Neonatal Screening for Cystic Fibrosis in Hungary—First-Year Experiences
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Study Design
2.2. Samples and Exclusion Criteria
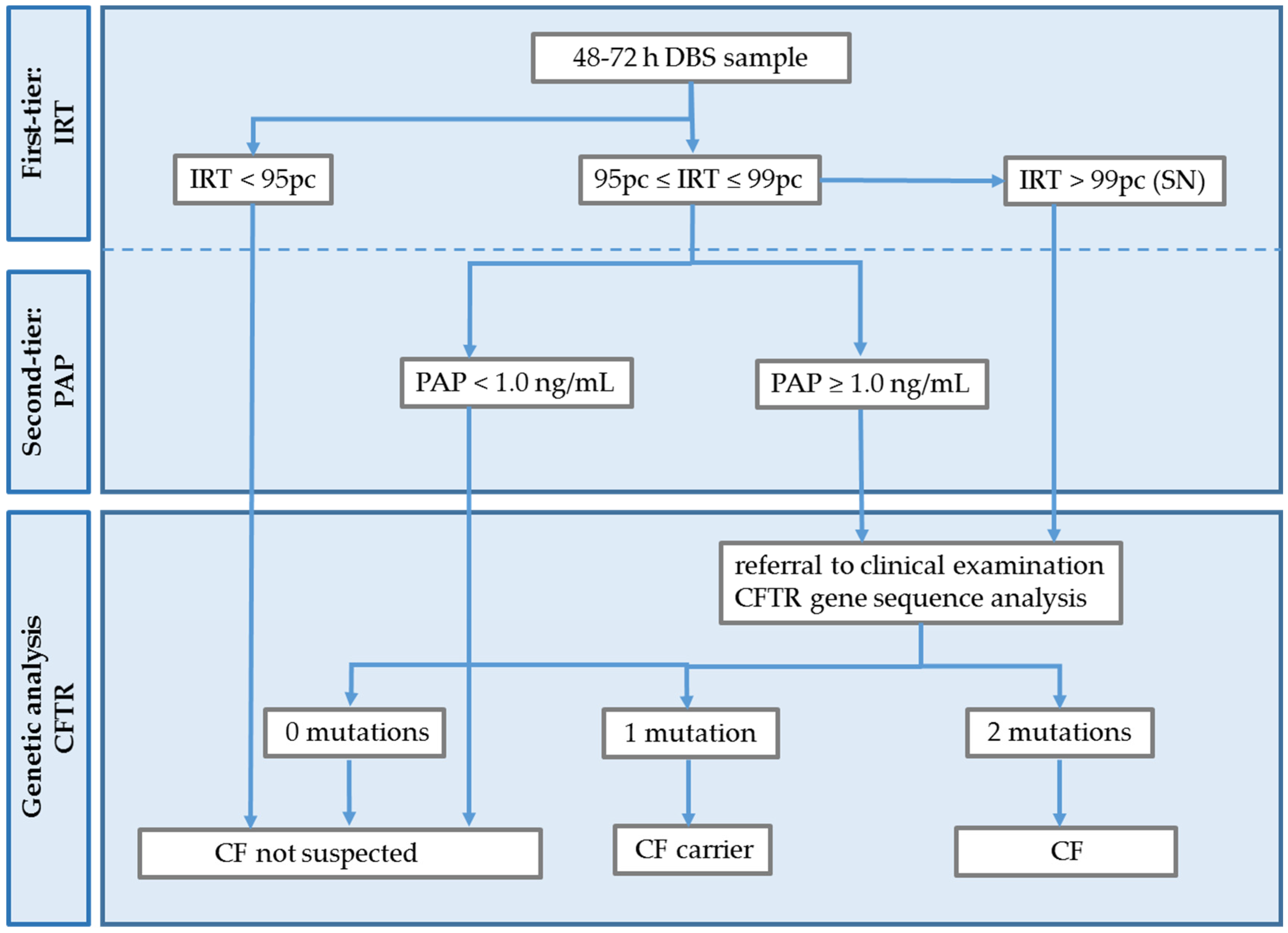
2.3. Screening Test Methods
2.4. CF Diagnosis
2.5. Data Analysis
3. Results
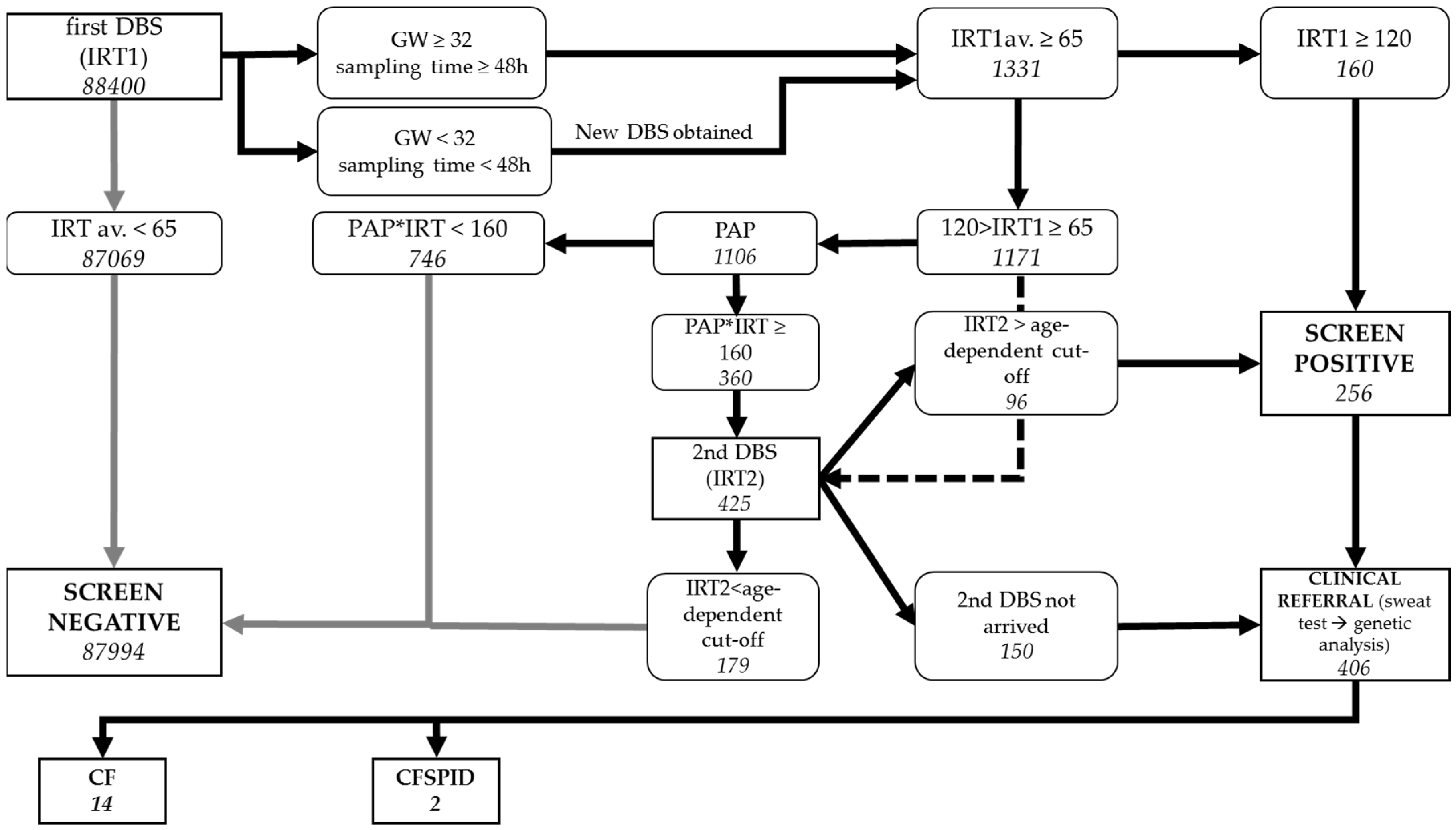
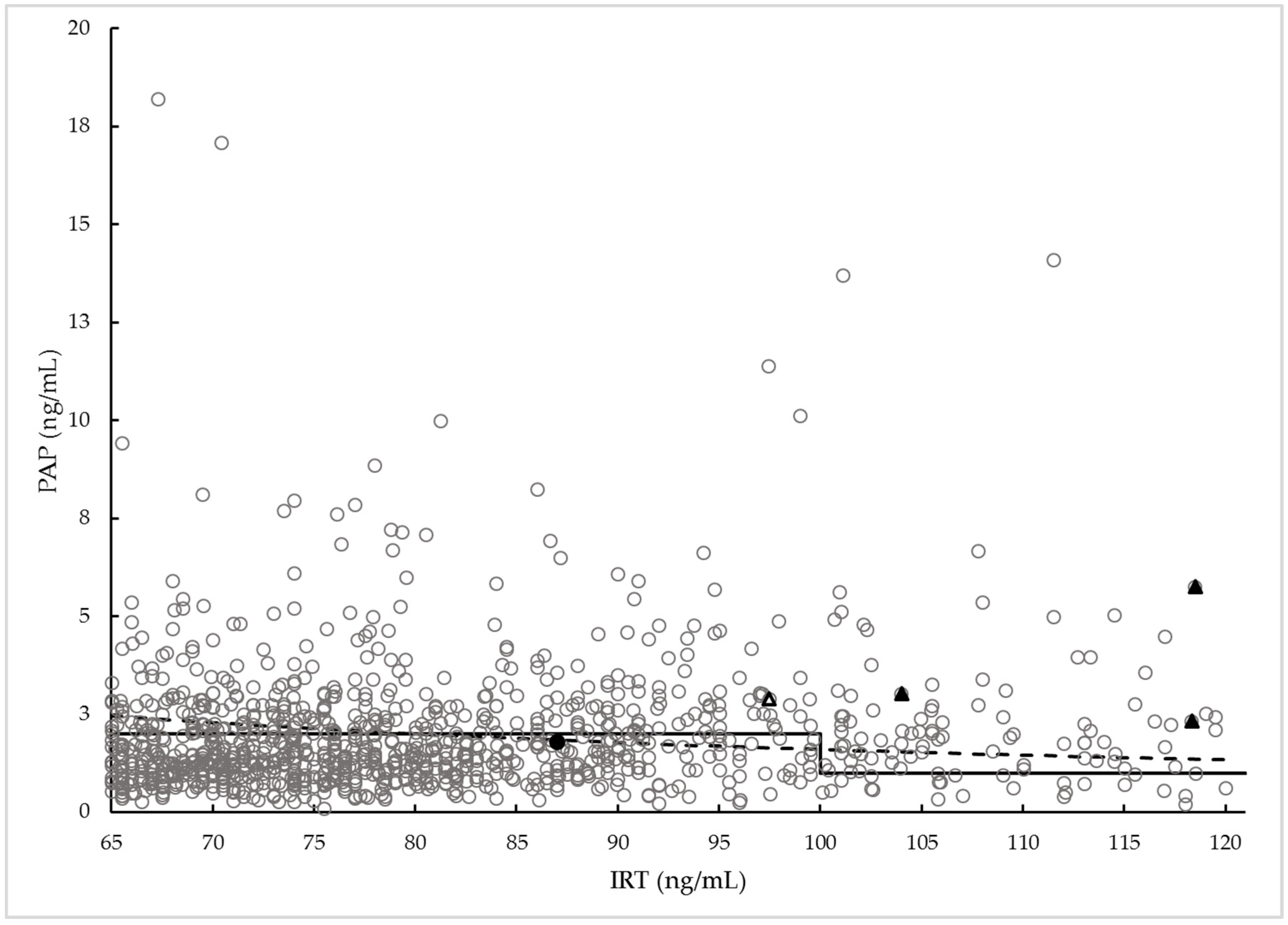
3.1. Pilot Study
3.2. CFNBS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goetz, D.; Ren, C.L. Review of Cystic Fibrosis. Pediatr. Ann. 2019, 48, e154–e161. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Kuca, K.; Novotny, M.; Maresova, P. Cystic Fibrosis Revisited—A Review Study. Med. Chem. 2017, 13, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Barben, J.; Chudleigh, J. Processing Newborn Bloodspot Screening Results for CF. Int. J. Neonatal Screen. 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Course, C.W.; Hanks, R. Newborn screening for cystic fibrosis: Is there benefit for everyone? Paediatr. Respir. Rev. 2019, 31, 3–5. [Google Scholar] [CrossRef]
- Lundman, E.; Gaup, H.J.; Bakkeheim, E.; Olafsdottir, E.J.; Rootwelt, T.; Storrosten, O.T.; Pettersen, R.D. Implementation of newborn screening for cystic fibrosis in Norway. Results from the first three years. J. Cyst. Fibros. 2016, 15, 318–324. [Google Scholar] [CrossRef]
- Torresani, T.; Fingerhut, R.; Rueegg, C.S.; Gallati, S.; Kuehni, C.E.; Baumgartner, M.R.; Barben, J. Newborn screening for cystic fibrosis in Switzerland—Consequences after analysis of a 4 months pilot study. J. Cyst. Fibros. 2013, 12, 667–674. [Google Scholar] [CrossRef][Green Version]
- Rehani, M.R.; Marcus, M.S.; Harris, A.B.; Farrell, P.M.; Ren, C.L. Variation in cystic fibrosis newborn screening algorithms in the United States. Pediatr. Pulmonol. 2023, 58, 927–933. [Google Scholar] [CrossRef]
- Sommerburg, O.; Hammermann, J.; Lindner, M.; Stahl, M.; Muckenthaler, M.; Kohlmueller, D.; Happich, M.; Kulozik, A.E.; Stopsack, M.; Gahr, M.; et al. Five years of experience with biochemical cystic fibrosis newborn screening based on IRT/PAP in Germany. Pediatr. Pulmonol. 2015, 50, 655–664. [Google Scholar] [CrossRef]
- Zeyda, M.; Schanzer, A.; Basek, P.; Bauer, V.; Eber, E.; Ellemunter, H.; Kallinger, M.; Riedler, J.; Thir, C.; Wadlegger, F.; et al. Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters. Diagnostics 2021, 11, 299. [Google Scholar] [CrossRef]
- Sadik, I.; Perez de Algaba, I.; Jimenez, R.; Benito, C.; Blasco-Alonso, J.; Caro, P.; Navas-Lopez, V.M.; Perez-Frias, J.; Perez, E.; Serrano, J.; et al. Initial Evaluation of Prospective and Parallel Assessments of Cystic Fibrosis Newborn Screening Protocols in Eastern Andalusia: IRT/IRT versus IRT/PAP/IRT. Int. J. Neonatal Screen. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Sontag, M.K.; Lee, R.; Wright, D.; Freedenberg, D.; Sagel, S.D. Improving the Sensitivity and Positive Predictive Value in a Cystic Fibrosis Newborn Screening Program Using a Repeat Immunoreactive Trypsinogen and Genetic Analysis. J. Pediatr. 2016, 175, 150–158.e1. [Google Scholar] [CrossRef] [PubMed]
- Sarles, J.; Berthezene, P.; Le Louarn, C.; Somma, C.; Perini, J.M.; Catheline, M.; Mirallie, S.; Luzet, K.; Roussey, M.; Farriaux, J.P.; et al. Combining immunoreactive trypsinogen and pancreatitis-associated protein assays, a method of newborn screening for cystic fibrosis that avoids DNA analysis. J. Pediatr. 2005, 147, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Weidler, S.; Stopsack, K.H.; Hammermann, J.; Sommerburg, O.; Mall, M.A.; Hoffmann, G.F.; Kohlmuller, D.; Okun, J.G.; Macek, M., Jr.; Votava, F.; et al. A product of immunoreactive trypsinogen and pancreatitis-associated protein as second-tier strategy in cystic fibrosis newborn screening. J. Cyst. Fibros. 2016, 15, 752–758. [Google Scholar] [CrossRef]
- Sommerburg, O.; Krulisova, V.; Hammermann, J.; Lindner, M.; Stahl, M.; Muckenthaler, M.; Kohlmueller, D.; Happich, M.; Kulozik, A.E.; Votava, F.; et al. Comparison of different IRT-PAP protocols to screen newborns for cystic fibrosis in three central European populations. J. Cyst. Fibros. 2014, 13, 15–23. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181S, S4–S15.e11. [Google Scholar] [CrossRef]
- Munck, A.; Mayell, S.J.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K.W. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. 2015, 14, 706–713. [Google Scholar] [CrossRef]
- Ren, C.L.; Borowitz, D.S.; Gonska, T.; Howenstine, M.S.; Levy, H.; Massie, J.; Milla, C.; Munck, A.; Southern, K.W. Cystic Fibrosis Transmembrane Conductance Regulator-Related Metabolic Syndrome and Cystic Fibrosis Screen Positive, Inconclusive Diagnosis. J. Pediatr. 2017, 181S, S45–S51.e41. [Google Scholar] [CrossRef]
- Sommerburg, O.; Hammermann, J. Pancreatitis-Associated Protein in Neonatal Screening for Cystic Fibrosis: Strengths and Weaknesses. Int. J. Neonatal Screen. 2020, 6, 28. [Google Scholar] [CrossRef]
- Kharrazi, M.; Sacramento, C.; Comeau, A.M.; Hale, J.E.; Caggana, M.; Kay, D.M.; Lee, R.; Reilly, B.; Thompson, J.D.; Nasr, S.Z.; et al. Missed Cystic Fibrosis Newborn Screening Cases due to Immunoreactive Trypsinogen Levels below Program Cutoffs: A National Survey of Risk Factors. Int. J. Neonatal Screen. 2022, 8, 58. [Google Scholar] [CrossRef]
- Deak, A.; Koczok, K.; Bessenyei, B.; Szucs, Z.; Madar, L.; Csorba, G.; Orosz, O.; Laki, I.; Halasz, A.; Marsal, G.; et al. [Genetic revision of the Hungarian Cystic Fibrosis Registry]. Orv. Hetil. 2022, 163, 2052–2059. [Google Scholar] [PubMed]
- Rock, M.J.; Mischler, E.H.; Farrell, P.M.; Wei, L.J.; Bruns, W.T.; Hassemer, D.J.; Laessig, R.H. Newborn Screening for Cystic-Fibrosis Is Complicated by Age-Related Decline in Immunoreactive Trypsinogen Levels. Pediatrics 1990, 85, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Jungner, Y.G. [Principles and practice of mass screening for disease]. Bol. Oficina Sanit. Panam. 1968, 65, 281–393. [Google Scholar] [PubMed]
- Kay, D.M.; Maloney, B.; Hamel, R.; Pearce, M.; DeMartino, L.; McMahon, R.; McGrath, E.; Krein, L.; Vogel, B.; Saavedra-Matiz, C.A.; et al. Screening for cystic fibrosis in New York State: Considerations for algorithm improvements. Eur. J. Pediatr. 2016, 175, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, R.; Rueegg, C.S.; Imahorn, O.; Pedersen, E.S.L.; Kuehni, C.E.; Gallati, S.; Regamey, N.; Barben, J. Immunoreactive trypsinogen in healthy newborns and infants with cystic fibrosis. Arch. Dis. Child Fetal Neonatal Ed. 2023, 108, 176–181. [Google Scholar] [CrossRef]
- Vernooij-van Langen, A.M.; Loeber, J.G.; Elvers, B.; Triepels, R.H.; Roefs, J.; Gille, J.J.; Reijntjens, S.; Dompeling, E.; Dankert-Roelse, J.E. The influence of sex, gestational age, birth weight, blood transfusion, and timing of the heel prick on the pancreatitis-associated protein concentration in newborn screening for cystic fibrosis. J. Inherit. Metab. Dis. 2013, 36, 147–154. [Google Scholar] [CrossRef]
- Rock, M.J.; Mischler, E.H.; Farrell, P.M.; Bruns, W.T.; Hassemer, D.J.; Laessig, R.H. Immunoreactive trypsinogen screening for cystic fibrosis: Characterization of infants with a false-positive screening test. Pediatr. Pulmonol. 1989, 6, 42–48. [Google Scholar] [CrossRef]
- Sommerburg, O.; Stahl, M.; Hammerling, S.; Gramer, G.; Muckenthaler, M.U.; Okun, J.; Kohlmuller, D.; Happich, M.; Kulozik, A.E.; Mall, M.A.; et al. Final results of the southwest German pilot study on cystic fibrosis newborn screening—Evaluation of an IRT/PAP protocol with IRT-dependent safety net. J. Cyst. Fibros. 2022, 21, 422–433. [Google Scholar] [CrossRef]
- Scotet, V.; Gutierrez, H.; Farrell, P.M. Newborn Screening for CF across the Globe-Where Is It Worthwhile? Int. J. Neonatal Screen. 2020, 6, 18. [Google Scholar] [CrossRef]
| Patient ID | IRT1 (ng/mL) | PAP (ng/mL) | IRT×PAP (ng2/mL2) | IRT2 (ng/mL) | ST (mmol/L) | Mutation | GW (Weeks) | BW (g) | Gender | Age at Diagnosis (Days) |
|---|---|---|---|---|---|---|---|---|---|---|
| CF1(FN) * | 121.0 | - | - | 36.0 | - | F508del/F508del | 38 | 2870 | F | 112 |
| CF2(MI) | 121.0 | - | - | 100.0 | - | F508del/F508del | 37 | 3250 | F | intrauterine |
| CF3 | 104.0 | 3.0 | 365 | 108.6 | Cl: 82 | F508del/F508del | 39 | 3090 | F | 85 |
| CF4 | 118.5 | 5.8 | 713 | 55.0 | F508del/F508del | 40 | 4150 | M | 134 | |
| CF5 | 143.5 | 6.5 | 933 | - | Cl: 71 | F508del/F508del | 41 | 3400 | F | 60 |
| CF6 | 140.0 | 11.0 | 1540 | - | Cl: 79 | F508del/E92 * | 40 | 3660 | F | 39 |
| CF7(FN) | 87.5 | 1.6 | 139 | F508del/F508del | 39 | 3000 | M | 109 | ||
| CF8 | 273.7 | 37.6 | 10,290 | - | F508del/2143delT | 34 | 2140 | F | 53 | |
| CF9 | 216.3 | 5.3 | 1149 | - | Cl: 96 | F508del/F508del | 38 | 2390 | F | 35 |
| CF10 | 359.0 | 28.2 | 10,124 | - | F508del/F508del | 38 | 2900 | M | 48 | |
| CF11 | 640.7 | 24.1 | 15,440 | - | F508del/F508del | 39 | 2650 | F | 24 | |
| CF12 | 283.0 | 4.9 | 1384 | - | F508del/2789+5G>A | 39 | 2920 | M | 39 | |
| CF13 | 118.3 | 2.3 | 275 | 106.5 | Cl: 80 | F508del/F508del | 38 | 2750 | F | 58 |
| CF14 | 342.3 | 8.8 | 2999 | - | Cl: 98 | F508del/G745X | 38 | 3870 | F | 37 |
| CF15 | 281.0 | 15.4 | 4327 | - | Cl: 35 | F508del/F508del | 39 | 3000 | M | 30 |
| CF16 ** | 143.0 | 2.0 | 286 | - | Cl: 50 | 3272-26A>G/C524X | 36 | 3060 | M | 190 |
| CFSPID1 *** | 97.5 | 2.9 | 282 | - | Cl: 52 | F508del/5TTG12 | 39 | 2900 | F | 96 |
| CFSPID2 | 215.0 | 4.5 | 968 | - | Cl: 21 | F508del/R117H;7T | 40 | 3480 | F | 118 |
| Screening for CF Characteristics | IRT/IRT×PAP/IRT+SN |
|---|---|
| Infants screened for CF | 88,400 |
| Infants with CF + CFSPID (% of infants screened) | 16 + 2 (0.02%) |
| CF screen-positive results (% of infants screened) | 256 (0.29%) |
| Infants referred to CF centre for sweat test | 406 |
| Detected cases with CF (% of infants with CF+CFSPID) | 14 (78%) |
| False-positive cases (% of infants screened) | 238 (0.27%) |
| Detected CF +CFSPID related to CF screen-positive cases | ~1:16 |
| CF screen-negative results (% of infants screened) | 87,994 (99.5%) |
| True-negative cases (% of infants screened) | 87,992 (99.5%) |
| False-negative results (% of infants with CF+CFSPID) | 2 (11%) |
| Sensitivity | 88% |
| Specificity | 99.7% |
| PPV | 5.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, A.; Lénárt, I.; Kincs, J.; Szabó, H.; Párniczky, A.; Balogh, I.; Deák, A.; Monostori, P.B.; Hegedűs, K.; Szabó, A.J.; et al. Neonatal Screening for Cystic Fibrosis in Hungary—First-Year Experiences. Int. J. Neonatal Screen. 2023, 9, 47. https://doi.org/10.3390/ijns9030047
Xue A, Lénárt I, Kincs J, Szabó H, Párniczky A, Balogh I, Deák A, Monostori PB, Hegedűs K, Szabó AJ, et al. Neonatal Screening for Cystic Fibrosis in Hungary—First-Year Experiences. International Journal of Neonatal Screening. 2023; 9(3):47. https://doi.org/10.3390/ijns9030047
Chicago/Turabian StyleXue, Andrea, István Lénárt, Judit Kincs, Hajnalka Szabó, Andrea Párniczky, István Balogh, Anna Deák, Péter Béla Monostori, Krisztina Hegedűs, Attila J. Szabó, and et al. 2023. "Neonatal Screening for Cystic Fibrosis in Hungary—First-Year Experiences" International Journal of Neonatal Screening 9, no. 3: 47. https://doi.org/10.3390/ijns9030047
APA StyleXue, A., Lénárt, I., Kincs, J., Szabó, H., Párniczky, A., Balogh, I., Deák, A., Monostori, P. B., Hegedűs, K., Szabó, A. J., & Szatmári, I. (2023). Neonatal Screening for Cystic Fibrosis in Hungary—First-Year Experiences. International Journal of Neonatal Screening, 9(3), 47. https://doi.org/10.3390/ijns9030047






