Foetal Haemoglobin as a Marker of Bone Marrow Suppression Secondary to Anti-Kell Alloimmunisation
Abstract
1. Introduction
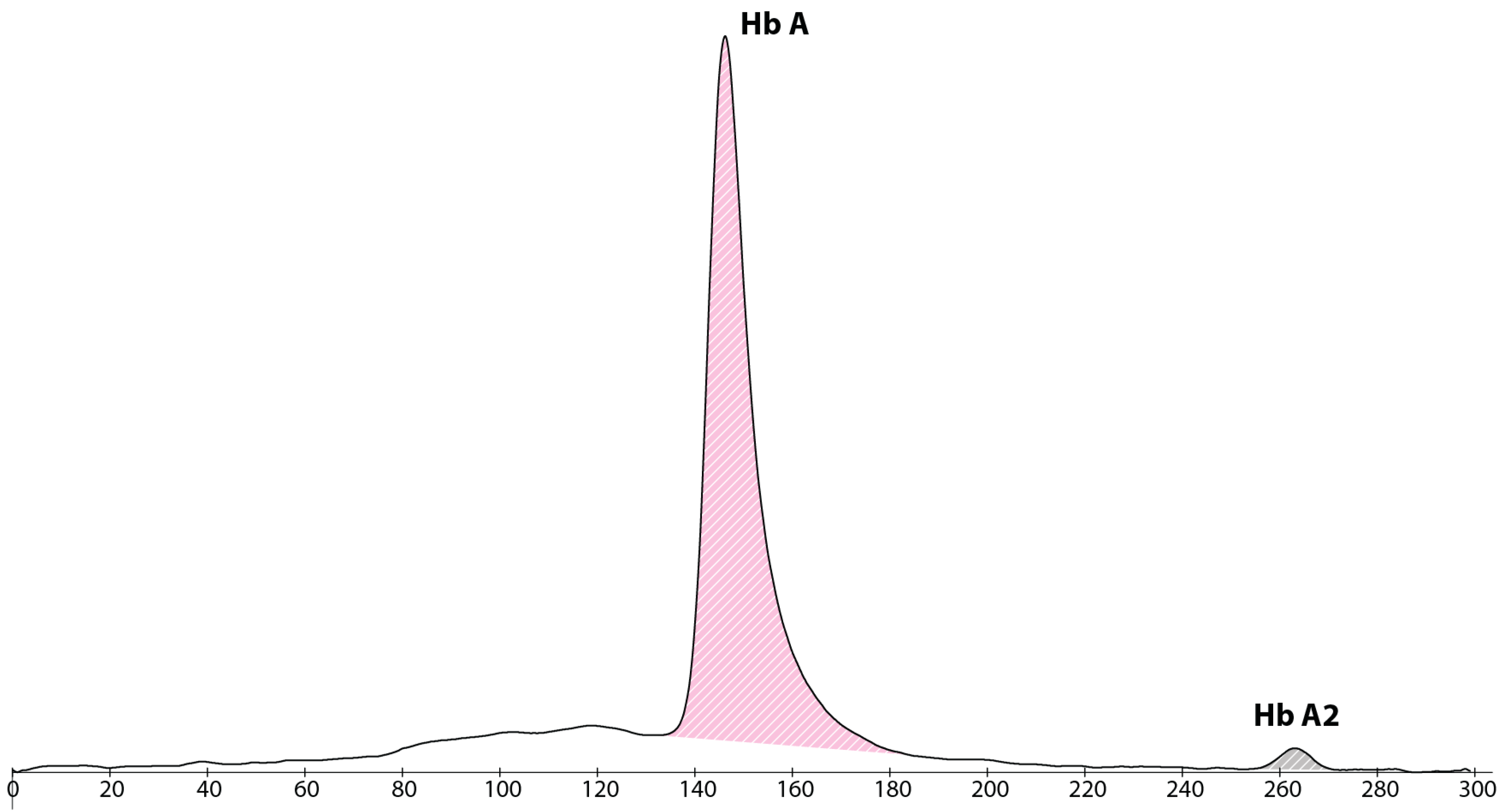
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slootweg, Y.M.; Lindenburg, I.T.; Koelewijn, J.M.; Van Kamp, I.L.; Oepkes, D.; De Haas, M. Predicting anti-Kell-mediated hemolytic disease of the fetus and newborn: Diagnostic accuracy of laboratory management. Am. J. Obstet. Gynecol. 2018, 219, 393.e1–393.e8. [Google Scholar] [CrossRef] [PubMed]
- Koelewijn, J.; Vrijkotte, T.; De Haas, M.; Van Der Schoot, C.; Bonsel, G. Risk factors for the presence of non-rhesus D red blood cell antibodies in pregnancy. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.I.; Manning, M.; Warwick, R.M.; Letsky, E.A.; Murray, N.A.; Roberts, I.A. Inhibition of erythroid progenitor cells by anti-Kell antibodies in fetal alloimmune anemia. N. Engl. J. Med. 1998, 338, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Mari, G.; Deter, R.L.; Carpenter, R.L.; Rahman, F.; Zimmerman, R.; Moise, K.J.; Dorman, K.F.; Ludomirsky, A.; Gonzalez, R.; Gomez, R.; et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N. Engl. J. Med. 2000, 342, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mari, G. Middle cerebral artery peak systolic velocity: Is it the standard of care for the diagnosis of fetal anemia? J. Ultrasound Med. 2005, 24, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Yinon, Y.; Visser, J.; Kelly, E.N.; Windrim, R.; Amsalem, H.; Seaward, P.G.R.; Ryan, G. Early intrauterine transfusion in severe red blood cell alloimmunization. Ultrasound Obstet. Gynecol. 2010, 36, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ree, I.M.; Lopriore, E.; Zwiers, C.; Böhringer, S.; Janssen, M.W.; Oepkes, D.; De Haas, M. Suppression of compensatory erythropoiesis in hemolytic disease of the fetus and newborn due to intrauterine transfusions. Am. J. Obstet. Gynecol. 2020, 223, 119.e1–119.e10. [Google Scholar] [CrossRef] [PubMed]
- Marín Soria, J.L.; López Galera, R.M.; Argudo Ramírez, A.; González de Aledo, J.M.; Pajares García, S.; Navarro Sastre, A.; Hernandez Pérez, J.M.; Ribes Rubio, A.; Gort Mas, L.; García Villoria, J.; et al. 50 años del Programa de Cribado Neonatal en Cataluña. Rev. Esp. Salud Pública 2020, 94, e202012177. [Google Scholar] [PubMed]
- Lobato, G.; Soncini, C.S. Fetal hematocrit decrease after repeated intravascular transfusions in alloimmunized pregnancies. Arch. Gynecol. Obstet. 2007, 276, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, K.M.; Blei, F. Treatment of hemolytic disease of the newborn caused by anti-Kell antibody with recombinant erythropoietin. J. Pediatr. Hematol. Oncol. 2001, 23, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Domínguez, E.; Pérez Fernández, J.M.; Figueras Aloy, J.; Estrany, X.C. Erythropoietin treatment for late anaemia after haemolytic disease of the newborn. An. De Pediatr. 2010, 73, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Zwiers, C.; Lindenburg, I.T.M.; Klumper, F.J.; de Haas, M.; Oepkes, D.; Van Kamp, I.L. Complications of intrauterine intravascular blood transfusion: Lessons learned after 1678 procedures. Ultrasound Obstet. Gynecol. 2017, 50, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Allaf, B.; Pondarre, C.; Allali, S.; De Montalembert, M.; Arnaud, C.; Barrey, C.; Benkerrou, M.; Benhaim, P.; Bensaid, P.; Brousse, V.; et al. Appropriate thresholds for accurate screening for β-thalassemias in the newborn period: Results from a French center for newborn screening. Clin. Chem. Lab. Med. 2020, 59, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.M.; Argudo-Ramírez, A.; García, S.P.; Castillo, J.G.D.A.; Quintero, Y.; Collado, T.; Galera, R.L. Reference values of haemoglobins A and F in newborns according to gestational age. Clin. Chim. Acta 2019, 493, S602. [Google Scholar] [CrossRef]


| Age (days) | 0–2 | 29 | 30 | 41 | 51 | 64 | 80 | 120 | |
|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||
| Weight (grams) | 2900–2720 | 3632 | 3632 | 4100 | 4550 | 5050 | 5490 | 6700 | |
| Haematocrit (%) | 40 | 14 | 33 | 25 | 22 | 21 | 28.4 | 34.7 | |
| Haemoglobin (g/dL) | 12.1 | 4.9 | 11.4 | 8.8 | 7.7 | 7.3 | 9.3 | 12.2 | |
| HbF (%) | 0 | 17.7 | |||||||
| Reticulocytes (%) | 2.6 | 1.5 | 3.2 | 7.3 | 8.6 | 1.11 | |||
| Total Bilirubin (mg/dL) | 2.7–6.2 | 2.3 | |||||||
| Treatment | PT | 20 mL/kg RBC transfusion | None | Oral iron and folic acid | +EPO thrice weekly | Same | STOP EPO Adjustment of oral iron | Same | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales Painamil, R.A.; de Aledo-Castillo, J.M.G.; Teresa-Palacio, M.; Argudo-Ramírez, A.; López-Galera, R.M.; Paredes-Fuentes, A.J.; Aldecoa-Bilbao, V.; Alsina-Casanova, M. Foetal Haemoglobin as a Marker of Bone Marrow Suppression Secondary to Anti-Kell Alloimmunisation. Int. J. Neonatal Screen. 2023, 9, 24. https://doi.org/10.3390/ijns9020024
Morales Painamil RA, de Aledo-Castillo JMG, Teresa-Palacio M, Argudo-Ramírez A, López-Galera RM, Paredes-Fuentes AJ, Aldecoa-Bilbao V, Alsina-Casanova M. Foetal Haemoglobin as a Marker of Bone Marrow Suppression Secondary to Anti-Kell Alloimmunisation. International Journal of Neonatal Screening. 2023; 9(2):24. https://doi.org/10.3390/ijns9020024
Chicago/Turabian StyleMorales Painamil, Rodrigo Alfredo, José Manuel González de Aledo-Castillo, Marta Teresa-Palacio, Ana Argudo-Ramírez, Rosa M. López-Galera, Abraham J. Paredes-Fuentes, Victoria Aldecoa-Bilbao, and Miguel Alsina-Casanova. 2023. "Foetal Haemoglobin as a Marker of Bone Marrow Suppression Secondary to Anti-Kell Alloimmunisation" International Journal of Neonatal Screening 9, no. 2: 24. https://doi.org/10.3390/ijns9020024
APA StyleMorales Painamil, R. A., de Aledo-Castillo, J. M. G., Teresa-Palacio, M., Argudo-Ramírez, A., López-Galera, R. M., Paredes-Fuentes, A. J., Aldecoa-Bilbao, V., & Alsina-Casanova, M. (2023). Foetal Haemoglobin as a Marker of Bone Marrow Suppression Secondary to Anti-Kell Alloimmunisation. International Journal of Neonatal Screening, 9(2), 24. https://doi.org/10.3390/ijns9020024






