Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010–2018
Abstract
1. Introduction
| Condition | Birth Prevalence (per 10,000 Births) in United States |
|---|---|
| Severe combined immunodeficiency (SCID) [11,12] | 0.2 |
| Critical congenital heart disease (CCHD) [13] | 20 |
| Glycogen storage disease, type II (Pompe) [12,14] | 0.3 |
| Mucopolysaccharidosis, type I (MPS I) [12,15] | 0.1 |
| X-linked adrenoleukodystrophy (X-ALD) [12,16] | 0.5 |
| Spinal muscular atrophy (SMA) [17] | 0.5 |
2. Conditions Added to the RUSP, 2010–2018
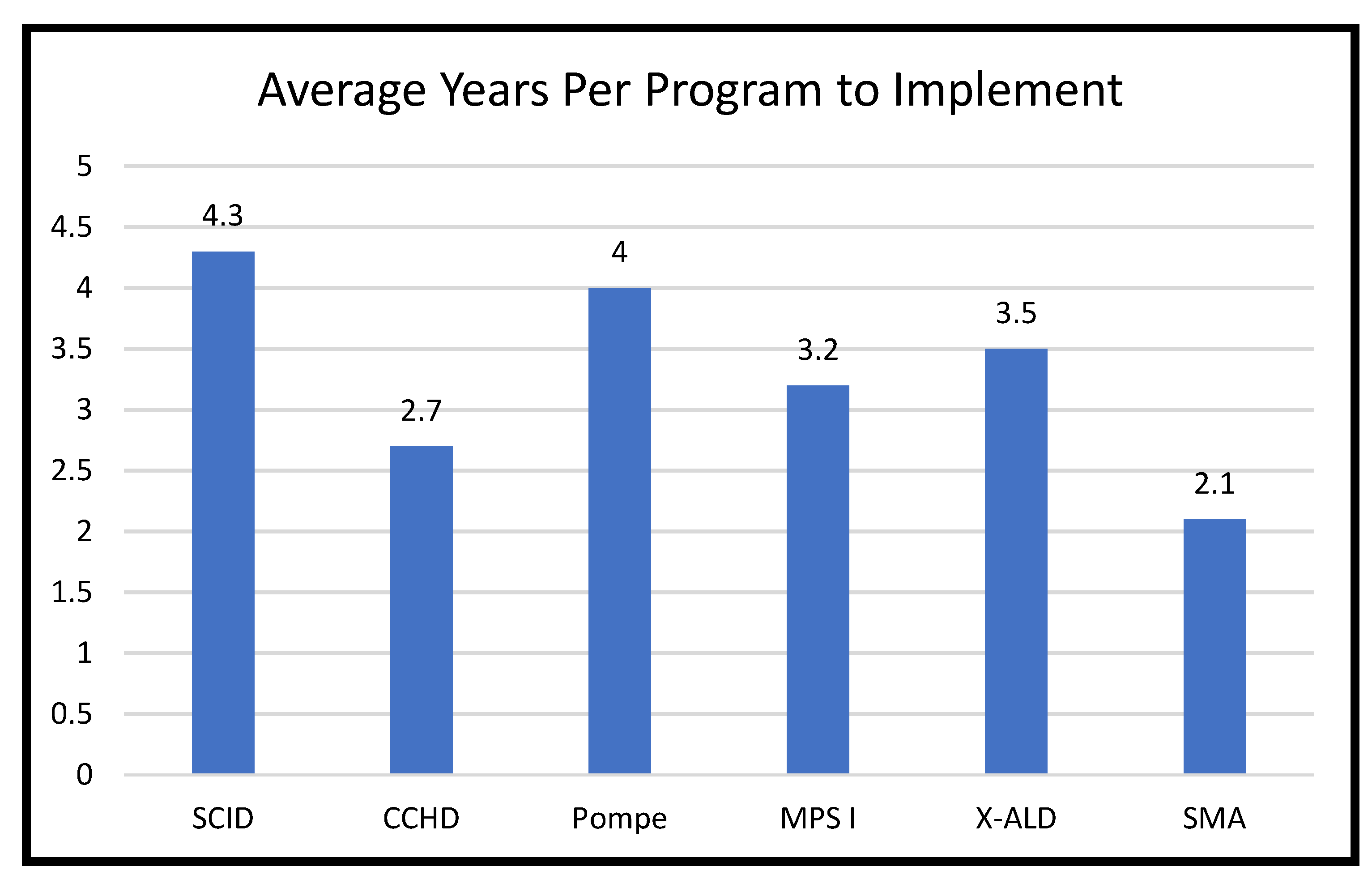
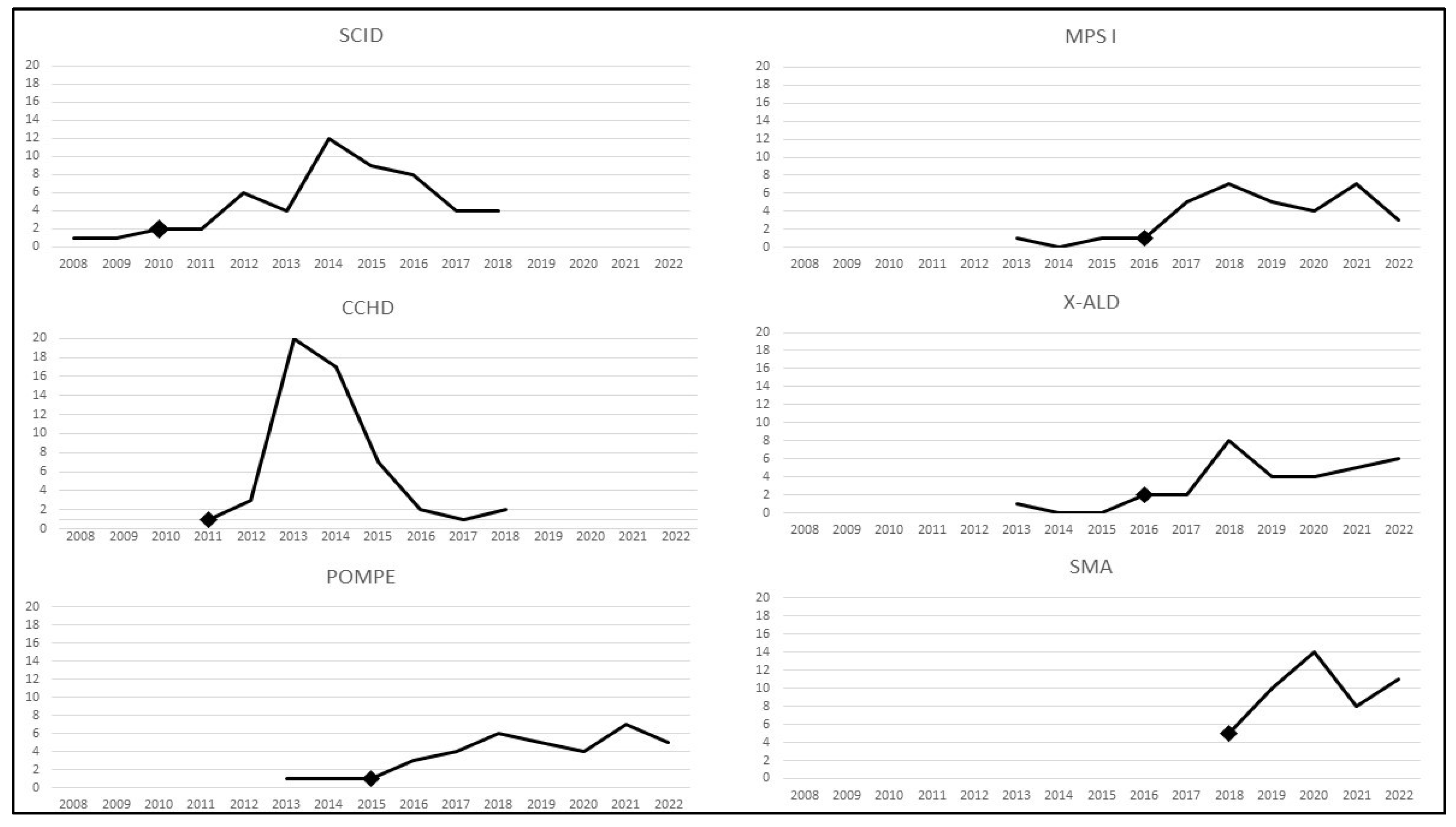
3. Implementation of Screening for Added RUSP Core Conditions in the United States, 2010–2022
3.1. Implementation of Screening for New Core Conditions
3.2. Potential Reasons for Differences in Implementation of New RUSP Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, M.S.; Lloyd-Puryear, M.A.; Howell, R.R. The progress and future of US newborn screening. Int. J. Neonatal Screen. 2022, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.S.; Mann, M.Y.; Lloyd-Puryear, M.A.; Rinaldo, P.; Howell, R.R. American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: Toward a uniform screening panel and system—Executive summary. Pediatrics 2006, 117 (Suppl. S3), S296–S307. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.; Green, N.; Calonge, N.; Lam, W.K.K.; Comeau, A.; Goldenberg, A.; Ojodu, J.; Prosser, L.A.; Tanksley, S.; Bocchini, J.A. Decision-making process for conditions nominated to the Recommended Uniform Screening Panel: Statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet. Med. 2014, 16, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Calonge, N.; Green, N.; Rinaldo, P.; Lloyd-Puryear, M.; Dougherty, D.; Boyle, C.; Watson, M.; Trotter, T.; Terry, S.; Howell, R.; et al. Committee report: Method for evaluating conditions nominated for population-based screening of newborns and children. Genet. Med. 2010, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Lam, W.K.K.; Ream, M.; Lennox, A. NBS Implementation for Conditions Added to the RUSP. In Proceedings of the ACHDNC Meeting; Rockville, MD, USA, 1 August 2019. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/meetings/kemper-implementation-rusp.pdf (accessed on 16 December 2022).
- Kemper, A.R.; Ream, M.; Lam, W.K.K. NBS Implementation for Conditions added to the RUSP: Review of the Previous Report. In Proceedings of the ACHDNC Meeting; Rockville, MD, USA, 1 December 2020. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/meetings/rusp-conditions.pdf (accessed on 16 December 2022).
- Advisory Committee on Heritable Disorders in Newborns and Children. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders (accessed on 16 December 2022).
- Ojodu, J.; Singh, S.; Kellar-Guenther, Y.; Yusuf, C.; Jones, E.; Wood, T.; Baker, M.; Sontag, M.K. NewSTEPs: The establishment of a national newborn screening technical assistance resource center. Int. J. Neonatal Screen. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Kellar-Guenther, Y.; McKasson, S.; Hale, K.; Singh, S.; Sontag, M.K.; Ojodu, J. Implementing statewide newborn screening for new disorders: U.S. program experiences. Int. J. Neonatal Screen. 2020, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Glidewell, J.; Grosse, S.D.; Riehle-Colarusso, T.; Pinto, N.; Hudson, J.; Daskalov, R.; Gaviglio, A.; Darby, E.; Singh, S.; Sontag, M. Actions in support of newborn screening for critical congenital heart disease—United States, 2011–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Lipstein, E.A.; Vorono, S.; Browning, M.F.; Green, N.S.; Kemper, A.R.; Knapp, A.A.; Prosser, L.A.; Perrin, J.M. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics 2010, 125, e1226–e1235. [Google Scholar] [CrossRef] [PubMed]
- Sontag, M.K.; Yusuf, C.; Grosse, S.D.; Edelman, S.; Miller, J.; McKasson, S.; Kellar-Guenther, Y.; Gaffney, M.; Hinton, C.F.; Cuthbert, C.; et al. Infants with congenital disorders identified through newborn screening—United States, 2015–2017. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Mahle, W.T.; Martin, G.R.; Cooley, W.C.; Kumar, P.; Morrow, W.R.; Kelm, K.; Pearson, G.D.; Glidewell, J.; Grosse, S.D.; et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics 2011, 128, e1259–e1267. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Comeau, A.M.; Green, N.S.; Goldenberg, A.; Ojodu, J.; Prosser, L.A.; Tanksley, S.; Weinreich, S.; Lam, K.K. Evidence report: Newborn screening for Pompe disease. In Proceedings of the ACHDNC Meeting; Rockville, MD, USA, 13 June 2013. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/pompe-external-evidence-review-report-2013.pdf (accessed on 16 December 2022).
- Grosse, S.D.; Lam, W.K.K.; Wiggins, L.; Kemper, A.R. Cognitive outcomes and age of detection of severe mucopolysaccharidosis type 1. Genet. Med. 2017, 19, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Brosco, J.; Comeau, A.M.; Green, N.S.; Grosse, S.D.; Jones, E.; Kwon, J.M.; Lam, W.K.K.; Ojodu, J.; Prosser, L.A.; et al. Newborn screening for X-linked adrenoleukodystrophy: Evidence summary and advisory committee recommendation. Genet. Med. 2017, 19, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.F.; Kwon, J.M. Spinal muscular atrophy: Past, present, and future. Neoreviews 2019, 20, e437–e451. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Riehle-Colarusso, T.; Gaffney, M.; Mason, C.A.; Shapira, S.K.; Sontag, M.K.; Braun, K.V.N.; Iskander, J. CDC grand rounds: Newborn screening for hearing loss and critical congenital heart disease. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Puck, J.M. Lessons for sequencing from the addition of severe combined immunodeficiency to newborn screening panels. Hastings Center Rep. 2018, 48 (Suppl. S2), S7–S9. [Google Scholar] [CrossRef] [PubMed]
- Furnier, S.M.; Durkin, M.S.; Baker, M.W. Translating molecular technologies into routine newborn screening practice. Int. J. Neonatal Screen. 2020, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Currier, R.J. Newborn screening is on a collision course with public health ethics. Int. J. Neonatal Screen. 2022, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Malvagia, S.; Forni, G.; Ombrone, D.; la Marca, G. Development of strategies to decrease false positive results in newborn screening. Int. J. Neonatal Screen. 2020, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Boyle, C.A.; Kenneson, A.; Khoury, M.J.; Wilfond, B.S. From public health emergency to public health service: The implications of evolving criteria for newborn screening panels. Pediatrics 2006, 117, 923–929. [Google Scholar] [CrossRef] [PubMed]
| Challenges | Facilitators | |
|---|---|---|
| SCID |
|
|
| CCHD |
|
|
| Pompe |
|
|
| MPS I |
|
|
| X-ALD |
|
|
| SMA |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Ojodu, J.; Kemper, A.R.; Lam, W.K.K.; Grosse, S.D. Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010–2018. Int. J. Neonatal Screen. 2023, 9, 20. https://doi.org/10.3390/ijns9020020
Singh S, Ojodu J, Kemper AR, Lam WKK, Grosse SD. Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010–2018. International Journal of Neonatal Screening. 2023; 9(2):20. https://doi.org/10.3390/ijns9020020
Chicago/Turabian StyleSingh, Sikha, Jelili Ojodu, Alex R. Kemper, Wendy K. K. Lam, and Scott D. Grosse. 2023. "Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010–2018" International Journal of Neonatal Screening 9, no. 2: 20. https://doi.org/10.3390/ijns9020020
APA StyleSingh, S., Ojodu, J., Kemper, A. R., Lam, W. K. K., & Grosse, S. D. (2023). Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010–2018. International Journal of Neonatal Screening, 9(2), 20. https://doi.org/10.3390/ijns9020020






