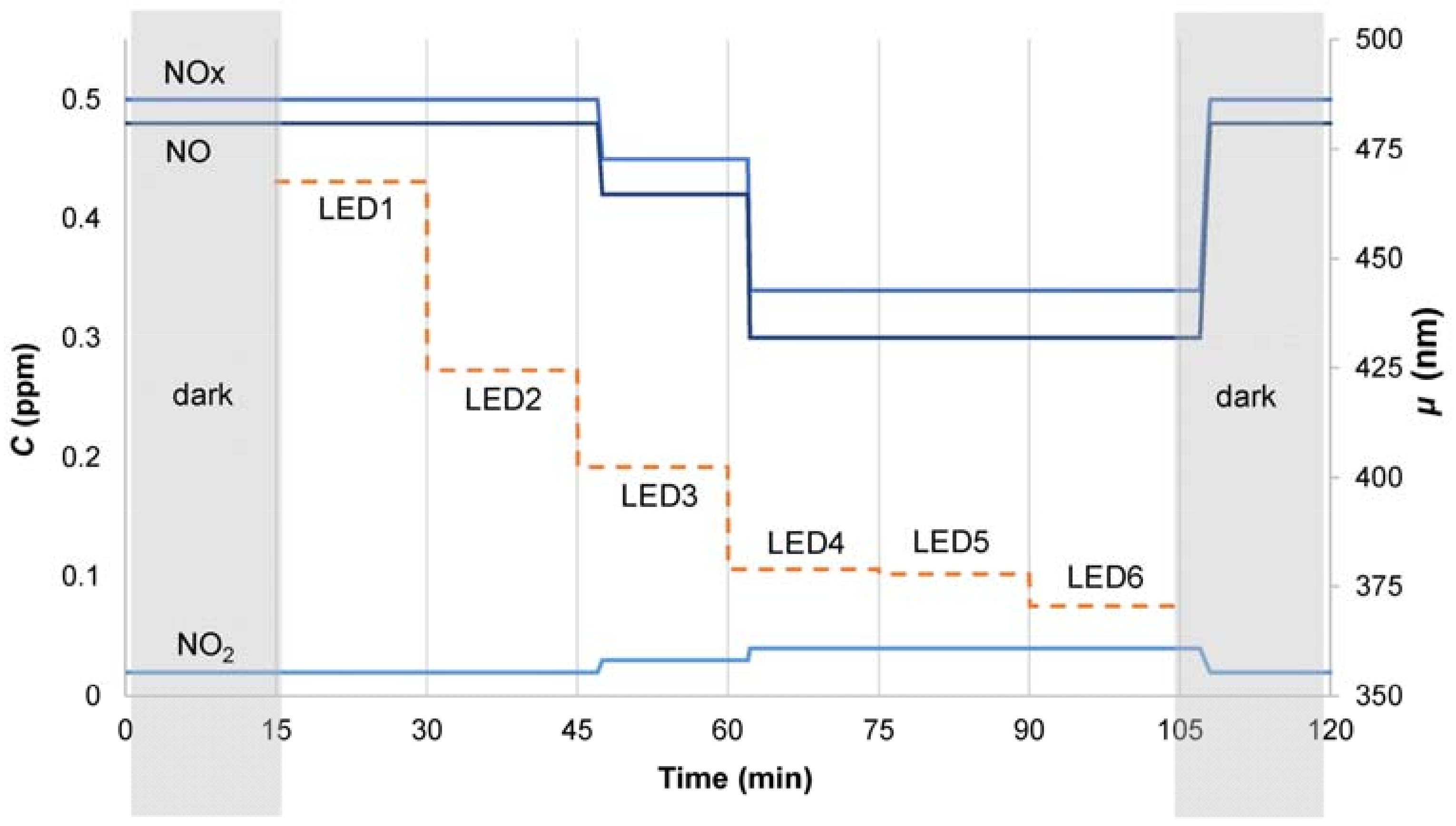
2.4. Test Procedure Description
2.5. Data Elaboration
3. Results
3.1. Characterization of TiO2-Based Materials
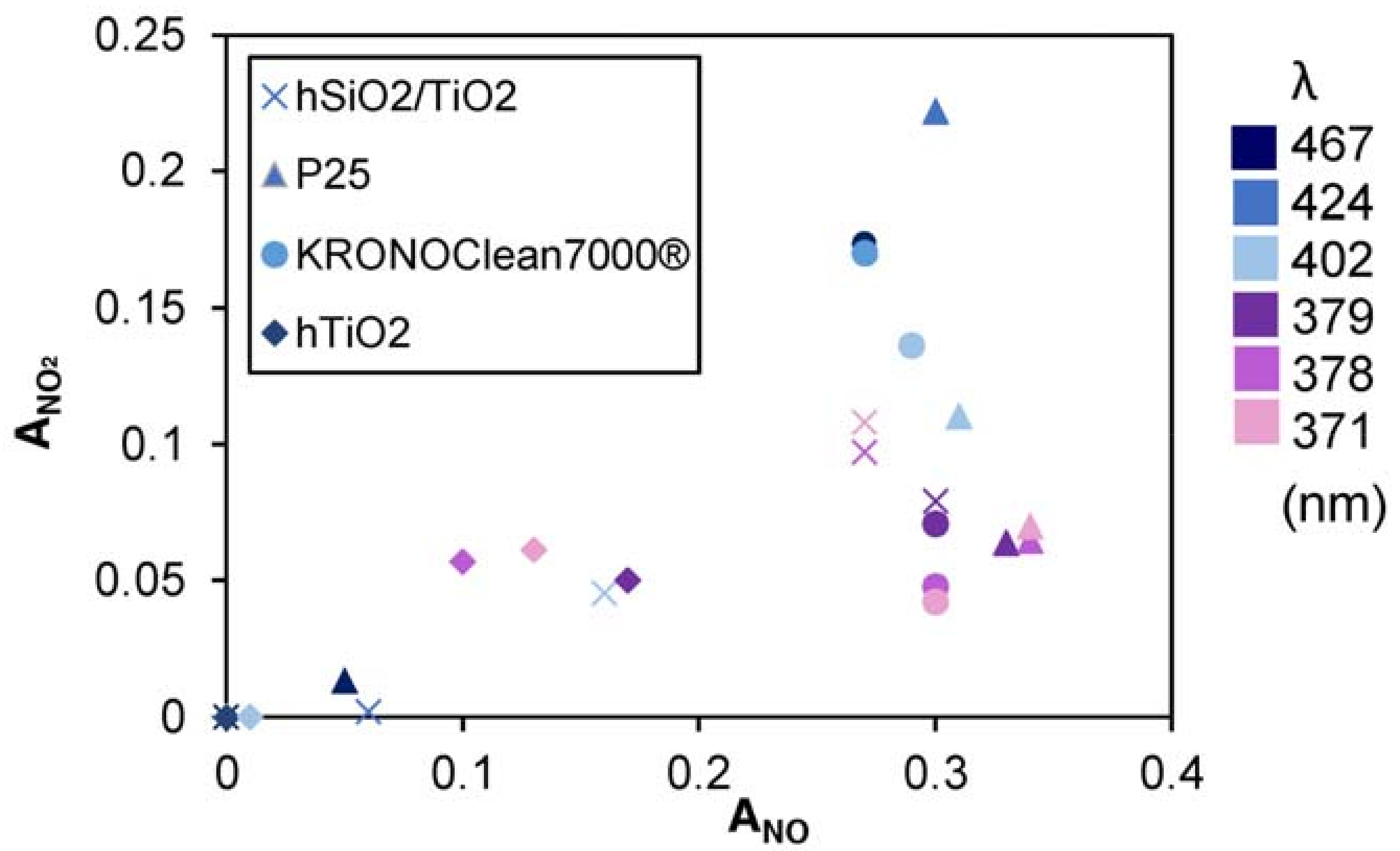
3.2. Nitrogen Oxides Abatement
- generally, the amount of NO2 generated is higher at higher wavelengths;
- generally, to a higher NO activity corresponds a higher production of NO2
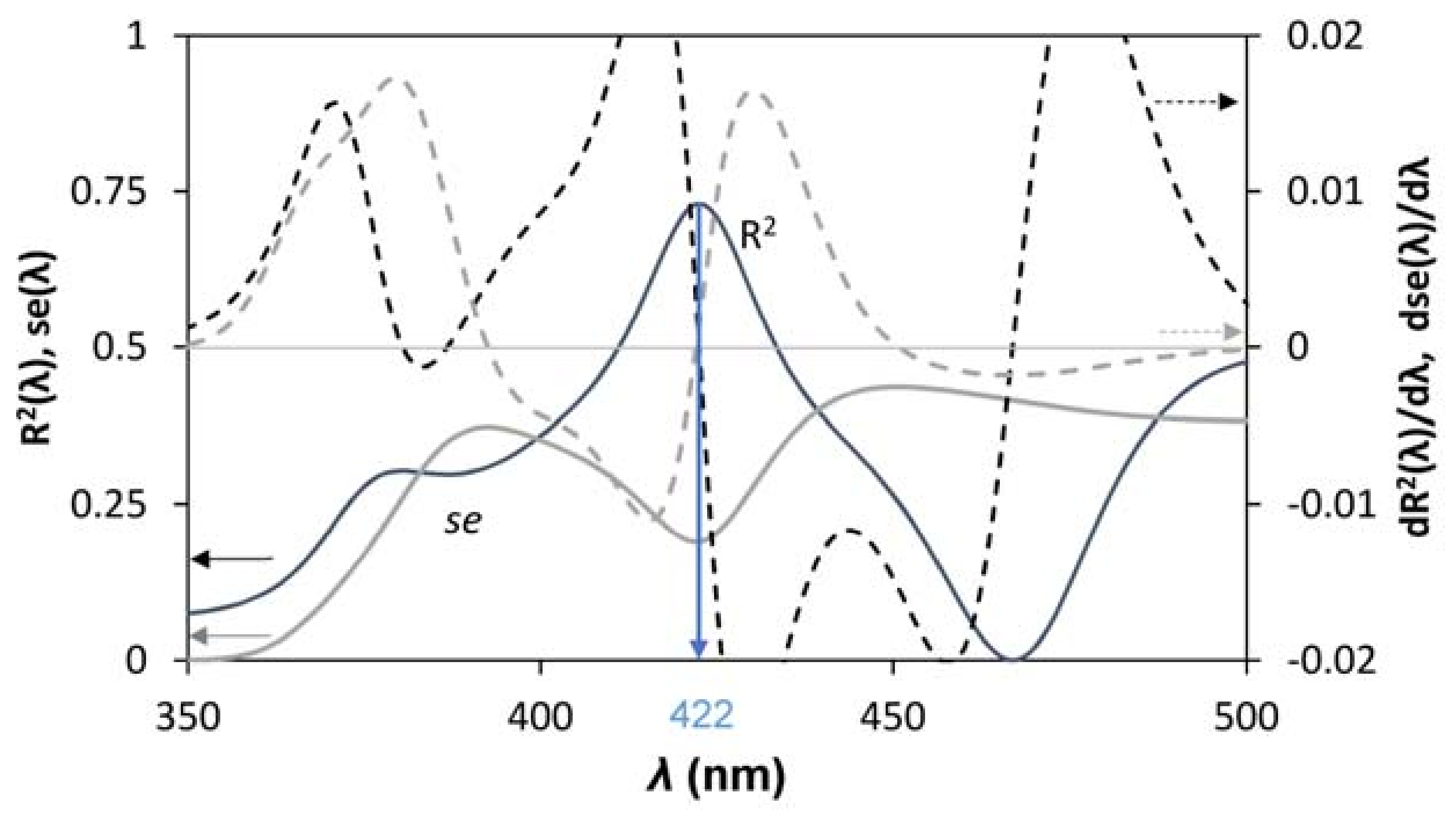
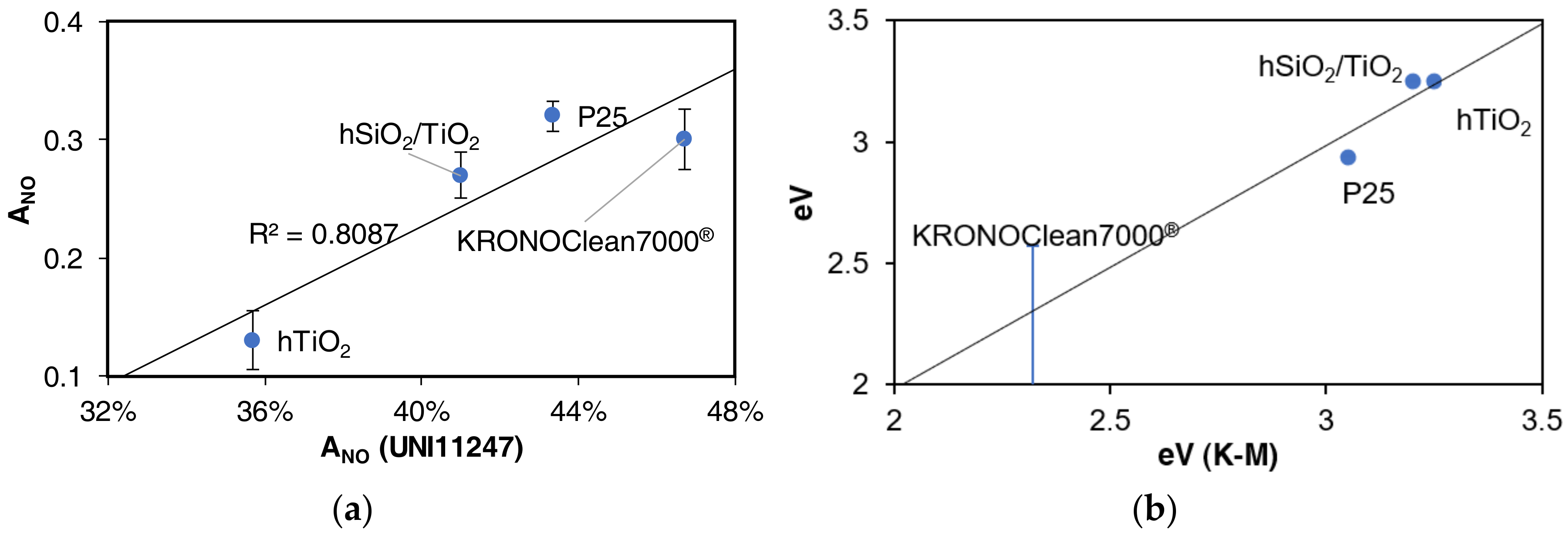
3.3. Bandgap Estimation
4. Discussion
4.1. The Method
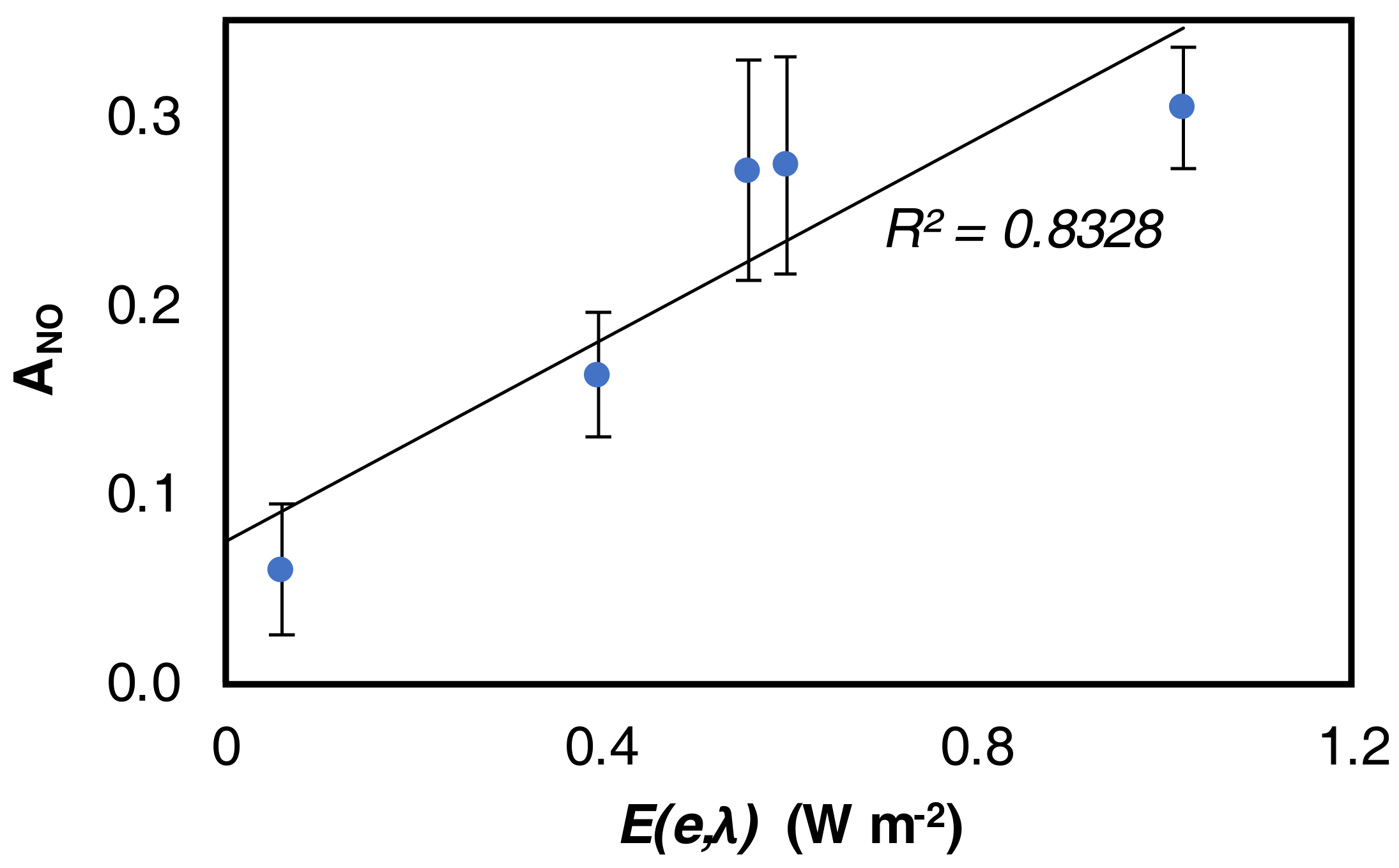
4.2. NO Activity
4.3. NO2 Selectivity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Ângelo, J.; Andrade, L.; Madeira, M.L.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Devahasdin, S.; Fan, C.; Li, K.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Giosuè, C.; Ruello, M.L.; Fava, G. Appraisal of a hybrid air cleaning process. Environ. Sci. Pollut. Res. 2017, 24, 12638–12645. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.W.; Tayade, J.R. New generation energy-efficient light source for photocatalysis: LEDs for environmental applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Levine, L.H.; Richards, J.T.; Coutts, J.L.; Soler, R.; Maxik, F.; Wheeler, R.M. Feasibility of Ultraviolet-Light-Emitting Diodes as an Alternative Light Source for Photocatalysis. J. Air Waste Manag. Assoc. 2011, 61, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Dai, Y.; Dong, W.; Zhang, S.; Chen, J. CFD modeling of a UV-LED photocatalytic odor abatement process in a continuous reactor. J. Hazard. Mater. 2012, 215–216, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lim, T.T. Solvothermal synthesis of C–N codoped TiO2 and photocatalytic evaluation for bisphenol A degradation using a visible-light irradiated LED photoreactor. Appl. Catal. B Environ. 2010, 100, 355–364. [Google Scholar] [CrossRef]
- Korovin, E.; Selishchev, D.; Besov, A.; Kozlov, D. UV-LED TiO2 photocatalytic oxidation of acetone vapor: Effect of high frequency controlled periodic illumination. Appl. Catal. B Environ. 2015, 163, 143–149. [Google Scholar] [CrossRef]
- Rojviroon, T.; Laobuthee, A.; Sirivithayapakorn, S. Photocatalytic activity of toluene under UV-LED light with TiO2 thin films. Int. J. Photoenergy 2012, 2012, 898464. [Google Scholar] [CrossRef]
- Yu, L.; Achari, G.; Langford, C.H. LED-based photocatalytic treatment of pesticides and chlorophenols. J. Environ. Eng. 2013, 139, 1146–1151. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Energy efficient UV-LED source and TiO2 nanotube array-based reactor for photocatalytic application. Ind. Eng. Chem. Res. 2011, 50, 7753–7762. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Pereira, M.F.R.; Castro-Silva, S.; Segundo, M.A.; et al. Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Res. 2016, 94, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Aponiene, K.; Luksiene, Z. Effective combination of LED-based visible light, photosensitizer and photocatalyst to combat Gram(−) bacteria. J. Photochem. Photobiol. B Biol. 2015, 142, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, N.; Castellote, M. Photocatalytic Activity for NO Degradation by Construction Materials: Parametric Study and Multivariable. J. Adv. Oxid. Technol. 2010, 13, 342–349. [Google Scholar]
- Herrmann, J.M. Fundamentals and misconceptions in photocatalysis. J. Photochem. Photobiol. A Chem. 2010, 216, 85–93. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, C.; Yeng, M.; Chiu, H. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Blöß, S.P. Visible Light Photo-catalysts Based on Titanium Dioxide and their Potential Applications: An Introduction. Tenside Surfactants Deterg. 2007, 44, 266–269. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.V.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Tang, H.; Sanjines, R.; Schmid, P.E.; Prasad, F.L. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042. [Google Scholar] [CrossRef]
- Bloh, J.Z.; Macphee, A.; Folli, D.E. Photocatalytic NOx abatement: Why the selectivity matters. RSC Adv. 2014, 4, 45726–45734. [Google Scholar] [CrossRef]
- Giosuè, C.; Pierpaoli, M.; Mobili, A.; Ruello, M.L.; Tittarelli, F. Influence of Binders and Lightweight Aggregates on the Properties of Cementitious Mortars: From Traditional Requirements to Indoor Air Quality Improvement. Materials 2017, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Kuo, C.; Huang, C.; Li, Y.; Chou, P.; Cheng, C.; Womg, M. Visible-light-responsive nano-TiO2 with mixed crystal lattice and its photocatalytic activity. Nanotechnology 2006, 17, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
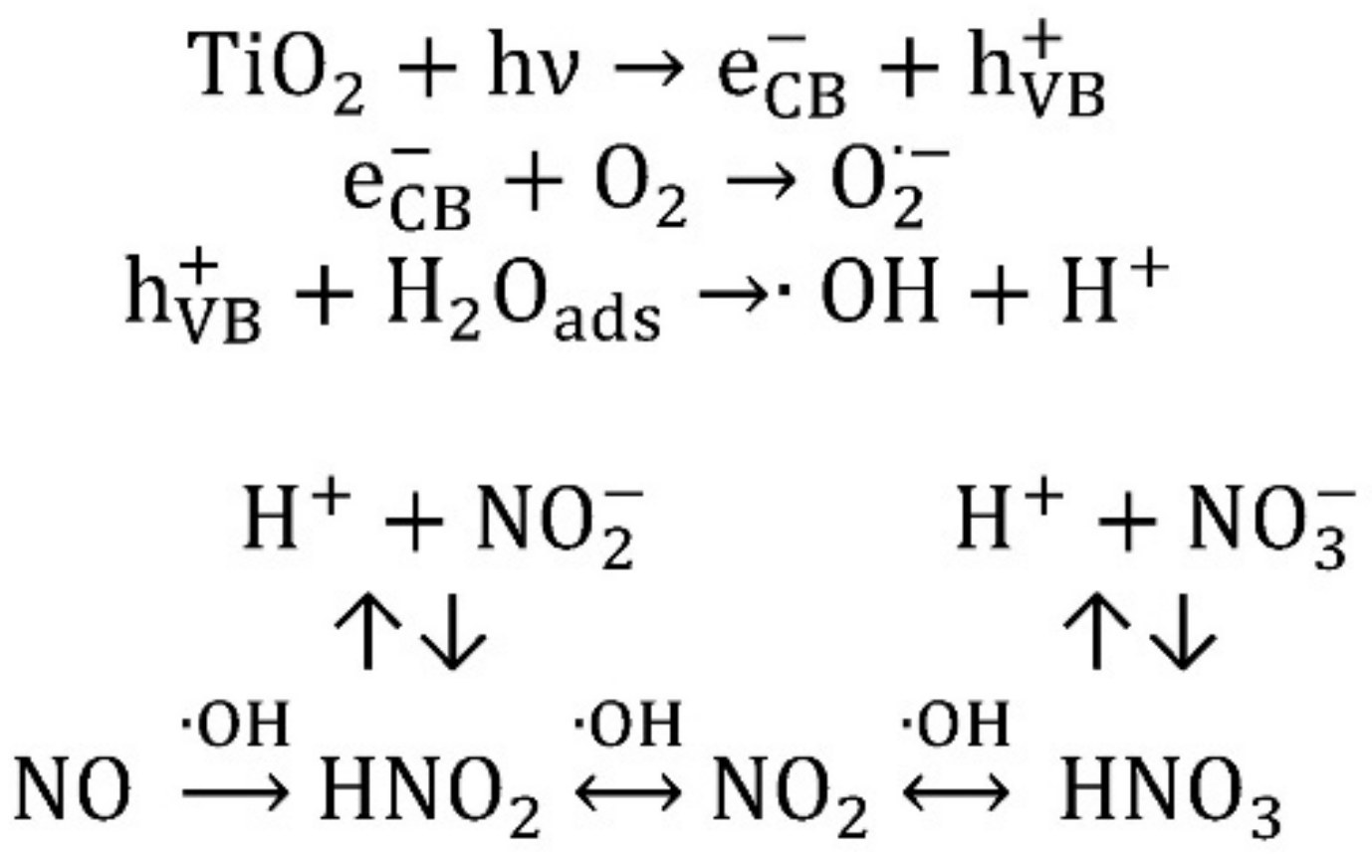
| Target Pollutant | Type of Irradiation | |
|---|---|---|
| UV | Visible | |
| NOx | UNI 11247 | DIS 17168-1 |
| ISO 22197-1 | ||
| DIN CEN/TS 16980-1 | ||
| JIS R 1701-1 | ||
| Acetaldehyde | ISO 22197-2 | DIS 17168-2 |
| JIS R 1701-2 | CD 19652 | |
| Toluene | ISO 22197-3 | DIS 17168-3 |
| JIS R 1701-3 | ||
| Formaldehyde | ISO 22197-4 | DIS 17168-4 |
| JIS R 1701-4 | ISO 18560-1 | |
| Methyl mercaptan | ISO 22197-5 | DIS 17168-5 |
| JIS R 1701-5 | ||
| µ, Spectrum Peak (nm) | σ2, Variance (nm2) | (W m−2 nm−1) | R2 | ||
|---|---|---|---|---|---|
| Declared * | Effective | ||||
| LED 1 | blue | 467.5 | 15.2 | 1.11 | 0.9860 |
| LED 2 | 420–430 | 424.4 | 10.5 | 1.10 | 0.9845 |
| LED 3 | 400–410 | 402.4 | 10.8 | 0.46 | 0.9927 |
| LED 4 | 385–390 | 378.9 | 7.2 | 0.74 | 0.9848 |
| LED 5 | 375–380 | 377.8 | 6.9 | 0.43 | 0.9782 |
| LED 6 | 365–370 | 370.6 | 7.0 | 0.40 | 0.9726 |
| LED | hSiO2/TiO2 | P25 | KRONOClean7000 | hTiO2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Peak (nm) | ANO | ANO | ANO | ANO2 | ANO | ANO2 | ANO | ANO2 |
| LED 1 | 467 | 0.00 | 0.00 | 0.05 | 0.01 | 0.27 | 0.17 | 0.00 | 0.00 |
| LED 2 | 424 | 0.06 | 0.00 | 0.30 | 0.22 | 0.27 | 0.17 | 0.00 | 0.00 |
| LED 3 | 402 | 0.16 | 0.05 | 0.31 | 0.11 | 0.29 | 0.14 | 0.01 | 0.00 |
| LED 4 | 379 | 0.30 | 0.08 | 0.33 | 0.06 | 0.30 | 0.07 | 0.17 | 0.05 |
| LED 5 | 378 | 0.27 | 0.10 | 0.34 | 0.06 | 0.30 | 0.05 | 0.10 | 0.06 |
| LED 6 | 371 | 0.27 | 0.11 | 0.34 | 0.07 | 0.30 | 0.04 | 0.13 | 0.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierpaoli, M.; Favoni, O.; Fava, G.; Ruello, M.L. A Novel Method for the Combined Photocatalytic Activity Determination and Bandgap Estimation. Methods Protoc. 2018, 1, 22. https://doi.org/10.3390/mps1020022
Pierpaoli M, Favoni O, Fava G, Ruello ML. A Novel Method for the Combined Photocatalytic Activity Determination and Bandgap Estimation. Methods and Protocols. 2018; 1(2):22. https://doi.org/10.3390/mps1020022
Chicago/Turabian StylePierpaoli, Mattia, Orlando Favoni, Gabriele Fava, and Maria Letizia Ruello. 2018. "A Novel Method for the Combined Photocatalytic Activity Determination and Bandgap Estimation" Methods and Protocols 1, no. 2: 22. https://doi.org/10.3390/mps1020022
APA StylePierpaoli, M., Favoni, O., Fava, G., & Ruello, M. L. (2018). A Novel Method for the Combined Photocatalytic Activity Determination and Bandgap Estimation. Methods and Protocols, 1(2), 22. https://doi.org/10.3390/mps1020022