Cord Blood-Based Neonatal Screening for Hemoglobinopathies in Northern Tunisia
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Laboratory Analyses
2.2.1. Hematological Analyses
2.2.2. Hemoglobin Electrophoresis
2.2.3. Molecular Analyses
2.3. Statistical Analyses
3. Results
3.1. Study Population and Initial Screening
3.2. Abnormal Patterns
3.3. Detection of β-Thalassemia Carriers
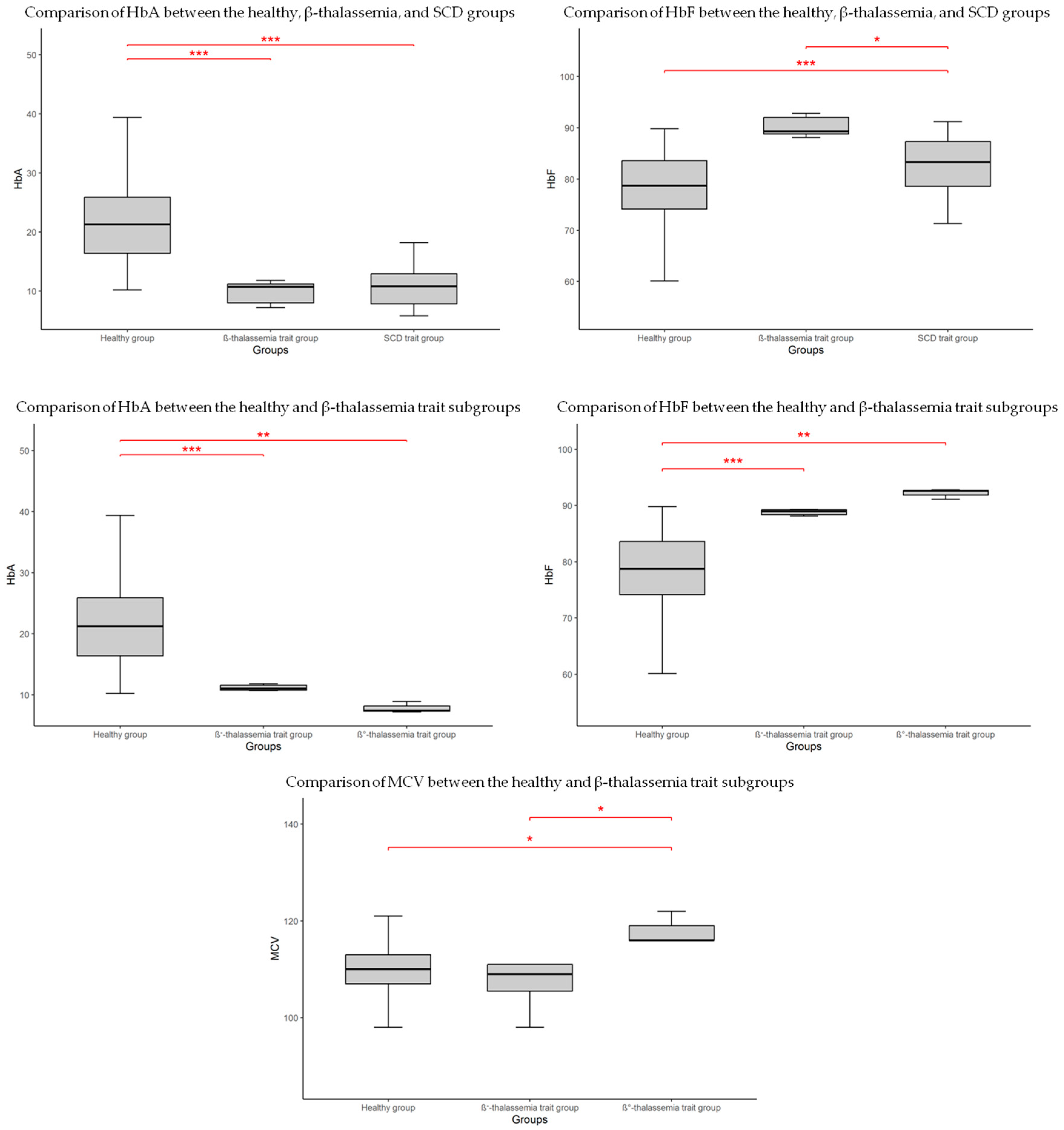
3.4. Hematological Comparisons Between Groups
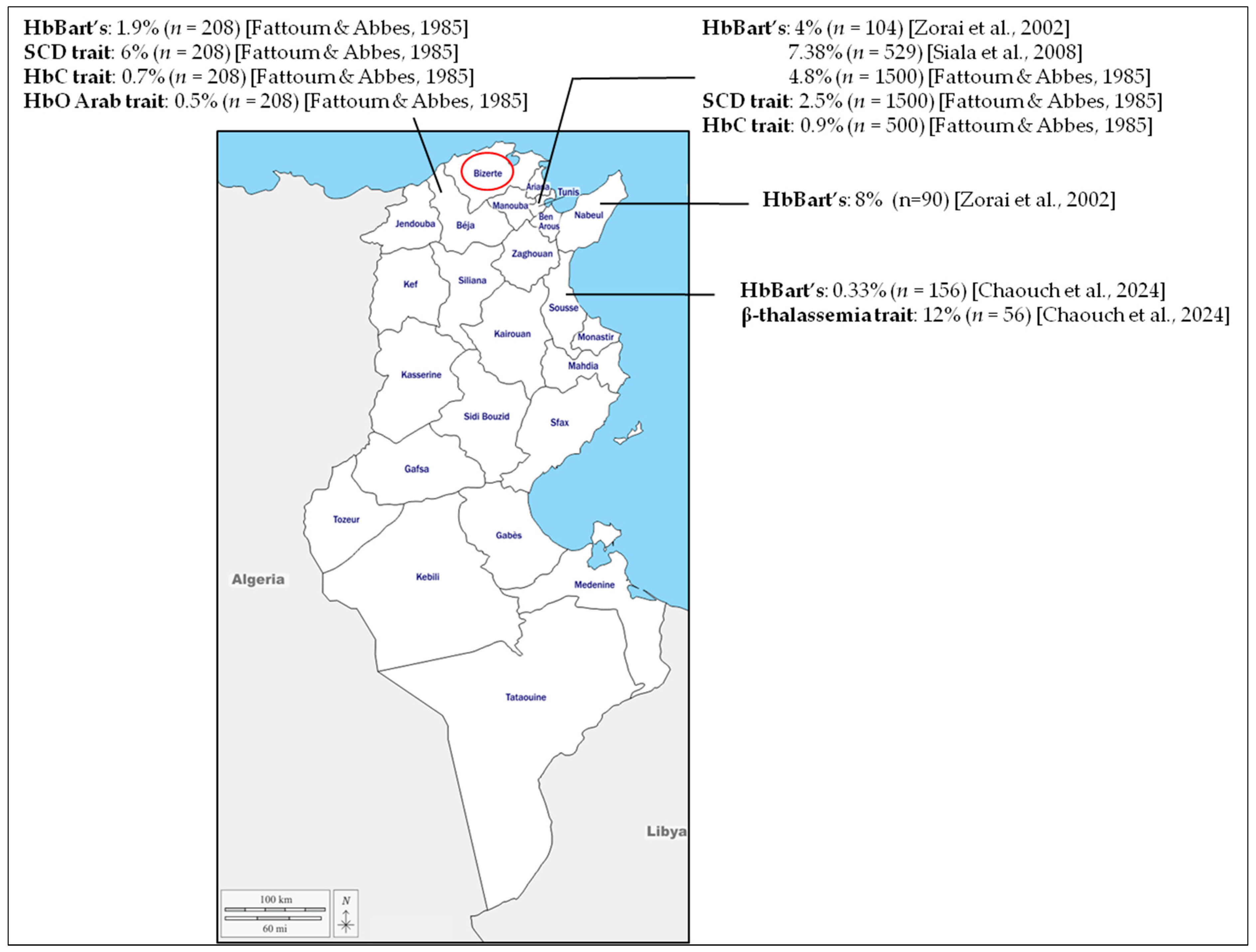
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hb | Hemoglobin |
| SCD | Sickle Cell Disease |
| CB | Cord Blood |
| CBC | Complete Blood Count |
| RBC | Red Blood Cell |
| Hct | Hematocrit |
| MCV | Mean Corpuscular Volume |
| MCH | Mean Corpuscular Hemoglobin |
| RDW | Red Cell Distribution Width |
| CE | Capillary Electrophoresis |
| SD | Standard Deviation |
References
- Weatherall, D.J.; Clegg, J.B. Inherited haemoglobin disorders: An increasing global health problem. Bull. World Health Organ. 2001, 79, 704–712. [Google Scholar]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef]
- Sani, A.; Idrees Khan, M.; Shah, S.; Tian, Y.; Zha, G.; Fan, L.; Zhang, Q.; Cao, C. Diagnosis and screening of abnormal hemoglobins. Clin. Chim. Acta 2024, 552, 117685. [Google Scholar] [CrossRef]
- Fattoum, S. Hemoglobinopathies in Tunisia. An updated review of the epidemiologic and molecular data. Tunis. Med. 2006, 84, 687–696. [Google Scholar]
- Farashi, S.; Harteveld, C.L. Molecular basis of α-thalassemia. Blood Cells Mol. Dis. 2018, 70, 43–53. [Google Scholar] [CrossRef]
- Viprakasit, V.; Ekwattanakit, S. Clinical classification, screening and diagnosis for thalassemia. Hematol. Oncol. Clin. N. Am. 2018, 32, 193–211. [Google Scholar] [CrossRef]
- Musallam, K.M.; Cappellini, M.D.; Coates, T.D.; Kuo, K.H.M.; Al-Samkari, H.; Sheth, S.; Viprakasit, V.; Taher, A.T. Alpha-thalassemia: A practical overview. Blood Rev. 2024, 64, 101165. [Google Scholar] [CrossRef]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Tesio, N.; Bauer, D.E. Molecular Basis and Genetic Modifiers of Thalassemia. Hematol. Oncol. Clin. N. Am. 2023, 37, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Needs, T.; Gonzalez-Mosquera, L.F.; Lynch, D.T. Beta Thalassemia; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. 2019, 14, 263–292. [Google Scholar] [CrossRef]
- Thein, S.L. Genetic Basis and Genetic Modifiers of β-Thalassemia and Sickle Cell Disease. Adv. Exp. Med. Biol. 2017, 1013, 27–57. [Google Scholar] [CrossRef]
- Kohne, E. Hemoglobinopathies: Clinical manifestations, diagnosis, and treatment. Dtsch. Arztebl. Int. 2011, 108, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Quarmyne, M.O.; Bock, F.; Lakshmanan, S.; Attell, B.K.; Snyder, A.; Boudreaux, J.; Sheth, S.; Bender, M.A.; Lal, A. Newborn Screening for Sickle Cell Disease and Thalassemia. JAMA Health Forum 2025, 6, e250064. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L. Newborn Screening for Hemoglobin Disorders. Clin. Perinatol. 2025, 52, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Tshilolo, L.; Kafando, E.; Sawadogo, M.; Cotton, F.; Vertongen, F.; Ferster, A.; Gulbis, B. Neonatal screening and clinical care programmes for sickle cell disorders in sub-Saharan Africa: Lessons from pilot studies. Public Health 2008, 122, 933–941. [Google Scholar] [CrossRef]
- El-Haj, N.; Hoppe, C.C. Newborn Screening for SCD in the USA and Canada. Int. J. Neonatal Screen. 2018, 4, 36. [Google Scholar] [CrossRef]
- Colah, R.B.; Mehta, P.; Mukherjee, M.B. Newborn Screening for Sickle Cell Disease: Indian Experience. Int. J. Neonatal Screen. 2018, 4, 31. [Google Scholar] [CrossRef]
- Shook, L.M.; Ware, R.E. Sickle cell screening in Europe: The time has come. Br. J. Haematol. 2018, 183, 534–535. [Google Scholar] [CrossRef]
- Lobitz, S.; Telfer, P.; Cela, E.; Allaf, B.; Angastiniotis, M.; Backman Johansson, C.; Badens, C.; Bento, C.; Bouva, M.J.; Canatan, D.; et al. Newborn screening for sickle cell disease in Europe: Recommendations from a Pan-European Consensus Conference. Br. J. Haematol. 2018, 183, 648–660. [Google Scholar] [CrossRef]
- Colombatti, R.; Cela, E.; Elion, J.; Lobitz, S. Editorial for Special Issue “Newborn Screening for Sickle Cell Disease and other Haemoglobinopathies”. Int. J. Neonatal Screen. 2019, 5, 36. [Google Scholar] [CrossRef]
- Therrell, B.L.; Padilla, C.D.; Borrajo, G.J.C.; Khneisser, I.; Schielen, P.C.J.I.; Knight-Madden, J.; Malherbe, H.L.; Kase, M. Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020–2023). Int. J. Neonatal Screen. 2024, 10, 38. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Chouk, I.; Daoud, B.B.; Mellouli, F.; Bejaoui, M.; Gerard, N.; Dellagi, K.; Abbes, S. Contribution to the description of the beta-thalassemia spectrum in Tunisia and the origin of mutation diversity. Hemoglobin 2004, 28, 189–195. [Google Scholar] [CrossRef]
- Chong, S.S.; Boehm, C.D.; Higgs, D.R.; Cutting, G.R. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood 2000, 95, 360–362. [Google Scholar] [CrossRef]
- Mantikou, E.; Arkesteijn, S.G.; Beckhoven van, J.M.; Kerkhoffs, J.L.; Harteveld, C.L.; Giordano, P.C. A brief review on newborn screening methods for hemoglobinopathies and preliminary results selecting beta thalassemia carriers at birth by quantitative estimation of the HbA fraction. Clin. Biochem. 2009, 42, 1780–1785. [Google Scholar] [CrossRef]
- Alkindi, S.; Pathare, A.; Al-Madhani, A.; Al-Zadjali, S.; Al-Haddabi, H.; Al-Abri, Q.; Gravell, D.; Mathew, M.; Krishnamoorthy, R. Neonatal Screening: Mean haemoglobin and red cell indices in cord blood from Omani neonates. Sultan Qaboos Univ. Med. J. 2011, 11, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Anwar, W.A.; Khyatti, M.; Hemminki, K. Consanguinity and genetic diseases in North Africa and immigrants to Europe. Eur. J. Public Health 2014, 1, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Weslati, R.; Ouederni, M.; Ruffo, G.; Khaled, M.B.; Kouki, R.; Di Girgenti, C.; Borsellino, Z.; Sammartano, I.; El Gazzah, M.; El-Bok, S.; et al. Consanguineous unions and endogamy in families of beta-thalassaemia patients from two Mediterranean populations: Tunisia and Italy. Ann. Hum. Biol. 2019, 46, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Zorai, A.; Harteveld, C.L.; Bakir, A.; Van Delft, P.; Falfoul, A.; Dellagi, K.; Abbes, S.; Giordano, P.C. Molecular spectrum of alpha-thalassemia in Tunisia: Epidemiology and detection at birth. Hemoglobin 2002, 26, 353–362. [Google Scholar] [CrossRef]
- Fattoum, S. Evolution of hemoglobinopathy prevention in Africa: Results, problems and prospect. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009005. [Google Scholar] [CrossRef]
- Almahmoud, R.; Hussein, A.; Khaja, F.A.; Soliman, A.F.; Dewedar, H.; Shareef, Z.A.; Mathai, S. Growth and endocrinopathies among children with β-Thalassemia major treated at Dubai Thalassemia centre. BMC Pediatr. 2024, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- Wolff, F.; Cotton, F.; Gulbis, B. Screening for haemoglobinopathies on cord blood: Laboratory and clinical experience. J. Med. Screen. 2012, 19, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.A.; Lucke, A.M.; Cummings, J.J. Committee on Fetus and Newborn. Postnatal Cord Blood Sampling: Clinical Report. Pediatrics 2025, 155, e2025071811. [Google Scholar] [CrossRef]
- Louahabi, A.; Philippe, M.; Lali, S.; Wallemacq, P.; Maisin, D. Evaluation of a new Sebia kit for analysis of hemoglobin fractions and variants on the Capillarys system. Clin. Chem. Lab. Med. 2006, 44, 340–345. [Google Scholar] [CrossRef]
- Shook, L.M.; Haygood, D.; Quinn, C.T. Clinical Utility of the Addition of Molecular Genetic Testing to Newborn Screening for Hemoglobinopathies for Confirmation of Alpha-Thalassemia Trait. Int. J. Neonatal Screen. 2025, 11, 12. [Google Scholar] [CrossRef]
- Pearson, H.A.; O’Brien, R.T. Sickle cell testing programs. J. Pediatr. 1972, 81, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.A.; O’Brien, R.T.; McIntosh, S.; Aspnes, G.T.; Yanget, M.M. Routine screening of umbilical cord blood for sickle cell diseases. JAMA 1974, 227, 420–421. [Google Scholar] [CrossRef]
- Fattoum, S.; Abbes, S. Some data on the epidemiology of hemoglobinopathies in Tunisia. Hemoglobin 1985, 9, 423–429. [Google Scholar] [CrossRef]
- Siala, H.; Ouali, F.; Messaoud, T.; Bibi, A.; Fattoum, S. Alpha-Thalassaemia in Tunisia: Some epidemiological and molecular data. J. Genet. 2008, 87, 229–234. [Google Scholar] [CrossRef]
- Chaouch, L.; Moumni, I.; Ben Abdallah, J.; Bouchahda, R.; Methlouthi, J.; Mahdhaoui, N.; Matamri, W.; Braham, N.; Bouguila, F.; Mejri, L.; et al. New Born Screening of Hemoglobinopathies in a Center Tunisian Population. J. Pediatr. Hematol. Oncol. 2024, 46, e296–e299. [Google Scholar] [CrossRef]
- Lin, C.K.; Chen, L.P.; Chang, H.L.; Sung, Y.C. Underestimation of the coexistence of iron deficiencies and thalassemia minors: A single institution experience in Taiwan. Kaohsiung. J. Med. Sci. 2014, 30, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Lie-Injo, L.E.; Solai, A.; Herrera, A.R.; Nicolaisen, L.; Kan, Y.W.; Wan, W.P.; Hasan, K. Hb Bart’s level in cord blood and deletions of α-globin genes. Blood 1982, 59, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Bain, B.J. Laboratory techniques for the identification of abnormalities of globin chain synthesis. In Haemoglobinopathy Diagnosis, 2nd ed.; Bain, B.J., Ed.; Black-Well Publishing: Oxford, UK, 2006; pp. 26–62. [Google Scholar]
- Uaprasert, N.; Settapiboon, R.; Amornsiriwat, S.; Sarnthammakul, P.; Thanapat, T.; Rojnuckarin, P.; Sutcharitchan, P. Diagnostic utility of isoelectric focusing and high performance liquid chromatography in neonatal cord blood screening for thalassemia and non-sickling hemoglobinopathies. Clin. Chim. Acta 2014, 427, 23–26. [Google Scholar] [CrossRef]
- Munkongdee, T.; Pichanun, D.; Butthep, P.; Klamchuen, S.; Chalermpolprapa, V.; Winichagoon, P.; Svasti, S.; Fucharoen, S. Quantitative analysis of Hb Bart’s in cord blood by capillary electrophoresis system. Ann. Hematol. 2011, 90, 741–746. [Google Scholar] [CrossRef]
- Laghmich, A.; Alaoui Ismaili, F.Z.; Barakat, A.; Ghailani Nourouti, N.; Khattab, M.; Bennani Mechita, M. Alpha-Thalassemia in North Morocco: Prevalence and Molecular Spectrum. Biomed. Res. Int. 2019, 2019, 2080352. [Google Scholar] [CrossRef]
- Zorai, A.; Moumni, I.; Mosbahi, I.; Douzi, K.; Chaouachi, D.; Guemira, F.; Abbes, S. Rare hemoglobin variants in Tunisian population. Int. J. Lab. Hematol. 2015, 37, 148–154. [Google Scholar] [CrossRef]
- Ouali, F.; Siala, H.; Bibi, A.; Hadj Fredj, S.; Dakhlaoui, B.; Othmani, R.; Ouenniche, F.; Zouari, F.; Bouguerra, B.; Rezigua, H.; et al. Prenatal diagnosis of hemoglobinopathies in Tunisia: An 18 years of experience. Int. J. Lab. Hematol. 2016, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]

| Hemoglobin Pattern | Frequency (n) | Percentage (%) |
|---|---|---|
| FA * | 315 | 96 |
| FAS | 7 | 2 |
| FAC | 2 | 0.6 |
| FA Bart’s | 3 | 0.9 |
| FAC Bart’s | 1 | 0.3 |
| Total | 328 | 100 |
| Genotype | Expected Phenotype (n) | Hb (g/dL) | RBC (106/µL) | Hct (%) | MCV (fL) | MCH (ρg) | RDW (%) | HbF (%) | HbA (%) | HbA2 (%) | HbBart’s (%) | HbS (%) | HbC (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| αα/αα β/β | Healthy (308) | 14.84 ± 2.34 | 4.20 ± 0.92 | 45.66 ± 7.48 | 109.64 ± 5.08 | 32.60 ± 0.84 | 11.62 ± 0.83 | 78.14 ± 7.25 | 21.81 ± 7.21 | 0.06 ± 0.15 | - | - | - |
| −α3.7/αα | Silent α-thalassemia (3) * | 14.7 13 16.2 | 4.74 4.62 4.93 | 46.6 41.4 51.3 | 102 90 104 | 31.3 13.4 13.6 | 11.7 12.9 11.3 | 81.2 64.8 76.9 | 18.3 33.3 22.8 | 0 | 0.5 1.9 0.3 | - | - |
| β/β+/0 | β-thalassemia trait (9) | 15.99 ± 2.60 | 4.41 ± 0.73 | 49.29 ± 8.46 | 111.89 ± 7.79 | 32.49 ± 0.56 | 12.09 ± 1.41 | 90.22 ± 1.90 | 9.76 ± 1.88 | 0 | - | - | - |
| β/β+ | β+-thalassemia trait (6) | 15.48 ± 3.01 | 4.40 ± 0.89 | 47.80 ± 9.81 | 108.83 ± 7.65 | 32.48 ± 0.68 | 12.55 ± 1.48 | 89.25 ± 1.43 | 10.72 ± 1.40 | 0 | - | - | - |
| β/β0 | β0-thalassemia trait (3) * | 18.3 15.4 17.3 | 4.91 4.05 4.36 | 56.8 46.8 53.2 | 116 116 122 | 32.2 32.8 32.5 | 11.5 11.7 10.3 | 92.8 92.6 91.1 | 7.2 7.4 8.9 | 0 | - | - | - |
| β/βS | HbS trait (7) | 15.61 ± 2.96 | 4.45 ± 0.87 | 48.23 ± 9.92 | 108.29 ± 3.90 | 32.47 ± 0.78 | 11.74 ± 0.80 | 82.50 ± 7.16 | 10.90 ± 4.35 | 0 | - | 6.60 ± 2.86 | - |
| β/βC | HbC trait (2) * | 15.9 15.7 | 4.67 4.59 | 50.2 49.8 | 107 105 | 31.7 31.8 | 12.3 12.1 | 64.4 82.8 | 21 10.5 | 0 | - | - | 13.6 6.7 |
| β/βC, −α3.7/αα | HbC trait, silent α-thalassemia (1) * | 10.2 | 3.7 | 33.2 | 90 | 30.7 | 12.9 | 81.4 | 2.1 | 0 | 0.8 | - | 15.7 |
| Mutation | HGVS Nomenclature | Mutation Type | Number of Carriers (n) | Ratio (%) | Hb (g/dL) | MCV (fL) | MCH (ρg) | HbF (%) | HbA (%) |
|---|---|---|---|---|---|---|---|---|---|
| IVS-I-110 G>A | c.93-21G>A | β+ | 3 | 33.3 | 16.2 12.7 12.8 | 11 121 98 | 32.1 33 33.1 | 92 89.1 88.1 | 8 10.9 11.7 |
| Cd39 C>T | c.118C>T | β0 | 2 | 22.2 | 15.4 17.3 | 116 122 | 37.9 39.7 | 92.6 91.1 | 7.4 8.9 |
| PolyA (T>A) | c.*110T>A | β+ | 1 | 1.1 | 15.5 | 105 | 31.5 | 88.8 | 11.2 |
| IVS-I-5 (G>A) | c.92+5G>A | β+ | 1 | 1.1 | 14.8 | 107 | 33.1 | 89.3 | 10.7 |
| IVS-I-6 T>C | c.92+6T>C | β+ | 1 | 1.1 | 20.9 | 111 | 32.1 | 88.2 | 11.8 |
| Cd 6 (−A) | c.20delA | β0 | 1 | 1.1 | 18.3 | 116 | 37.3 | 92.2 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouragini, H.; Halim, N.B.; Zitouni, S.; Chaouachi, D.; Boudrigua, I.; Saidani, N.; Kraiem, I.; Ayachi, A.; Abbes, S.; Mourali, M.; et al. Cord Blood-Based Neonatal Screening for Hemoglobinopathies in Northern Tunisia. Int. J. Neonatal Screen. 2025, 11, 107. https://doi.org/10.3390/ijns11040107
Ouragini H, Halim NB, Zitouni S, Chaouachi D, Boudrigua I, Saidani N, Kraiem I, Ayachi A, Abbes S, Mourali M, et al. Cord Blood-Based Neonatal Screening for Hemoglobinopathies in Northern Tunisia. International Journal of Neonatal Screening. 2025; 11(4):107. https://doi.org/10.3390/ijns11040107
Chicago/Turabian StyleOuragini, Houyem, Nizar Ben Halim, Sana Zitouni, Dorra Chaouachi, Imen Boudrigua, Naima Saidani, Imen Kraiem, Amira Ayachi, Salem Abbes, Mechaal Mourali, and et al. 2025. "Cord Blood-Based Neonatal Screening for Hemoglobinopathies in Northern Tunisia" International Journal of Neonatal Screening 11, no. 4: 107. https://doi.org/10.3390/ijns11040107
APA StyleOuragini, H., Halim, N. B., Zitouni, S., Chaouachi, D., Boudrigua, I., Saidani, N., Kraiem, I., Ayachi, A., Abbes, S., Mourali, M., & Menif, S. (2025). Cord Blood-Based Neonatal Screening for Hemoglobinopathies in Northern Tunisia. International Journal of Neonatal Screening, 11(4), 107. https://doi.org/10.3390/ijns11040107






