Identifying Clinical Criteria for an Expanded Targeted Approach to Screening for Congenital Cytomegalovirus Infection—A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
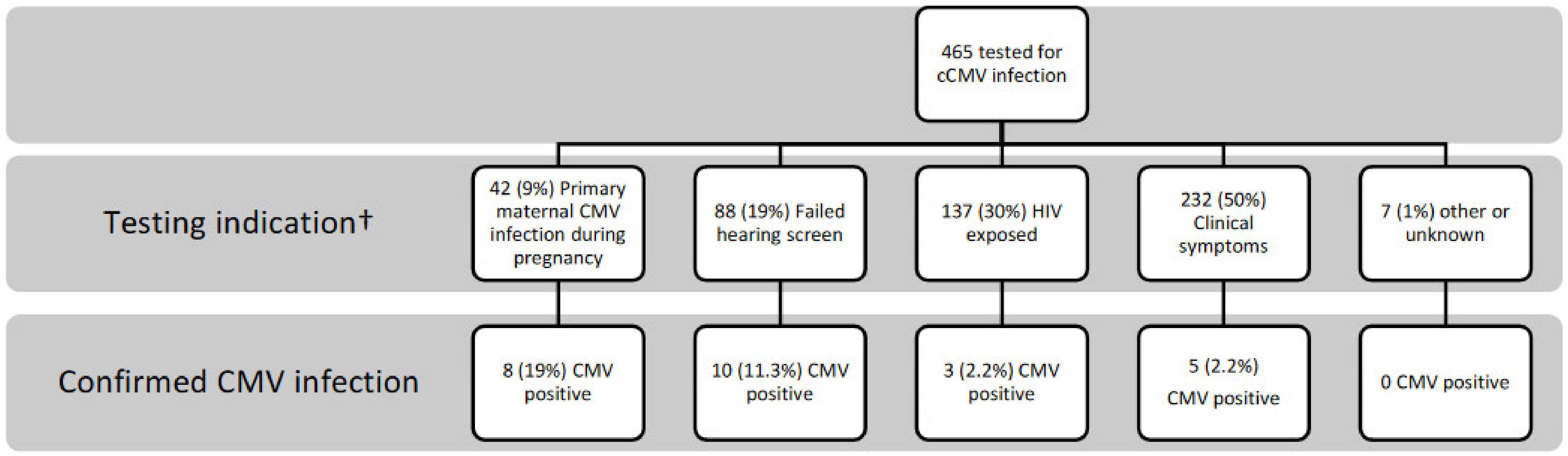
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Ross, S.A.; Shimamura, M.; Palmer, A.L.; Ahmed, A.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; Tolan, R.W.; Novak, Z.; et al. Saliva Polymerase-Chain-Reaction Assay for Cytomegalovirus Screening in Newborns. N. Engl. J. Med. 2011, 364, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Dollard, S.C.; Dreon, M.; Hernandez-Alvarado, N.; Amin, M.M.; Wong, P.; Lanzieri, T.M.; Osterholm, E.A.; Sidebottom, A.; Rosendahl, S.; McCann, M.T.; et al. Sensitivity of Dried Blood Spot Testing for Detection of Congenital Cytomegalovirus Infection. JAMA Pediatr. 2021, 175, e205441. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries with Universal Screening. JAMA Net. Open 2021, 4, e2120736. [Google Scholar] [CrossRef]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef]
- Grosse, S.D.; Ross, D.S.; Dollard, S.C. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: A quantitative assessment. J. Clin. Virol. 2008, 41, 57–62. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Chung, W.; Flores, M.; Blum, P.; Caviness, A.C.; Bialek, S.R.; Grosse, S.D.; Miller, J.A.; Demmler-Harrison, G. Hearing Loss in Children With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics 2017, 139, 20162610. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Barnett, E.D.; Lynfield, R.; Sawyer, M.H. Cytomegalovirus Infection. In Red Book: 2021–2024 Report of the Committee on Infectious Diseases; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- Grosse, S.D.; Leung, J.; Lanzieri, T.M. Identification of congenital CMV cases in administrative databases and implications for monitoring prevalence, healthcare utilization, and costs. Curr. Med. Res. Opin. 2021, 37, 769. [Google Scholar] [CrossRef]
- Sorichetti, B.; Goshen, O.; Pauwels, J.; Kozak, F.K.; Tilley, P.; Krajden, M.; Gantt, S. Symptomatic Congenital Cytomegalovirus Infection Is Underdiagnosed in British Columbia. J. Pediatr. 2016, 169, 316. [Google Scholar] [CrossRef]
- Cannon, M.J.; Griffiths, P.D.; Aston, V.; Rawlinson, W.D. Universal newborn screening for congenital CMV infection: What is the evidence of potential benefit? Rev. Med. Virol. 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Jester, P.M.; Sánchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for Symptomatic Congenital Cytomegalovirus Disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Dionne, F.; Kozak, F.K.; Goshen, O.; Goldfarb, D.M.; Park, A.H.; Boppana, S.B.; Fowler, K. Cost-effectiveness of Universal and Targeted Newborn Screening for Congenital Cytomegalovirus Infection. JAMA Pediatr. 2016, 170, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.L.; Zick, C.D.; McVicar, S.B.; Boettger, J.; Park, A.H. Outcomes From a Hearing-Targeted Cytomegalovirus Screening Program. Pediatrics 2017, 139, e20160789. [Google Scholar] [CrossRef]
- Grosse, S.D.; Dollard, S.C.; Kimberlin, D.W. Screening for Congenital Cytomegalovirus after Newborn Hearing Screening: What Comes Next? Pediatrics 2017, 139, e20163837. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; McCollister, F.P.; Sabo, D.L.; Shoup, A.G.; Owen, K.E.; Woodruff, J.L.; Cox, E.; Mohamed, L.S.; Choo, D.I.; Boppana, S.B. A Targeted Approach for Congenital Cytomegalovirus Screening Within Newborn Hearing Screening. Pediatrics 2017, 139, e20162128. [Google Scholar] [CrossRef] [PubMed]
- Smiljkovic, M.; Le Meur, J.-B.; Malette, B.; Boucoiran, I.; Minsart, A.-F.; Lamarre, V.; Tapiero, B.; Renaud, C.; Kakkar, F. Blood viral load in the diagnostic workup of congenital cytomegalovirus infection. J. Clin. Virol. 2020, 122, 104231. [Google Scholar] [CrossRef]
- Gantt, S.; Bitnun, A.; Renaud, C.; Kakkar, F.; Vaudry, W. Diagnosis and management of infants with congenital cytomegalovirus infection. Paediatr. Child Health 2017, 22, 72. [Google Scholar] [CrossRef]
- Barton, M.; Forrester, A.M.; McDonald, J. Update on congenital cytomegalovirus infection: Prenatal prevention, newborn diagnosis, and management. Paediatr. Child Health 2020, 25, 395–396. [Google Scholar] [CrossRef]
- Ellington, S.R.; Clarke, K.E.; Kourtis, A.P. Cytomegalovirus Infection in Human Immunodeficiency Virus (HIV)-Exposed and HIV-Infected Infants: A Systematic Review. J. Infect. Dis. 2016, 213, 891–900. [Google Scholar] [CrossRef]
- Adachi, K.; Xu, J.; Ank, B.; Watts, D.H.; Camarca, M.; Mofenson, L.M.; Pilotto, J.H.; Joao, E.; Gray, G.; Theron, G.; et al. Congenital Cytomegalovirus and HIV Perinatal Transmission. Pediatr. Infect. Dis. J. 2018, 37, 1016–1021. [Google Scholar] [CrossRef]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv (accessed on 1 March 2023).
- Hyde De Souza, H.; Boucoiran, I.; Valois, S.; Taillefer, S.; Boucher, M.; Soudeyns, H.; Renaud, C.; Lamarre, V.; Kakkar, F. Double Trouble: Increased Risk of Congenital Cytomegalovirus Infection among HIV-exposed Newborns. In Proceedings of the 23rd International AISDS Conference (AIDS 2020), Virtual, 6–10 July 2020; p. 329. [Google Scholar]
- Stagno, S. Primary Cytomegalovirus Infection in Pregnancy. JAMA 1986, 256, 1904. [Google Scholar] [CrossRef] [PubMed]
- Shahar-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: A randomised, double-blind, placebo-controlled trial. Lancet 2020, 396, 779. [Google Scholar] [CrossRef] [PubMed]
- Boucoiran, I.; Boucoiran, I.; Yudin, M.; Poliquin, V.; Caddy, S.; Gantt, S.; Castillo, E. Guideline No. 420: Cytomegalovirus Infection in Pregnancy. J. Obstet. Gynaecol. Can. 2021, 43, 893. [Google Scholar] [CrossRef]
- Roth, D.A.-E.; Lubin, D.; Kuint, J.; Teperberg-Oikawa, M.; Mendelson, E.; Strauss, T.; Barkai, G. Contribution of targeted saliva screening for congenital CMV-related hearing loss in newborns who fail hearing screening. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, 519. [Google Scholar] [CrossRef]
- Vancor, E.; Shapiro, E.D.; Loyal, J. Results of a Targeted Screening Program for Congenital Cytomegalovirus Infection in Infants Who Fail Newborn Hearing Screening. J. Pediatr. Infect. Dis. Soc. 2019, 8, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Stehel, E.K.; Shoup, A.G.; Owen, K.E.; Jackson, G.L.; Sendelbach, D.M.; Boney, L.F.; Sánchez, P.J. Newborn Hearing Screening and Detection of Congenital Cytomegalovirus Infection. Pediatrics 2008, 121, 970. [Google Scholar] [CrossRef]
- Chung, P.K.; Schornagel, F.; Oudesluys-Murphy, A.M.; De Vries, L.S.; Soede, W.; Van Zwet, E.; Vossen, A. Targeted screening for congenital cytomegalovirus infection: Clinical, audiological and neuroimaging findings. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 302–308. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Renaud, C.; Tapiero, B.; Lamarre, V.; Kakkar, F. Head ultrasound, CT or MRI? The choice of neuroimaging in the assessment of infants with congenital cytomegalovirus infection. BMC Pediatr. 2019, 19, 180. [Google Scholar] [CrossRef]
- Suarez, D.; Nielson, C.; McVicar, S.B.; Sidesinger, M.; Ostrander, B.; O’Brien, E.; Ampofo, K.; Ling, C.Y.; Miner, L.J.; Park, A.H. Analysis of an Expanded Targeted Early Cytomegalovirus Testing Program. Otolaryngol. Head Neck Surg. 2023, in press. [Google Scholar] [CrossRef]
- Lamarre, V.; Gilbert, N.L.; Rousseau, C.; Gyorkos, T.W.; Fraser, W.D. Seroconversion for cytomegalovirus infection in a cohort of pregnant women in Québec, 2010–2013. Epidemiol. Infect. 2016, 144, 1701–1709. [Google Scholar] [CrossRef]
- Balegamire, S.J.; Renaud, C.; Mâsse, B.; Zinszer, K.; Gantt, S.; Giguere, Y.; Forest, J.-C.; Boucoiran, I. Frequency, timing and risk factors for primary maternal cytomegalovirus infection during pregnancy in Quebec. PLoS ONE 2021, 16, e0252309. [Google Scholar] [CrossRef]
| CMV Positive Infants | Reason for Testing | Newborn Hearing Screen Results | Symptoms |
|---|---|---|---|
| 1 | Primary maternal CMV infection | Pass | - |
| 2 | Primary maternal CMV infection | Bilateral fail | Growth impairment, Thrombocytopenia, Petechia |
| 3 | Primary maternal CMV infection | Pass | - |
| 4 | Primary maternal CMV infection | Unilateral | Hepatomegaly, Hypotonia |
| 5 | Primary maternal CMV infection | Pass | Thrombocytopenia |
| 6 | Primary maternal CMV infection | Pass | - |
| 7 | Primary maternal CMV infection | Pass | - |
| 8 | Primary maternal CMV infection | Unilateral fail | Growth impairment, Microcephaly, Petechia |
| 9 | Intrauterine HIV exposure | Pass | Growth impairment, Prematurity |
| 10 | Intrauterine HIV exposure | Unilateral fail | - |
| 11 | Intrauterine HIV exposure | Pass | - |
| 12 | Clinical symptoms | Pass | Growth impairment |
| 13 | Clinical symptoms | Pass | Growth impairment, Thrombocytopenia, Prematurity |
| 14 | Clinical symptoms | Pass | Growth impairment, Thrombocytopenia, Microcephaly |
| 15 | Clinical symptoms | Pass | Growth impairment, Thrombocytopenia, Prematurity, Hypotonia |
| 16 | Clinical symptoms | Pass | Growth impairment, Thrombocytopenia, Petechia, Splenomegaly |
| 17 | Failed hearing screen | Unilateral fail | Pneumonitis, Thrombocytopenia |
| 18 | Failed hearing screen | Unilateral fail | Growth impairment, Microcephaly |
| 19 | Failed hearing screen | Unilateral fail | - |
| 20 | Failed hearing screen | Bilateral fail | Growth impairment, Thrombocytopenia, Petechia, Hyperbilirubinemia, Splenomegaly |
| 21 | Failed hearing screen | Bilateral fail | Growth impairment |
| 22 | Failed hearing screen | Unilateral fail | Hypotonia |
| Indications for Testing | Tested (N) | Positive (N) | Percentage (%) |
|---|---|---|---|
| Maternal primary CMV infection (total) | 42 | 8 | 19.1 |
| Maternal primary CMV infection only | 29 | 5 | 17.2 |
| Maternal primary CMV infection + failed hearing screen | 8 | 3 | 37.5 |
| Maternal primary CMV infection + HIV exposed | 5 | 0 | 0 |
| Failed hearing screen (total) | 88 | 10 | 11.4 |
| Failed hearing screen only | 52 | 6 | 11.5 |
| Failed hearing screen + Maternal primary CMV infection. | 8 | 3 | 37.5 |
| Failed hearing screen + HIV exposed | 28 | 1 | 3.6 |
| HIV exposed (Total) | 137 | 3 | 2.2 |
| HIV exposed only | 104 | 2 | 1.9 |
| HIV exposed + Failed hearing screen | 28 | 1 | 3.6 |
| HIV exposed + Maternal primary CMV infection | 5 | 0 | 0 |
| Clinical Symptoms † | 232 | 5 | 2.2 |
| Indication for Testing | Symptoms Alone (n = 232) | All Indication (n = 465) | ||||
|---|---|---|---|---|---|---|
| Symptom | Newborns Tested | CMV+ | Newborns Tested | CMV+ | ||
| N | N | % | N | N | % | |
| Enlarged liver and/or spleen | 7 | 1 | 14.3 | 9 | 3 | 33.3 |
| Microcephaly | 16 | 1 | 6.3 | 32 | 3 | 9.4 |
| Growth impairment | 172 | 5 | 2.9 | 256 | 11 | 4.3 |
| Thrombocytopenia | 102 | 4 | 3.9 | 132 | 8 | 6 |
| Petechia | 8 | 1 | 12.5 | 12 | 4 | 33.3 |
| Hypotonia | 47 | 1 | 2.1 | 74 | 3 | 4 |
| Prematurity | 81 | 2 | 2.5 | 106 | 3 | 2.8 |
| Direct hyperbilirubinemia | 10 | 0 | 0 | 13 | 1 | 7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiva, M.H.; Hyde-De Sousa, H.; Lamarre, V.; Boucoiran, I.; Gantt, S.; Renaud, C.; Kakkar, F. Identifying Clinical Criteria for an Expanded Targeted Approach to Screening for Congenital Cytomegalovirus Infection—A Retrospective Study. Int. J. Neonatal Screen. 2023, 9, 40. https://doi.org/10.3390/ijns9030040
Akiva MH, Hyde-De Sousa H, Lamarre V, Boucoiran I, Gantt S, Renaud C, Kakkar F. Identifying Clinical Criteria for an Expanded Targeted Approach to Screening for Congenital Cytomegalovirus Infection—A Retrospective Study. International Journal of Neonatal Screening. 2023; 9(3):40. https://doi.org/10.3390/ijns9030040
Chicago/Turabian StyleAkiva, Maya Heled, Hannah Hyde-De Sousa, Valerie Lamarre, Isabelle Boucoiran, Soren Gantt, Christian Renaud, and Fatima Kakkar. 2023. "Identifying Clinical Criteria for an Expanded Targeted Approach to Screening for Congenital Cytomegalovirus Infection—A Retrospective Study" International Journal of Neonatal Screening 9, no. 3: 40. https://doi.org/10.3390/ijns9030040
APA StyleAkiva, M. H., Hyde-De Sousa, H., Lamarre, V., Boucoiran, I., Gantt, S., Renaud, C., & Kakkar, F. (2023). Identifying Clinical Criteria for an Expanded Targeted Approach to Screening for Congenital Cytomegalovirus Infection—A Retrospective Study. International Journal of Neonatal Screening, 9(3), 40. https://doi.org/10.3390/ijns9030040





