Abstract
Genomic advances have contributed to a proliferation of newborn screening (NBS) programs. Psychosocial consequences of NBS have been identified as risks to these public health initiatives. Following PRISMA guidelines, this systematic review synthesizes findings from 92 evidence-based, peer-reviewed research reports published from 2000 through 2020 regarding psychosocial issues associated with NBS. Results describe parents’ knowledge of and attitudes towards NBS, reactions to and understanding of positive NBS results, experiences of communication with health providers, decisions about carrier testing, and future pregnancies. Findings also explain the impact of positive NBS results on parent–child relationships, child development, informing children about carrier status, family burden, quality of life, and disparities. In conclusion, psychosocial consequences of receiving unexpected neonatal screening results and unsolicited genetic information remain significant risks to expansion of NBS. Findings suggest that risks may be mitigated by improved parent NBS education, effective communication, individualized genetic counseling, and anticipatory developmental guidance. Clinicians need to take extra measures to ensure equitable service delivery to marginalized subpopulations. Future investigations should be more inclusive of culturally and socioeconomically diverse families and conducted in low-resource countries. Providing these countries with adequate resources to develop NBS programs is an essential step towards achieving international health equity.
1. Introduction
Newborn screening (NBS) programs are public health initiatives that screen infants shortly after birth to identify those at risk for serious health conditions, most of which are genetic. Early diagnosis and treatment can reduce infant mortality, morbidity, and long-term complications [1]. In 1962, the United States (US) implemented a mass NBS program for phenylketonuria (PKU) [2], marking a paradigm shift from diagnosis based on clinical presentation to identifying pre-symptomatic infants. In 1968, the World Health Organization (WHO) established criteria requiring conditions on NBS panels to have established treatments [3]. In 2004, the American College of Medical Genetics surveyed experts and stakeholders to develop new NBS criteria, and recommended a core panel of 29 conditions for all states [4]. The new criteria included conditions for which early detection offered potential long-term benefits to children and families. The WHO criteria were expanded in 2008 to include less well-known conditions, which created opportunities to document the natural clinical course of such conditions, produced more precise diagnostic tests, and led to more effective treatments [5].
Countries worldwide have NBS programs that screen for a variety of conditions. Most panels include core conditions and secondary conditions [6]. Core conditions are those for which there is “compelling evidence of benefit” for early detection, intervention, and treatment. Secondary conditions are identified incidentally, and have less evidence of benefit from early detection [7,8].
Since its inception, NBS has generated debate. Proponents point to the benefits of early treatment to improve the health and lifespans of affected children, while families avoid a costly, stressful, and protracted diagnostic process. Preventing diagnostic delays in underrepresented or economically underserved populations also advances health equity [9]. Identifying more complex inherited disorders through tandem mass spectrometry and other novel genetic technologies further expands knowledge of risk beyond single-gene conditions [10,11]. Future use of whole genome sequencing in NBS could exponentially increase the number of conditions identified, benefiting ever-increasing numbers of children.
Conversely, concerns about the psychosocial ramifications of NBS for parents and infants with abnormal (hereafter, “positive”) results remain. Tandem mass spectrometry detects mild conditions that require no treatment. Advanced technologies identify unaffected heterozygote carriers, for whom NBS may provide no known health benefits. Findings can also reveal non-paternity. Parents’ lack of knowledge about NBS and genetics lead to confusion about results and the need for subsequent diagnostic testing—all of which can engender parental distress and worry about the infant’s health [1,12].
A systematic review of 28 studies published between 1981–2000 found that positive results were associated with parental emotional distress due to poor provider communication, particularly for false-positive results [12]. In many European countries, parental consent is required [13]. In other countries, such as the US, consent is not required and NBS is mandatory except for religious exemptions. Thus, results come as a shock to parents during a vulnerable time in their lives when they are likely to feel fatigued and overwhelmed and may be struggling with postpartum depression [14]. Unsolicited genetic information from NBS can also impact parents’ future reproductive decisions, raising concerns about state interference with private family matters [15]. While experts recognize the importance of understanding the psychosocial consequences of imparting unexpected genetic risk information to parents, and the effects on their relationships with their child [16], no global reviews of such issues within the context of NBS have been completed since the expansion of NBS in the early 2000s. The purpose of this systematic literature review was to synthesize the most recent empirical evidence regarding psychosocial issues associated with NBS in the era of genetic technologies.
2. Methods
A team of content and methods experts followed PRISMA guidelines in conducting this systematic review of peer-reviewed research publications that focused on psychosocial issues associated with NBS [17]. For this project, psychosocial was defined as the “interaction of social, cultural, and environmental influences on the mind and behavior” [18], and also included factors related to human emotion, cognition, child development, and interpersonal relationships [19].
We included qualitative, quantitative, and mixed methods studies in which participants were parents of any age with children of any age who underwent NBS. We also included third party observations of families affected by positive NBS results. All articles were written in English and published between 2000 and 2020 in peer-reviewed journals. We excluded systematic or narrative reviews, opinion papers, study proposals, secondary sources, dissertations, and parent reports about hypothetical situations or opinions about NBS if they did not have a child who underwent NBS. See Table 1 for terms and databases used for this literature review.

Table 1.
Databases and Search Terms.
Using an eligibility checklist, two team members screened each article first by title, then abstract, and finally full text, sorting each article into three categories: yes (include), maybe (might include), and no (exclude) [20]. Discrepancies were addressed during team meetings. We repeated these procedures with an ancestry search of references from included articles. Figure 1 illustrates the screening and selection process.
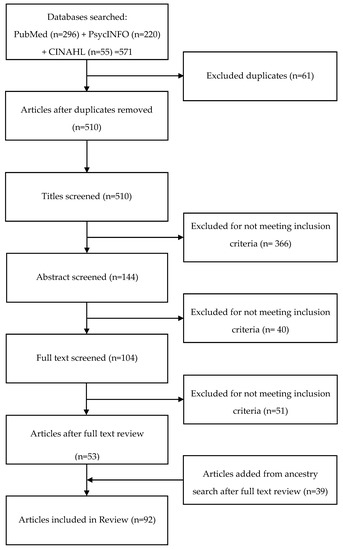
Figure 1.
Screening Flow Chart.
Data Extraction, Analysis and Synthesis
Data extracted from each article were stored in separate Word documents. To ensure accuracy and reliability, two team members extracted data independently. Regular team meetings were held to discuss and resolve inconsistencies and confirm accuracy of extracted data. Concurrent with data extraction, we conducted thematic analyses to inductively identify categories and manifest patterns in summary data [21] and incorporated sub-themes to describe specific issues. Finally, we noted gaps in the empirical evidence and directions for future NBS research.
3. Results
3.1. Search and Screening Results
The initial search of PubMed, PyscINFO, and CINAHL produced 571 articles, with 511 remaining after duplicates were removed (Figure 1, Table 2). The screening process reduced the number of included articles to 53. An ancestry search of reference lists from these articles produced an additional 39 articles. Thus, the final review included 92 articles listed in Table 3.

Table 2.
Inclusion/Exclusion Criteria.

Table 3.
Study Characteristics.
Studies were conducted in 12 countries, though the majority took place in North America (59.8%, n = 55) and Europe (26.1%, n = 24). Conditions screened included cystic fibrosis (CF, n = 53), sickle cell anemia and other hemoglobinopathies (SCD, n = 13), metabolic disorders (n = 13), type 1 diabetes (T1D, n = 9), congenital hypothyroidism (CH, n = 6), fragile X syndrome (FXS, n = 3), Duchenne and Becker muscular dystrophy (DMD/BMD, n = 2), and NBS in general (n = 10). Most studies used cross-sectional designs (87%, n = 80) and quantitative data (42%, n = 39), with fewer using qualitative (30%, n = 28) or mixed methods approaches (27%, n = 25). A few studies involved video observations (7%, n = 6). More than half of included articles focused on infants with false-positive NBS results.
3.2. Parent Knowledge of and Attitudes about NBS and/or Dried Blood Spots
Research repeatedly showed that parents were uninformed about NBS, conditions screened, implications of positive results, or the storage of dried blood spots (DBS) for future research [34,48,49,66,70,74]. In one study, most postpartum women with healthy infants [74] reported that they had not received prenatal education about NBS, did not know how they would receive results, and did not know that abnormal results had implications for parents. About half were unsure or did not know where to take their baby for follow-up testing if required and many did not understand that NBS identifies serious genetic disorders in infants. Postnatal education on NBS, provided by postpartum nurses or midwives, was common [23,74,75,92]. Short postpartum hospital stays, mothers’ physiological needs, and time constraints of caring for their newborns posed challenges to effective postnatal parent NBS education [74,75]. Recall improved when parents received a pamphlet about NBS postpartum, vs. prenatally [49], though findings could reflect a recency effect. By contrast, another study showed that parents preferred receiving NBS information prenatally [39].
To address gaps in parent knowledge, researchers recommended that knowledgeable health professionals provide written and verbal information [23,92], offer more information on DBS [38], and accommodate parent sociodemographics, such as income, primary language, and education [23,29,31]. Parents expressed an interest in receiving more information about the reasons for and benefits of NBS, conditions identified, how to interpret positive results, the probability of positive results, timing of receiving results, and the possibility of additional testing [92]. Expanding responsibility for family education about NBS to include primary care and prenatal providers [23,39], and improving parents’ understanding of false-positive and true-positive results, were also proposed [23]. Relying on printed material and referring parents to online resources were identified as ineffective approaches to parental education [49].
Despite the anxiety and distress associated with their experiences of NBS, parents of children with false-positive results [41,79,80,104] and children diagnosed with a condition [33,37,38] supported routine NBS. Reasons included satisfaction with communication and gratitude for receiving information about their child’s health [35,39,40] or their own carrier status [70]. Parents believed that NBS was in children’s best interest because they could benefit from early treatment [76]. Additional benefits were avoiding the emotional and financial costs of diagnostic delays, receiving information relevant to future reproductive decisions, and opportunity to prepare for having a child with special health needs [78]. However, they also highlighted the importance of parental choice [78].
Parent support for NBS programs was not universal. Mothers whose newborns were screened for FXS through a research study did so because it posed minimal risk, provided information about their child’s health and development, could inform future reproductive decisions, and was offered at no cost [88]. Reasons for declining included not wanting to know or worry, concerns about genetic testing of children, no family history of FXS, partner disapproval, infant’s apparent good health, and no cure for condition [88]. Findings point to a need for NBS protocols that guide information dissemination about essential follow-up assessments, potential treatments, ongoing family support, and communicating NBS results to all health care providers involved in the child’s care [50,78].
Parents of infants screened for genetic risk of developing T1D using next generation sequencing were less enthusiastic about expanding NBS than parents in the general population [61]. Parents voiced concerns about difficulty understanding genetic information, unclear rationale for testing at birth rather than later in childhood, limited predictive value, whether behavioral changes could mitigate risk, increased parental worry or overprotectiveness, and potential adverse effects on family functioning. Yet, parents also recognized the benefits of potentially preventing disease, particularly for conditions for which risk can be minimized [61]. Variability in parents’ support for NBS highlights the need to explore ways to help them make informed choices for their families.
Retention of dried blood spot (DBS) samples for future research raised unique concerns. Parents asserted their desire to be involved in making decisions about retaining DBS [74]. In another study, 95% of new mothers supported use of DBS for medical research, contingent upon parental informed consent and de-identification of samples [38].
3.3. Parent Reactions to NBS Results
3.3.1. Positive (Initial) Results
Parents of infants whose NBS results were positive for SCD [22], CF [14,53,68,72,86,106], FXS [24], metabolic disorders [41,52], CH [86,92], or increased risk of T1D [16,61] reported short-term anxiety and/or stress. One study found that identifying increased genetic risk of T1D was not associated with elevated maternal anxiety [26], and another found elevated depressive symptoms in mothers with a history of postpartum depression [56]. Delays for confirmatory diagnostic testing were especially troubling for parents, some of whom reported clinical levels of depression while waiting for diagnostic sweat test results [14]. In other studies, parents reported substantial emotional distress, such as fear, shock, and worry, while waiting for results of diagnostic testing [36,51,56]. Parents whose newborns needed additional testing (e.g., a second sweat test) experienced continued distress [81], often anticipating a CF diagnosis [79]. Parents also varied in their response to uncertainty during diagnostic sweat tests [42]. Some tried to reduce uncertainty by seeking information while others maintained uncertainty by avoiding new information. Both strategies aimed to reduce or manage anxiety about a potential CF diagnosis.
In response, researchers identified ways to mitigate initial parental distress. Some occurred naturally within families. For example, spousal support was associated with less depression and stress among mothers identified as FXS carriers [24]. Interventions by health care providers included developing culturally sensitive counseling techniques [14,41], tailoring the timing, content, and method of communication to match each family’s needs for emotional support and information [14], and having knowledgeable, trained professionals communicate test results [14,53]. For CF, recommendations also included minimizing the time between receipt of NBS results and confirmatory sweat testing [14,39,47].
3.3.2. True-Positive Results, Confirmed Diagnosis
A confirmed diagnosis following positive NBS results was devastating to parents who thought they had a healthy infant. The diagnosis generated a mix of shock, fear, anxiety, and disbelief [42,58,59]. Mothers of infants diagnosed with CF were more likely to have clinical levels of anxiety and depression than mothers of infants diagnosed with CH, identified as CF carriers, or with normal NBS results [99]. Delays in receiving additional information and follow-up were particularly anxiety-provoking, especially for parents who had to wait over a weekend. This prompted many to search for information from potentially unreliable online sources [57]. Parents of infants with CF, in particular, were overwhelmed by the implications of the diagnosis and the need to engage with a large and diverse health care team [57]. Grob [51] found that parents of infants diagnosed with CF through NBS felt emotionally unprepared due to lack of knowledge. They were challenged by the complexity of CF genetics and physiology and overwhelmed by the home care required to keep their child healthy, though these feelings diminished over time. The diagnosis also changed parents’ perceptions of their child from “healthy and normal” to being a child in need of additional medical care. Other responses differed, often by condition. For example, despite experiencing initial emotional distress, parents of infants with FXS viewed early diagnosis as an opportunity to prepare for their child’s future needs and to make informed reproductive plans [33].
3.3.3. False-Positive Results
False-positive NBS results were particularly confusing for parents. For some conditions, such as CF and SCD, false-positive results often identified infants who are heterozygote carriers, which was difficult for parents to understand [22,95]. Many did not realize that one or both parents could also be carriers, nor did they know about carrier testing to determine risks to future pregnancies [68]. Parents of infants with positive NBS for metabolic disorders had difficulty understanding the need for additional confirmatory testing [52]. Many perceived their children as being vulnerable to health problems, and, as a result, children with false-positive results were more likely to be hospitalized or have more health care encounters than infants with normal results [52,54,59,101,107]. However, another study found that after adjusting for differences in sample demographics, there were no significant differences in health care utilization between children with false-positive CF results and those with normal results [69]. Providers’ understanding of false-positive results may have contributed to health care overutilization. Those who were less familiar with conditions identified by NBS were more likely to hospitalize children, especially when children exhibited signs or symptoms of the condition [59]. Over time parents of infants with false-positive CF results (identified as CF carriers) viewed their children as having very good or excellent health [79,81], though some reported feeling guilty for passing a mutation to their child [95].
Parents of infants with false-positive results repeatedly called for more factual information and guidance about the health implications for their child and family [35,63,65]. Tluczek et al. [95] found that some parents questioned the accuracy of false-positive results and believed their child might have CF, which led to increased vigilance for CF signs and symptoms. However, such concerns tended to abate over time when children remained healthy. While parents felt relieved that their child did not have CF, and empathy for parents of children diagnosed with CF, they also felt guilt for passing what they perceived to be defective genes to their offspring. Some took on new identities as “CF carriers”.
3.3.4. Inconclusive or Intermediary Results
Follow-up diagnostic sweat tests for CF occasionally produce inconclusive or intermediary results. These include NBS results showing one or no CFTR mutations, with a sweat chloride level above the normal range but below a confirmatory CF diagnosis, or two mutations with a sweat chloride level within the normal range. Such results are classified as CF transmembrane conductance regulator-related metabolic syndrome (CRMS) in the US, and CF screen positive, inconclusive diagnosis (CFSPID) in Europe [110,111]. Most infants are healthy in early life, though some develop signs and symptoms of CF or receive a CF diagnosis later in childhood, and there are few data on long-term prognosis [110,111].
Parents of infants classified as having CRMS/CFSPID were uncertain about whether their child would develop CF-related health problems [55,93]. In one study, they reported lower levels of distress than parents of infants with a definitive CF diagnosis and considered their infants as healthy as parents of infants with negative NBS results [80]. By contrast, in another study, parents of children with this CRMS/CFSPID viewed their children as more vulnerable than parents of healthy children, but less so than parents of children with a CF diagnosis [100]. Parents struggled to understand uncertain results, worried about their infant’s health, and were vigilant for signs of CF [55,93]. Some reported frustration due to the lack of information about their child’s mutations and felt isolated because there were no national organizations or parent groups focused on intermediate results for CF [93].
3.3.5. Comparisons across Groups
Studies that compared parent reactions to different types of NBS results showed similarities and nuanced differences based on the condition and results of confirmation testing. Parents of infants diagnosed with CF and those whose children had intermediate results were more worried than parents of infants who received normal sweat test results [29] or parents of healthy controls [80]. By contrast, another study found no differences in anxiety, depression, or stress between parents of infants with true-positive and false-positive results for CF [77]. Beucher et al. [27] compared the emotional responses of parents of infants identified as CF carriers to those of infants with persistent hypertrypsinemia but no CF mutations. Parents in both groups reported anxiety while awaiting confirmatory test results. However, at the two-year follow-up, some parents of children who were heterozygote carriers still expressed anxiety, particularly when their child was ill, while none of the parents of children with persistent hypertrypsinemia expressed concerns. Persistent anxiety was associated with lack of knowledge about CF, potential health implications for CF carriers, and transmission of CF mutations to future generations, as well as doubts about the accuracy of test results [27]. In another study, parents of infants with false-positive results for metabolic disorders who had other conditions reported the most stress, while parents in the false-positive/healthy group experienced anxiety while waiting for results, misunderstood the results, and expressed concern about the child’s health [73]. One study found that infants identified as CF carriers and infants with CF had more illnesses than the healthy comparison group [97]. However, it was unclear whether illness frequency in CF carriers was due to parents’ perceptions of child vulnerability or the physiological consequences of one CF mutation [97].
3.4. Parent Understanding of NBS Results
Research showed a link between parents’ understanding of test results and their levels of anxiety or stress. Gurian et al. [52] found that mothers who understood their child’s false-positive NBS results for metabolic disorders had lower levels of stress than mothers who did not understand the results. Many parents sought information prior to follow-up appointments from the internet, health care providers, family or friends, or books. Although source quality varied, those who accessed information prior to genetic counseling had higher levels of knowledge post-counseling [45,89,102].
Genetic counseling sessions improved understanding in many parents, though knowledge often declined over time [31,36]. In an intervention study, parents of infants with mutations for hemoglobinopathies who received genetic counseling were less anxious, had higher knowledge, and were more likely to discuss findings with other family members [63]. Carrier testing was also significantly higher among parents who received genetic counseling following false-positive NBS for CF [65,109]. Parents of infants with false-positive CF results who received information about the results also reported less anxiety and depression [106]. Receiving tailored written and video-recorded information two weeks after genetic counseling significantly improved parents’ understanding of their child’s CF carrier status [81].
However, even with genetic counseling, some parents of children identified as carriers for CF or SCD mistakenly believed their infant might develop the condition [47], or were confused about the implications of the test results [67]. Parents of infants with false-positive results for CF had difficulty understanding the genetics of autosomal recessive conditions [36]. In another study, about three-quarters of mothers of infants at increased risk for T1D accurately reported their infant’s risk shortly after receiving NBS results, but over time this rate declined to two-thirds [31]. Difficulty retaining and understanding information from genetic counseling sessions was attributed to parental anxiety and concerns about the child’s health [67].
3.5. Parent Education following Confirmed Diagnosis
Parent education is critical following a confirmed diagnosis. Jessup et al. [57] found that parents wanted to know the severity of the condition, effect on their child’s life, and practical information about keeping their child well, followed by education throughout the child’s first year of life. Parents also welcomed messages that engendered confidence in their ability to care for their child and conveyed hopefulness for their child’s future. They advocated for adapting educational approaches to each family, considering parents’ life experiences, knowledge, education, and family circumstances. Timing and mode of parent education affected parents’ desire to engage with care teams and their ability to retain information. Parents may need a few days to absorb the diagnosis before participating in intensive education. Sawyer and Glazner [84] compared parents’ experiences of residential education and outpatient teaching following a CF diagnosis. While residential programs saved repeated travel to and from the facility, they also had to take time off from work and find childcare for siblings. Despite the challenges, all parents said they would recommend the residential program.
3.6. Communication
3.6.1. Provider-Parent Communication
Parents’ preferences for communication with providers varied depending on the condition and type of results and may reflect perceptions about the seriousness of the condition. For example, parents of children with positive results for CF preferred in-person disclosure, while parents of infants with CH were comfortable with disclosure by phone [83]. Parents of infants diagnosed with CF or SCD who received false reassurance of likely negative NBS results from their primary care providers experienced distress when results were positive [34]. Being notified by letter of positive NBS results for CF or SCD was also distressing, as letters failed to provide adequate explanations about test results or reasons for required follow-up [58]. If the provider was known, telephone communication was acceptable, but face-to-face communication was preferred for unfamiliar providers. Follow-up phone calls were helpful for parents of infants with CF or SCD and those who received positive NBS results, providing opportunities to answer questions, clarify misunderstandings, and learn about other resources [64,91].
Many factors contributed to the quality of provider-parent communication. For parents, limited knowledge of NBS, misunderstanding screening tests, emotional distress, and having only one parent attend the appointment impeded communication [44]. Parents preferred to receive initial results from professionals knowledgeable about NBS and the condition for which their infant screened positive and who could communicate clearly and empathically. Ideally, such individuals would be known, trusted providers, though being knowledgeable superseded familiarity [22,45,51,73,87,88,93,94]. Parents wanted providers to communicate factual information in simple, jargon-free language with sensitivity to parents’ emotional state [22,35,36,91,94]. Receiving NBS results by voicemail, or before weekends or holidays, was extremely troubling because questions and concerns could not be addressed in a timely manner [49,59,94]. Disruptions during genetic counseling sessions also adversely affected parents’ recall [43].
3.6.2. Informing Children of Their Carrier Status
Parents of infants identified as carriers for a genetic condition faced the additional dilemma of when and how to inform their children about the NBS results. Parents believed that their children had a right to know their carrier status, but worried that this information might damage the child’s self-esteem and adversely impact their child’s opportunities to find a partner and have a family [32,103]. Consequently, parents wanted to emphasize that the child was “normal” and that no one is to blame [103].
Parents also carefully considered the timing and approach to informing their child about their carrier status and its potential impact on the child’s reproductive future. They wanted additional support and guidance as they navigated the communication process [95,104].
A subset worried about stigma or rejection from potential partners [95]. Some tried to normalize their child’s carrier status by making the telling part of the child’s birth story. Others identified strategic opportunities, such as when children learned about genetics, when teens began dating, or when young adult children began planning their own families [32,103]. Some parents planned to inform their children themselves, believing that they know their children best, while others welcomed the assistance of knowledgeable health professionals [103].
3.6.3. Communicating NBS Results to Family Members
Parents of children with CF were comfortable sharing the diagnosis with family members and friends, but reported difficulty answering questions about genetics. In the same study, parents of children with SCD were more cautious about sharing the diagnosis for fear of stigma and discrimination [34]. New genetic information affected extended family relationships, as some parents wondered if family members might have CF or be carriers. This posed moral dilemmas for some parents, who needed to decide whether and how to share genetic information with biological relatives who might be at risk for having a child with the condition. Sharing genetic information with extended family was an opportunity for some and a burden for others, depending on relationship quality. Parents were concerned that the information could provoke anxiety in at risk family members. Such decisions were particularly challenging when parents had strained relationships with extended family members [95,104]. Many parents chose not to share their child’s CF carrier status with health care providers, health insurance companies, children’s classmates or teachers or their spiritual leaders [67].
3.7. Parent Decisions about Carrier Testing and Future Pregnancies
The impact of genetic NBS results on carrier testing uptake and future reproductive decisions varied based on type of result and conditions identified [78]. Following positive screens for CF and SCD, parents expressed interest in and uptake of carrier testing; however, higher anxiety among mothers of infants identified as SCD carriers was associated with genetic test avoidance [22]. Concerns about recurrence prompted many parents to decide against having more children. Others obtained prenatal testing for subsequent pregnancies, and some opted to terminate affected pregnancies [46,73,78,80,85,87]. Interestingly, one study showed that parents of infants with false-positive results for metabolic or endocrine disorders were more likely to consider not having more children than parents of infants with true-positive results [73]. In another study, positive NBS results for CF prompted some parents to have subsequent prenatal testing, in order to choose to terminate an affected pregnancy or prepare to have a child with special needs [85]. In the same study, reasons for not pursuing prenatal testing included disapproval of termination, belief that the condition was not serious, or concerns about health risks associated with prenatal testing.
3.8. Child and Family Outcomes
3.8.1. Parent-Child Relationships
The impact of NBS results on parents’ relationships with their children and perceptions of their vulnerability varied. Parents of infants diagnosed with CF were “excessively protective and indulgent” of affected children, while relationships with infants who had inconclusive results were less affected [80]. Parents of infants with intermediate CF results reported “delayed infant bonding” and changes in parenting behavior, though these issues abated over time [55]. However, other studies found no differences in self-reported rejection or protective parenting behaviors among mothers of infants diagnosed with DMD or CF or those identified as CF carriers, compared to mothers in the general population [79]. Parents of infants diagnosed with DMD or CF did not believe that the diagnosis would influence their relationship with or their plans for raising their child [35,40].
Anxiety and stress due to confusion about test results adversely affected parents’ interactions with their children [53,63,66]. In two studies [52,101], parents of infants with false-positive results for metabolic disorders perceived their children as being vulnerable to illness, which was associated with parental overprotectiveness and a focus on physical symptoms. Compared to parents of infants with normal NBS, these parents reported more challenging interactions with their children and higher levels of parenting stress, and described their children as being more difficult and requiring more care [52]. Mothers of children diagnosed with CF were more likely to be protective of their children, compared to mothers of infants with positive NBS for other conditions [34,36,72]. Parents who perceived their child as vulnerable were also more likely to view their child as being less attached than parents who identified their children as less vulnerable [99]. Parents of pre-school children at increased risk of T1D perceived their children as vulnerable to illness and altered their parenting behavior accordingly [60]. However, by age 12 there were no differences between low and high risk groups in parenting styles [62]. Parents of 12-year-olds were also more likely to underestimate their child’s risk, compared to when their children were infants, which could affect parenting behavior. By contrast, Baughcum et al. [25] found that family history of T1D and high maternal anxiety led parents to engage in more prevention behaviors. While some were beneficial (i.e., watching for signs of diabetes, providing healthy foods) [25,61], others were potentially harmful (i.e., limiting contact with other children, administering insulin unnecessarily) [25]. Some parents also chose not to share risk-related information with children who were at increased risk for T1D [61]. In another study [94], mothers of infants diagnosed with CF through NBS were less likely to breastfeed than mothers of infants with normal NBS. Bottle feeding was also associated with less responsive and more task-oriented feeding behaviors than breastfeeding. This has implications for maternal bonding and child health because of the significant immunologic properties of human milk [112]. Another study found that parents of infants with CH and those in a healthy control group had similar interactions with their infants, compared to parents of infants diagnosed with CF or identified as CF carriers, suggesting the severity of the condition identified through NBS may influence parents’ perceptions of and relationships and interactions with their infants [97].
3.8.2. Child Development, Family Burden, and Quality of Life
Effects of positive NBS results on child development, family burden, and quality of life varied depending on the disorder identified, the child’s age, and the outcomes measured. School-aged children with CH diagnosed though NBS were at increased risk for behavioral problems, which dissipated as children became more psychologically mature [28]. Similarly, although young adults with CH diagnosed through NBS showed lower health-related quality of life, they had no significant differences in happiness, autonomy, psychosexual development, gross motor function, educational attainment, or marital status compared to healthy peers [105]. Parents of children with metabolic disorders identified through NBS [50] reported that the child’s developmental delays contributed to family burden and parental stress. Despite these challenges, parents maintained positive expectations about their children’s developmental capacities and futures. Parents of children diagnosed with metabolic disorders through NBS also reported improved child development and less parenting stress than parents of children diagnosed clinically [107,108], while parents of children with DMD diagnosed through NBS reported similar family quality of life to parents of children diagnosed clinically [35]. Studies found no differences in health-related quality of life or psychosocial function in youth with CF diagnosed through NBS compared with those diagnosed clinically [96,98]. However, parents of youth with CF diagnosed through NBS were at increased risk of depressive symptoms [98]. Parents of infants at increased risk of T1D reported no significant concerns about their children’s psychosocial development or self-concept twelve years after neonatal identification of T1D risk [62].
3.9. Disparities
Several studies uncovered disparities based on parents’ age, race, marital status, insurance, immigration status, and primary language. Postpartum women who were predominately African American had significant gaps in their general knowledge of NBS [66]. Another study found that parents who were single, non-white, had less than a college education, did not have private health insurance, and had their children early in life lacked knowledge about false-positive NBS results for CF [67]. Black mothers were less likely to allow their newborns to be screened for FXS than Hispanic or white mothers, possibly reflecting cultural differences or historical mistrust of health care systems [88].
Sociodemographic factors also influenced parents’ outcomes. Maternal risk for anxiety [26] or depression [56] following identification of increased risk of T1D was associated with being single, of Hispanic ethnicity, low education, and/or having female infants. Although most parents were concerned about their infant’s positive NBS results for CF, parents of first-born children and those who were immigrants were even more worried [29]. In another study, African American and Hispanic mothers and those with less than a college education were least likely to accurately report their infant’s risk for developing T1D [31]. Spanish-speaking and bilingual parents living in the US also identified language barriers to learning about NBS [39]. Providers seldom spoke Spanish and most written information was only available in English. In Spanish, NBS is referred to as “blood test” and can be easily confused with other blood-based tests performed on newborns. Parents who had low health literacy and identified as multiracial reported more dissatisfaction with their NBS experience [47]. Younger parents of infants found to be SCD carriers, and parents of CF carriers who were biracial or multiracial, were more likely to have misconceptions about NBS results.
Parental knowledge and acceptance of NBS results also varied according to sociodemographic characteristics. Parents who had never heard of CF were more likely to have less education, speak English as a second language, and were less likely to have private insurance [48]. In one of the few studies comparing fathers and mothers, fathers were more likely to be completely uninformed about NBS [92], which likely amplified their emotional reactions to receiving positive NBS results. Mothers with lower incomes were almost four times less likely to receive information prenatally and more likely to be informed during the suboptimal postpartum time than their higher income counterparts [92]. Additionally, parents of varied demographic backgrounds expressed concerns about potential societal stigma and discrimination associated with the diagnostic labels of “cystic fibrosis” and “sickle cell disease” or being “carriers” of genetic mutations for either of these conditions [22,56,62,71,111].
4. Discussion
This comprehensive systematic review synthesizes twenty years of research that investigated psychosocial issues associated with NBS at the dawn of genomic technologies. Despite the global increase in NBS programs over the last five decades, many new parents remain uninformed about the purpose of NBS and the potential implications of positive results for their families. Findings support expanded, comprehensive parent education that includes information about use of DBS for research.
4.1. Parents’ Psychosocial Response to NBS
Although the optimal time and approach to educating parents about NBS is still under debate, parents clearly believed receiving comprehensive information was important. Those who had already experienced NBS advocated for offering information before and during pregnancy, as well as at the time of specimen collection. Communicating information in multiple ways is also helpful. Results of a randomized controlled intervention found that women who received information via videos and brochures during the last trimester of pregnancy had significantly greater knowledge of and more positive attitudes about NBS and storage of DBS for research than the control group, which received only brochures [113]. Clinicians need to carefully time postpartum education to be sure that parents are sufficiently rested and alert to comprehend and retain information. In every interaction, clinicians must take time to discuss the purpose of NBS, conditions identified, when and how to expect results, the meaning of positive results, and the relatively low risk of receiving positive results [92]. Improving parents’ awareness and understanding of NBS may also reduce the shock they feel when results are positive.
Regardless of the condition, a positive NBS result is associated with a range of parental emotions, including relief, guilt, shock, and denial [34,83]. Kerruish et al. [16] suggest that research might underestimate the extent of parental distress, because the most distressed parents might have declined to participate. Parents’ emotions may not be reflected in standard measures of anxiety and stress, highlighting the value of qualitative approaches that elicit their thoughts and feelings associated with their child’s NBS results. Findings underscore a link between parental distress and misunderstanding of NBS results, particularly false-positive results; however, education and counseling can lessen persistent confusion and anxiety. Early involvement of specialists, reducing wait time for confirmatory testing, and continued monitoring by trusted providers can reduce parental anxiety following positive NBS.
Research suggests that neonatal diagnosis of serious health conditions could impact parents’ perceptions of their child’s vulnerability and alter their approach to parenting. Emphasizing the benefits of normative childhood experiences, peer relationships, and pastimes is an important element of anticipatory guidance for families following diagnosis of potentially serious conditions or false-positive results through NBS. Comprehensive multidisciplinary care and enhanced psychological and social support can also contribute to positive family outcomes.
4.2. Communication of NBS Results
We encourage clinicians to assess parents’ emotional states and most immediate concerns as part of every communication of positive NBS results. In our intervention work [114], asking parents to rate their level of worry on a scale of 1–10 at the beginning of a genetic counseling session for follow-up sweat testing for a positive CF screening result was an efficient way to gauge their distress. We then inquired about what parents were “most worried about” and immediately addressed that issue. Reducing some of parents’ initial distress may improve their capacities to receive new information.
We agree with the conclusions of Buchbinder and Timmermans [30] that effectively communicating “bad news” to new parents continues to challenge clinicians. Despite providers’ diligent efforts to explain NBS results to parents, parents remained confused about test results and related implications. A clear deficit in parental knowledge of the rationale for follow-up testing of positive results was evident in this review. Thus, we urge clinicians to explain the difference between “screening” tests and “diagnostic” tests so parents will understand the rationale for additional testing following positive NBS results.
Table 4 lists recommendations based on available research to improve parents’ comprehension and retention of test results and their experiences with NBS. Parents’ retention of information received during counseling sessions could be improved by providing additional resources before, during, or after sessions. These could include packets of written information or access to educational videos [81,89]. Parents who found information on their own, before counseling, were more likely to retain information received during the session [89]. Unfortunately, such information was generally not offered to parents before follow-up appointments, and its quality cannot be assured, as information was found by parents, not professionals. Systematically sending information to parents before genetic counseling sessions might improve their short and long-term understanding of critical NBS facts. Given the multi-step process required for establishing or ruling out a diagnosis following a positive NBS, effective communication and ongoing education throughout the process are critical to successful NBS programs. Collaboration among regional laboratories, specialists, and primary care providers and effective provider–parent communication are paramount for ensuring parents understand NBS results and for mitigating their distress following positive results.

Table 4.
Recommendations.
4.3. Molecular Genetic Technologies in NBS
Evidence highlights the unique nature of NBS results that rely on molecular genetic technologies that can identify infants who are carriers of recessive conditions such as CF and SCD, or at risk for developing conditions like T1D, as well as those with health conditions. These results also provide information about parents’ carrier status for recessive conditions. While this unsolicited information can be critical for future reproductive decisions, it also creates challenges in sharing the implications with their children and extended family members. Use of molecular genetics in NBS was largely responsible for identifying the broad spectrum of CF phenotypes now classified as CRMS/CFSPID. While these new discoveries offer the benefits of improved clinical monitoring, they also impose a psychosocial burden of prognostic uncertainty.
Parents typically rely on clinicians to help them understand and apply these complex findings in their own families. Communicating genetic test results requires translation of complicated genetic concepts and understanding of the sociocultural context in which parents receive such information, making genetic counseling absolutely imperative.
Knowledge about the implications of heterozygosity for recessive conditions is expanding rapidly. Heterozygote carriers of some recessive mutations may show some clinical signs and symptoms of the condition. For example, those with one copy of a mutated allele for SCD, an autosomal recessive condition, may demonstrate the effects of the mutation under certain environmental conditions [115]. In another example, children who are heterozygote carriers of a CF mutation may receive NBS results indicating that they are unaffected carriers, or that they have inconclusive NBS results. Children in either group could develop symptoms in the future [115].
Although expanded genetic NBS that identifies potential risk for developing later-onset disorders is currently limited, whole-genome or whole-exome sequencing for NBS is likely to become more common. The implications of this expansion are immense and raise important questions for parents, clinicians, researchers, and policymakers. Exploring stakeholders’ perspectives, particularly parents’, on the use of genomic sequencing for NBS is essential to ensure ethical and equitable future policies [116,117]. Our review suggested that genetic NBS to determine T1D risk may have less public support than conditions that manifest early in life [61]. By contrast, another recent report noted that parents and the public may view genomic data as empowering and important. Yet, providers may be more reticent to employ broader genomic testing that provides substantial information about future disease risk and may find it challenging to explain such information to parents [118]. Concerns about the utility of genomic sequencing for underrepresented populations and issues of privacy remain [118,119]. Also of paramount importance is equitable access to genetic counseling resources [116].
Clinicians must ensure that parents receive up to date information about NBS and that they understand the potential implications for their child, themselves, and other family members. This information must be presented in ways that do not provoke undue psychological distress. As each family is unique, the amount and type of information and the sequence in which it is presented should be tailored to match parents’ needs. For example, evidence shows that during follow-up testing for CF, parents’ preferences may differ depending on their perception of the child’s risk of actually having CF. Parents may also not be ready to receive all relevant information immediately after receiving test results. Clinicians must remain cognizant that potentially upsetting NBS results were not solicited by parents and are often shared at a time when they may be sleep deprived and overwhelmed by caring for their newborn. Some may also suffer from postpartum depressive symptoms, which can be exacerbated by receiving positive NBS results. These factors can impair parents’ capacities to absorb and assimilate new information.
This review also identified disparities in understanding among marginalized subpopulations, which further supports the need for clinicians to tailor information to parents’ existing knowledge and immediate needs, involve professional interpreters in counseling, reinforce information offered at the initial counseling session, and provide ongoing access to additional counseling. Parents of newly diagnosed infants with CF valued initial genetic counseling sessions, but did not identify genetic information as the most important information received at the time of diagnosis [84]. Thus, clinicians also need to adjust the content of each educational or counseling session to accommodate parent preferences. Results of genetic NBS also have important implications for other children in the family. NBS is a process with a series of integrated procedures. For this reason, it is imperative that genetic counselors support long-term follow-up of genetic NBS results [116].
4.4. Limitations
Most studies were conducted in high resource countries in North America and Europe. Most used cross-sectional designs with non-standardized researcher-designed assessments without documented psychometric properties, relied on convenience samples that were often small and homogenous (i.e., mostly white educated mothers when demographic data were documented), and lacked a theoretical basis. There were only six intervention studies. Few studies included participants who spoke a language other than the national language of the country in which the study was conducted. Despite a proliferation of NBS programs that identify heterozygote carriers of autosomal recessive conditions, only two studies examined parents’ perspectives about or experiences of communicating this information to their children. Parents’ reasons for supporting NBS were articulated in multiple studies, but opposition to or ambivalence about NBS were seldom explored, suggesting researcher bias in favor of NBS. The majority of studies also focused on NBS for CF and SCD, with few comparisons across conditions. Molecular genetic screening tests for these conditions, which detect carriers, were the first such tests introduced in practice, which is likely why most research has focused on them. Studies should be expanded to include a greater diversity of conditions, to determine if results are consistent across conditions. Authors acknowledge that there could have been research articles not captured by the search and screen process used for this report.
4.5. Implications for Future Research
Future investigations must be more inclusive of culturally and socioeconomically diverse families and should examine psychosocial effects of NBS in low-resource countries. Researchers need to design interventions that explicitly identify ways to address the informational needs of families with low health literacy and those in marginalized populations. We agree with O’Connor et al. [77] that further research should focus on the “marital relationship, paternal NBS experiences and continual parental education”. Research must also establish evidence-based guidelines for timing and modes of communication about NBS and DBS retention to optimize parents’ comprehension and retention of information. We agree with Farrell et al. [115] that, while there may be health and psychosocial benefits to identifying carriers of recessive conditions, it is imperative to explore parents’ reasons for receiving or not receiving such information and at what time this information should be shared, if at all. Such research is essential to informing counseling approaches that respect parents’ preferences while ensuring their comprehensive understanding of the implications for their child, themselves, and for future pregnancies. Process research using video-recorded counseling sessions can identify communication skills that effectively address parents’ needs for emotional support while providing factual information. Such research can inform assessment techniques that guide clinical judgment regarding when and how much information and/or emotional support to offer parents. Finally, empirical evidence is needed to help guide parents in deciding when and how to inform and educate their children about their genetic status and related reproductive implications.
5. Conclusions
The psychosocial consequences of an unexpected neonatal diagnosis and unsolicited genetic information remain significant risks to NBS. Adverse sequalae may be reduced by providing comprehensive, multimodal parent education about NBS throughout pregnancy and the postpartum period, delivering tailored genetic counseling to parents, and offering anticipatory developmental guidance and support for families receiving positive NBS results. Clinicians must recognize the unique needs of families with marginalized identities and take measures to ensure equitable service delivery. Future intervention research needs to be more inclusive of culturally and socioeconomically diverse families as well as families from low-resource countries. Finally, it is critical that resources be allocated to low-income nations and disenfranchised communities to achieve equitable access to NBS.
Author Contributions
A.T. conceptualized and designed this systematic review. She participated in every aspect of the literature search, screen, and review process as well as data extraction, data synthesis and manuscript preparation. A.L.E. participated in the literature review, data extraction, data synthesis, and manuscript preparation. She made substantive intellectual contributions to every component of the manuscript. S.L. participated in the literature search, screen, and review process, as well as data extraction. She assisted with technical aspects of manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, because it was not human subject research.
Informed Consent Statement
Not relevant.
Acknowledgments
We gratefully acknowledge Phillip M. Farrell for sharing his wisdom with us during the final preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fabie, N.A.V.; Pappas, K.B.; Feldman, G.L. The Current State of Newborn Screening in the United States. Pediatr. Clin. N. Am. 2019, 66, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, R.; Susi, A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef]
- Wilson, J.M.G.; Jungner, G.; Principles and Practice of Screening for Disease. World Health Organization. 1968. Available online: https://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf (accessed on 5 August 2022).
- Watson, M.S.; Mann, M.Y.; Lloyd-Puryear, M.A.; Rinaldo, P.; Howell, R.R.; American College of Medical Genetics Newborn Screening Expert Group. Newborn Screening: Toward a Uniform Screening Panel and System—Executive Summary. Pediatrics 2006, 117 (Suppl. S3), S296–S307. [Google Scholar] [CrossRef] [PubMed]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A.; Calonge, N.; Teutsch, S.M.; Botkin, J.R. Expanding Newborn Screening: Process, Policy, and Priorities. Häst. Cent. Rep. 2008, 38, 32–39. [Google Scholar] [CrossRef]
- Johnson, T.; Wile, M. State Newborn Health Screening Policies. NCSL Legisbrief 2017, 25, 1–2. [Google Scholar]
- Farrell, M.; Farrell, P.M. Newborn screening for cystic fibrosis: Ensuring more good than harm. J. Pediatr. 2003, 143, 707–712. [Google Scholar] [CrossRef]
- Tarini, B.A.; Christakis, D.A.; Welch, H.G. State Newborn Screening in the Tandem Mass Spectrometry Era: More Tests, More False-Positive Results. Pediatrics 2006, 118, 448–456. [Google Scholar] [CrossRef]
- Hollegaard, M.V.; Grove, J.; Grauholm, J.; Kreiner-Møller, E.; Bønnelykke, K.; Nørgaard, M.; Benfield, T.L.; Nørgaard-Pedersen, B.; Mortensen, P.B.; Mors, O.; et al. Robustness of genome-wide scanning using archived dried blood spot samples as a DNA source. BMC Genet. 2011, 12, 58. [Google Scholar] [CrossRef][Green Version]
- Green, J.M.; Hewison, J.; Bekker, H.L.; Bryant, L.D.; Cuckle, H.S. Psychosocial aspects of genetic screening of pregnant women and newborns: A systematic review. Health Technol. Assess. 2004, 8, iii–ix. [Google Scholar] [CrossRef] [PubMed]
- Loeber, J.G.; Burgard, P.; Cornel, M.C.; Rigter, T.; Weinreich, S.S.; Rupp, K.; Hoffmann, G.F.; Vittozzi, L. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1—From blood spot to screening result. J. Inherit. Metab. Dis. Off. J. Soc. Study Inborn Errors Metab. 2012, 35, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Koscik, R.L.; Farrell, P.M.; Rock, M.J. Psychosocial Risk Associated With Newborn Screening for Cystic Fibrosis: Parents’ Experience While Awaiting the Sweat-Test Appointment. Pediatrics 2005, 115, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Grob, R. Parenting in the genomic age: The ‘cursed blessing’of newborn screening. New Genet. Soc. 2006, 25, 159–170. [Google Scholar] [CrossRef]
- Kerruish, N.J.; Campbell-Stokes, P.L.; Gray, A.; Merriman, T.R.; Robertson, S.P.; Taylor, B.J. Maternal Psychological Reaction to Newborn Genetic Screening for Type 1 Diabetes. Pediatrics 2007, 120, e324–e335. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- American Psychological Association. APA Dictionary of Psychology. 2022. Available online: https://dictionary.apa.org/psychosocial (accessed on 10 February 2022).
- National Research Council; Committee on Population. Psychosocial Concepts in Humanitarian Work with Children: A Review of the Concepts and Related Literature; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Bettany-Saltikov, J. EBOOK: How to Do a Systematic Literature Review in Nursing: A Step-By-Step Guide; McGraw-Hill Education: Berkshire, UK, 2016. [Google Scholar]
- Kiger, M.E.; Varpio, L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med. Teach. 2020, 42, 846–854. [Google Scholar] [CrossRef]
- Ahmad, N.Y.; Farrell, M.H. Linguistic markers of emotion in mothers of sickle cell carrier infants: What are they and what do they mean? Patient Educ. Couns. 2014, 94, 128–133. [Google Scholar] [CrossRef]
- Araia, M.H.; Wilson, B.J.; Chakraborty, P.; Gall, K.; Honeywell, C.; Milburn, J.; Ramsay, T.; Potter, B.K. Factors associated with knowledge of and satisfaction with newborn screening education: A survey of mothers. Genet. Med. 2012, 14, 963–970. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Wheeler, A.; Berry-Kravis, E.; Hagerman, R.; Tassone, F.; Powell, C.M.; Roche, M.; Gane, L.W.; Sideris, J. Maternal Consequences of the Detection of Fragile X Carriers in Newborn Screening. Pediatrics 2015, 136, e433–e440. [Google Scholar] [CrossRef]
- Baughcum, A.E.; Johnson, S.B.; Carmichael, S.K.; Lewin, A.B.; She, J.-X.; Schatz, D.A. Maternal Efforts to Prevent Type 1 Diabetes in At-Risk Children. Diabetes Care 2005, 28, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.B.; Baughcum, A.E.; Carmichael, S.K.; She, J.-X.; Schatz, D.A. Maternal Anxiety Associated With Newborn Genetic Screening for Type 1 Diabetes. Diabetes Care 2004, 27, 392–397. [Google Scholar] [CrossRef]
- Beucher, J.; Leray, E.; Deneuville, E.; Roblin, M.; Pin, I.; Bremont, F.; Turck, D.; Giniès, J.-L.; Foucaud, P.; Rault, G.; et al. Psychological Effects of False-Positive Results in Cystic Fibrosis Newborn Screening: A Two-Year Follow-Up. J. Pediatr. 2010, 156, 771–776.e1. [Google Scholar] [CrossRef] [PubMed]
- Bisacchi, N.; Bal, M.O.; Nardi, L.; Bettocchi, I.; D’Addabbo, G.; Conti, V.; Monti, S.; D’Alberton, F.; Cicognani, A.; Cassio, A. Psychological and behavioural aspects in children and adolescents with congenital hypothyroidism diagnosed by neonatal screening: Comparison between parents’ and children’s perceptions. Eur. J. Endocrinol. 2011, 164, 269–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brockow, I.; Nennstiel, U. Parents’ experience with positive newborn screening results for cystic fibrosis. Eur. J. Pediatr. 2019, 178, 803–809. [Google Scholar] [CrossRef]
- Buchbinder, M.; Timmermans, S. Newborn screening for metabolic disorders: Parental perceptions of the initial communication of results. Clin. Pediatr. 2012, 51, 739–744. [Google Scholar] [CrossRef]
- Carmichael, S.K.; Johnson, S.B.; Baughcum, A.; North, K.; Hopkins, D.; Dukes, M.G.; She, J.-X.; Schatz, D.A. Prospective assessment in newborns of diabetes autoimmunity (PANDA): Maternal understanding of infant diabetes risk. Genet. Med. 2003, 5, 77–83. [Google Scholar] [CrossRef]
- Cavanagh, L.; Compton, C.J.; Tluczek, A.; Brown, R.L.; Farrell, P.M. Long-term Evaluation of Genetic Counseling Following False-Positive Newborn Screen for Cystic Fibrosis. J. Genet. Couns. 2010, 19, 199–210. [Google Scholar] [CrossRef]
- Christie, L.; Wotton, T.; Bennetts, B.; Wiley, V.; Wilcken, B.; Rogers, C.; Boyle, J.; Turner, C.; Hansen, J.; Hunter, M.; et al. Maternal attitudes to newborn screening for fragile X syndrome. Am. J. Med. Genet. Part A 2013, 161, 301–311. [Google Scholar] [CrossRef]
- Chudleigh, J.; Buckingham, S.; Dignan, J.; O’Driscoll, S.; Johnson, K.; Rees, D.; Wyatt, H.; Metcalfe, A. Parents’ Experiences of Receiving the Initial Positive Newborn Screening (NBS) Result for Cystic Fibrosis and Sickle Cell Disease. J. Genet. Couns. 2016, 25, 1215–1226. [Google Scholar] [CrossRef]
- Chung, J.; Smith, A.L.; Hughes, S.C.; Niizawa, G.; Abdel-Hamid, H.Z.; Naylor, E.W.; Hughes, T.; Clemens, P.R. Twenty-year follow-up of newborn screening for patients with muscular dystrophy. Muscle Nerve 2015, 53, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Ciske, D.J.; Haavisto, A.; Laxova, A.; Rock, L.Z.M.; Farrell, P.M. Genetic Counseling and Neonatal Screening for Cystic Fibrosis: An Assessment of the Communication Process. Pediatrics 2001, 107, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.L.; La Pean, A.; O’Tool, F.; Eskra, K.L.; Roedl, S.J.; Tluczek, A.; Farrell, M.H. Factors that influence parents’ experiences with results disclosure after newborn screening identifies genetic carrier status for cystic fibrosis or sickle cell hemoglobinopathy. Patient Educ. Couns. 2012, 90, 378–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davey, A.; French, D.; Dawkins, H.; O’Leary, P. New mothers’ awareness of newborn screening and attitudes to retention of samples for research. Genom. Soc. Policy 2005, 1, 41–51. [Google Scholar] [CrossRef]
- Davis, T.C.; Humiston, S.G.; Arnold, C.L.; Bocchini, J.A.; Bass, P.F.; Kennen, E.M.; Bocchini, A.; Williams, D.; Kyler, P.; Lloyd-Puryear, M. Recommendations for Effective Newborn Screening Communication: Results of Focus Groups With Parents, Providers, and Experts. Pediatrics 2006, 117, S326–S340. [Google Scholar] [CrossRef]
- De Monestrol, I.; Brucefors, A.B.; Sjöberg, B.; Hjelte, L. Parental support for newborn screening for cystic fibrosis. Acta Paediatr. 2010, 100, 209–215. [Google Scholar] [CrossRef]
- DeLuca, J.M.; Kearney, M.H.; Norton, S.A.; Arnold, G.L. Parents’ Experiences of Expanded Newborn Screening Evaluations. Pediatrics 2011, 128, 53–61. [Google Scholar] [CrossRef]
- Dillard, J.P.; Carson, C.L. Uncertainty Management Following a Positive Newborn Screening for Cystic Fibrosis. J. Health Commun. 2005, 10, 57–76. [Google Scholar] [CrossRef]
- Dillard, J.P.; Shen, L.; Tluczek, A.; Modaff, P.; Farrell, P. The Effect of Disruptions During Counseling on Recall of Genetic Risk Information: The Case of Cystic Fibrosis. J. Genet. Couns. 2007, 16, 179–190. [Google Scholar] [CrossRef]
- Dillard, J.P.; Shen, L.; Laxova, A.; Farrell, P. Potential Threats to the Effective Communication of Genetic Risk Information: The Case of Cystic Fibrosis. Health Commun. 2008, 23, 234–244. [Google Scholar] [CrossRef]
- Dillard, J.P.; Shen, L.; Robinson, J.D.; Farrell, P.M. Parental Information Seeking Following a Positive Newborn Screening for Cystic Fibrosis. J. Health Commun. 2010, 15, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Dudding, T.; Wilcken, B.; Burgess, B.; Hambly, J.; Turner, G. Reproductive decisions after neonatal screening identifies cystic fibrosis. Arch. Dis. Child.-Fetal Neonatal Ed. 2000, 82, 124F–127F. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.H.; Kirschner, A.L.P.; Tluczek, A.; Farrell, P.M. Experience with Parent Follow-Up for Communication Outcomes after Newborn Screening Identifies Carrier Status. J. Pediatr. 2020, 224, 37–43.e2. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Linnane, B.; Heery, E.; Conneally, N.; George, S.; Fitzpatrick, P. Newborn bloodspot screening for cystic fibrosis: What do antenatal and postnatal women know about cystic fibrosis? J. Cyst. Fibros. 2016, 15, 436–442. [Google Scholar] [CrossRef]
- Fitzpatrick, P.; Fitzgerald, C.; Somerville, R.; Linnane, B. Parental awareness of newborn bloodspot screening in Ireland. Ir. J. Med. Sci. 2018, 188, 921–923. [Google Scholar] [CrossRef]
- Gramer, G.; Haege, G.; Glahn, E.M.; Hoffmann, G.F.; Lindner, M.; Burgard, P. Living with an inborn error of metabolism detected by newborn screening—Parents’ perspectives on child development and impact on family life. J. Inherit. Metab. Dis. 2013, 37, 189–195. [Google Scholar] [CrossRef]
- Grob, R. Is my sick child healthy? Is my healthy child sick?: Changing parental experiences of cystic fibrosis in the age of expanded newborn screening. Soc. Sci. Med. 2008, 67, 1056–1064. [Google Scholar] [CrossRef]
- Gurian, E.A.; Kinnamon, D.D.; Henry, J.J.; Waisbren, S.E. Expanded Newborn Screening for Biochemical Disorders: The Effect of a False-Positive Result. Pediatrics 2006, 117, 1915–1921. [Google Scholar] [CrossRef]
- Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Kerr, E.; Tam, K.; Carroll, J.C.; Potter, B.K.; Chakraborty, P.; Davies, C.; et al. Parent Experience With False-Positive Newborn Screening Results for Cystic Fibrosis. Pediatrics 2016, 138, e20161052. [Google Scholar] [CrossRef]
- Hayeems, R.Z.; Miller, F.A.; Vermeulen, M.; Potter, B.K.; Chakraborty, P.; Davies, C.; Carroll, J.C.; Ratjen, F.; Guttmann, A. False-Positive Newborn Screening for Cystic Fibrosis and Health Care Use. Pediatrics 2017, 140, e20170604. [Google Scholar] [CrossRef]
- Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Carroll, J.C.; Tam, K.; Kerr, E.; Chakraborty, P.; Potter, B.K.; Patton, S.; et al. Psychosocial Response to Uncertain Newborn Screening Results for Cystic Fibrosis. J. Pediatr. 2017, 184, 165–171.e1. [Google Scholar] [CrossRef] [PubMed]
- Hood, K.K.; Johnson, S.B.; Carmichael, S.K.; Laffel, L.M.; She, J.-X.; Schatz, D.A. Depressive Symptoms in Mothers of Infants Identified as Genetically at Risk for Type 1 Diabetes. Diabetes Care 2005, 28, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Jessup, M.; Douglas, T.; Priddis, L.; Branch-Smith, C.; Shields, L. Parental Experience of Information and Education Processes Following Diagnosis of Their Infant With Cystic Fibrosis Via Newborn Screening. J. Pediatr. Nurs. 2015, 31, e233–e241. [Google Scholar] [CrossRef]
- Kai, J.; Ulph, F.; Cullinan, T.; Qureshi, N. Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: Qualitative study of experience and practice. Health Technol. Assess. 2009, 13. [Google Scholar] [CrossRef]
- Karaceper, M.D.; on behalf of the Canadian Inherited Metabolic Diseases Research Network; Chakraborty, P.; Coyle, D.; Wilson, K.; Kronick, J.B.; Hawken, S.; Davies, C.; Brownell, M.; Dodds, L.; et al. The health system impact of false positive newborn screening results for medium-chain acyl-CoA dehydrogenase deficiency: A cohort study. Orphanet J. Rare Dis. 2016, 11, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kerruish, N.J. Parents’ experiences of newborn screening for genetic susceptibility to type 1 diabetes. J. Med. Ethic. 2011, 37, 348–353. [Google Scholar] [CrossRef]
- Kerruish, N. Parents’ experiences 12 years after newborn screening for genetic susceptibility to type 1 diabetes and their attitudes to whole-genome sequencing in newborns. Genet. Med. 2016, 18, 249–258. [Google Scholar] [CrossRef]
- Kerruish, N.J.; Healey, D.M.; Gray, A.R. Psychosocial effects in parents and children 12 years after newborn genetic screening for type 1 diabetes. Eur. J. Hum. Genet. 2017, 25, 397–403. [Google Scholar] [CrossRef]
- Kladny, B.; Williams, A.; Gupta, A.; Gettig, E.A.; Krishnamurti, L. Genetic counseling following the detection of hemoglobinopathy trait on the newborn screen is well received, improves knowledge, and relieves anxiety. Genet. Med. 2011, 13, 658–661. [Google Scholar] [CrossRef]
- La Pean, A.; Collins, J.L.; Christopher, S.A.; Eskra, K.L.; Roedl, S.J.; Tluczek, A.; Farrell, M.H. A qualitative secondary evaluation of statewide follow-up interviews for abnormal newborn screening results for cystic fibrosis and sickle cell hemoglobinopathy. Genet. Med. 2011. [Google Scholar] [CrossRef]
- Lagoe, E.; Labella, S.; Arnold, G.; Rowley, P.T. Cystic Fibrosis Newborn Screening: A Pilot Study to Maximize Carrier Screening. Genet. Test. 2005, 9, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.W.; Stark, A.; Acharya, K.; Ross, L.F. Maternal knowledge and attitudes about newborn screening for sickle cell disease and cystic fibrosis. Am. J. Med. Genet. Part A 2009, 149A, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.W.; McColley, S.A.; Lester, L.A.; Ross, L.F. Parental Understanding of Newborn Screening for Cystic Fibrosis After a Negative Sweat-Test. Pediatrics 2011, 127, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Curnow, L.; Ross, M.; Massie, J. Parental attitudes to the identification of their infants as carriers of cystic fibrosis by newborn screening. J. Paediatr. Child. Health 2006, 42, 533–537. [Google Scholar] [CrossRef]
- Lipstein, E.A.; Perrin, J.M.; Waisbren, S.E.; Prosser, L.A. Impact of false-positive newborn metabolic screening results on early health care utilization. Genet. Med. 2009, 11, 716–721. [Google Scholar] [CrossRef]
- Locock, L.; Kai, J. Parents’ experiences of universal screening for haemoglobin disorders: Implications for practice in a new genetics era. Br. J. Gen. Pract. 2008, 58, 161–168. [Google Scholar] [CrossRef][Green Version]
- Miller, F.A.; Paynter, M.; Hayeems, R.Z.; Little, J.; Carroll, J.C.; Wilson, B.J.; Allanson, J.; Bytautas, J.P.; Chakraborty, P. Understanding sickle cell carrier status identified through newborn screening: A qualitative study. Eur. J. Hum. Genet. 2009, 18, 303–308. [Google Scholar] [CrossRef]
- Moran, J.; Quirk, K.; Duff, A.J.; Brownlee, K.G. Newborn screening for CF in a regional paediatric centre: The psychosocial effects of false-positive IRT results on parents. J. Cyst. Fibros. 2007, 6, 250–254. [Google Scholar] [CrossRef]
- Morrison, D.; Clayton, E. False Positive Newborn Screening Results Are Not Always Benign. Public Health Genom. 2010, 14, 173–177. [Google Scholar] [CrossRef]
- Newcomb, P.; True, B.; Walsh, J.; Dyson, M.; Lockwood, S.; Douglas, B. Maternal Attitudes and Knowledge about Newborn Screening. MCN Am. J. Matern. Nurs. 2013, 38, 289–294. [Google Scholar] [CrossRef]
- Nicholls, S.G.; Southern, K.W. Parental information use in the context of newborn bloodspot screening. An exploratory mixed methods study. J. Community Genet. 2012, 3, 251–257. [Google Scholar] [CrossRef][Green Version]
- Nicholls, S.G.; Southern, K.W. Parental Decision-Making and Acceptance of Newborn Bloodspot Screening: An Exploratory Study. PLoS ONE 2013, 8, e79441. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.; Jukes, T.; Goobie, S.; DiRaimo, J.; Moran, G.; Potter, B.K.; Chakraborty, P.; Rupar, C.A.; Gannavarapu, S.; Prasad, C. Psychosocial impact on mothers receiving expanded newborn screening results. Eur. J. Hum. Genet. 2018, 26, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Clarke, A.J.; Hood, K.; Lycett, E.; Bradley, D.M. Newborn screening for Duchenne muscular dystrophy: A psychosocial study. Arch. Dis. Child.-Fetal Neonatal Ed. 2002, 86, 91F–95F. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Bradley, D.M. Psychosocial issues in newborn screening for cystic fibrosis. Paediatr. Respir. Rev. 2003, 4, 285–292. [Google Scholar] [CrossRef]
- Perobelli, S.; Zanolla, L.; Tamanini, A.; Rizzotti, P.; Assael, B.M.; Castellani, C. Inconclusive Cystic Fibrosis neonatal screening results: Long-term psychosocial effects on parents. Acta Paediatr. 2009, 98, 1927–1934. [Google Scholar] [CrossRef]
- Quigley, S.J.; Linnane, B.; Connellan, S.; Ward, A.; Ryan, P. Psychosocial Distress and Knowledge Deficiencies in Parents of Children in Ireland Who Carry an Altered Cystic Fibrosis Gene. J. Genet. Couns. 2017, 27, 589–596. [Google Scholar] [CrossRef]
- Rueegg, C.S.; Barben, J.; Hafen, G.M.; Moeller, A.; Jurca, M.; Fingerhut, R.; Kuehni, C.E. Newborn screening for cystic fibrosis—The parent perspective. J. Cyst. Fibros. 2016, 15, 443–451. [Google Scholar] [CrossRef]
- Salm, N.; Yetter, E.; Tluczek, A. Informing parents about positive newborn screen results: Parents’ recommendations. J. Child. Health Care 2012, 16, 367–381. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Glazner, J.A. What Follows Newborn Screening? An Evaluation of a Residential Education Program for Parents of Infants With Newly Diagnosed Cystic Fibrosis. Pediatrics 2004, 114, 411–416. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Cerritelli, B.; Carter, L.S.; Cooke, M.; Glazner, J.A.; Massie, J. Changing Their Minds With Time: A Comparison of Hypothetical and Actual Reproductive Behaviors in Parents of Children With Cystic Fibrosis. Pediatrics 2006, 118, e649–e656. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.L.; Castellanos-Brown, K.; Childress, S.; Bonhomme, N.; Oktay, J.S.; Terry, S.F.; Kyler, P.; Davidoff, A.; Greene, C. The impact of false-positive newborn screening results on families: A qualitative study. Genet. Med. 2012, 14, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; de Braekeleer, M.; Roussey, M.; Rault, G.; Parent, P.; Dagorne, M.; Journel, H.; Lemoigne, A.; Codet, J.-P.; Catheline, M.; et al. Neonatal screening for cystic fibrosis in Brittany, France: Assessment of 10 years’ experience and impact on prenatal diagnosis. Lancet 2000, 356, 789–794. [Google Scholar] [CrossRef]
- Skinner, D.; Choudhury, S.; Sideris, J.; Guarda, S.; Buansi, A.; Roche, M.; Powell, C.; Bailey, D.B. Parents’ Decisions to Screen Newborns for FMR1 Gene Expansions in a Pilot Research Project. Pediatrics 2011, 127, e1455–e1463. [Google Scholar] [CrossRef]
- Temme, R.; Gruber, A.; Johnson, M.; Read, L.; Lu, Y.; McNamara, J. Assessment of Parental Understanding of Positive Newborn Screening Results and Carrier Status for Cystic Fibrosis with the use of a Short Educational Video. J. Genet. Couns. 2014, 24, 473–481. [Google Scholar] [CrossRef]
- Timmermans, S.; Buchbinder, M. Patients-in-waiting: Living between sickness and health in the genomics era. J. Health Soc. Behav. 2010, 51, 408–423. [Google Scholar] [CrossRef]
- Tluczek, A.; Koscik, R.L.; Modaff, P.; Pfeil, D.; Rock, M.J.; Farrell, P.M.; Lifchez, C.; Freeman, M.E.; Gershan, W.; Zaleski, C.; et al. Newborn Screening for Cystic Fibrosis: Parents’ Preferences Regarding Counseling At the Time of Infants’ Sweat Test. J. Genet. Couns. 2006, 15, 277–291. [Google Scholar] [CrossRef]
- Tluczek, A.; Orland, K.M.; Nick, S.W.; Brown, R.L. Newborn screening: An appeal for improved parent education. J. Périnat. Neonatal Nurs. 2009, 23, 326–334. [Google Scholar] [CrossRef]
- Tluczek, A.; McKechnie, A.C.; Lynam, P.A. When the Cystic Fibrosis Label Does Not Fit: A Modified Uncertainty Theory. Qual. Health Res. 2010, 20, 209–223. [Google Scholar] [CrossRef]
- Tluczek, A.; Clark, R.; McKechnie, A.; Orland, K.M.; Brown, R.L. Task-Oriented and Bottle Feeding Adversely Affect the Quality of Mother-Infant Interactions After Abnormal Newborn Screens. J. Dev. Behav. Pediatr. 2010, 31, 414–426. [Google Scholar] [CrossRef]
- Tluczek, A.; Orland, K.M.; Cavanagh, L. Psychosocial Consequences of False-Positive Newborn Screens for Cystic Fibrosis. Qual. Health Res. 2010, 21, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Becker, T.; Laxova, A.; Grieve, A.; Gilles, C.N.R.; Rock, M.J.; Gershan, W.M.; Green, C.G.; Farrell, P.M. Relationships Among Health-Related Quality of Life, Pulmonary Health, and Newborn Screening for Cystic Fibrosis. Chest 2011, 140, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; McKechnie, A.; Brown, R.L. Factors Associated With Parental Perception of Child Vulnerability 12 Months After Abnormal Newborn Screening Results. Res. Nurs. Health 2011, 34, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Laxova, A.; Grieve, A.; Heun, A.; Brown, R.L.; Rock, M.J.; Gershan, W.M.; Farrell, P.M. Long-term follow-up of cystic fibrosis newborn screening: Psychosocial functioning of adolescents and young adults. J. Cyst. Fibros. 2013, 13, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Clark, R.; McKechnie, A.C.; Brown, R.L. Factors Affecting Parent-Child Relationships One Year After Positive Newborn Screening for Cystic Fibrosis or Congenital Hypothyroidism. J. Dev. Behav. Pediatr. 2015, 36, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Levy, H.; Rock, M.J.; Ondoma, C.; Brown, R.L. Impact of Intermediate Cystic Fibrosis Classification on Parents’ Perceptions of Child Vulnerability and Protectiveness. J. Fam. Nurs. 2019, 25, 287–313. [Google Scholar] [CrossRef]
- Tu, W.-J.; He, J.; Chen, H.; Shi, X.-D.; Li, Y. Psychological Effects of False-Positive Results in Expanded Newborn Screening in China. PLoS ONE 2012, 7, e36235. [Google Scholar] [CrossRef]
- Ulph, F.; Cullinan, T.; Qureshi, N.; Kai, J. Familial influences on antenatal and newborn haemoglobinopathy screening. Ethn. Health 2011, 16, 361–375. [Google Scholar] [CrossRef]
- Ulph, F.; Cullinan, T.; Qureshi, N.; Kai, J. Informing children of their newborn screening carrier result for sickle cell or cystic fibrosis: Qualitative study of parents’ intentions, views and support needs. J. Genet. Couns. 2014, 23, 409–420. [Google Scholar] [CrossRef]
- Ulph, F.; Cullinan, T.; Qureshi, N.; Kai, J. Parents’ responses to receiving sickle cell or cystic fibrosis carrier results for their child following newborn screening. Eur. J. Hum. Genet. 2014, 23, 459–465. [Google Scholar] [CrossRef]
- Veer, L.V.D.S.; Kempers, M.J.E.; Last, B.F.; Vulsma, T.; Grootenhuis, M.A. Quality of Life, Developmental Milestones, and Self-Esteem of Young Adults with Congenital Hypothyroidism Diagnosed by Neonatal Screening. J. Clin. Endocrinol. Metab. 2008, 93, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Langen, A.V.-V.; van der Pal, S.; Reijntjens, A.; Loeber, J.; Dompeling, E.; Dankert-Roelse, J. Parental knowledge reduces long term anxiety induced by false-positive test results after newborn screening for cystic fibrosis. Mol. Genet. Metab. Rep. 2014, 1, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of Expanded Newborn Screening for Biochemical Genetic Disorders on Child Outcomes and Parental Stress. JAMA 2003, 290, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, S.E.; Rones, M.; Read, C.Y.; Marsden, D.; Levy, H.L. Brief Report: Predictors of Parenting Stress Among Parents of Children With Biochemical Genetic Disorders. J. Pediatr. Psychol. 2004, 29, 565–570. [Google Scholar] [CrossRef]
- Wheeler, P.G.; Smith, R.; Dorkin, H.; Parad, R.B.; Comeau, A.M.; Bianchi, D.W. Genetic counseling after implementation of statewide cystic fibrosis newborn screening: Two years’ experience in one medical center. Genet. Med. 2001, 3, 411–415. [Google Scholar] [CrossRef]
- Munck, A.; Mayell, S.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. 2015, 14, 706–713. [Google Scholar] [CrossRef]
- Ren, C.L.; Borowitz, D.S.; Gonska, T.; Howenstine, M.S.; Levy, H.; Massie, J.; Milla, C.; Munck, A.; Southern, K.W. Cystic Fibrosis Transmembrane Conductance Regulator-Related Metabolic Syndrome and Cystic Fibrosis Screen Positive, Inconclusive Diagnosis. J. Pediatr. 2017, 181, S45–S51.e1. [Google Scholar] [CrossRef]
- Jadin, S.A.; Wu, G.S.; Zhang, Z.; Shoff, S.M.; Tippets, B.M.; Farrell, P.M.; Miller, T.; Rock, M.J.; Levy, H.; Lai, H.J. Growth and pulmonary outcomes during the first 2 y of life of breastfed and formula-fed infants diagnosed with cystic fibrosis through the Wisconsin Routine Newborn Screening Program. Am. J. Clin. Nutr. 2011, 93, 1038–1047. [Google Scholar] [CrossRef]
- Botkin, J.R.; Rothwell, E.; Anderson, R.A.; Rose, N.C.; Dolan, S.; Kuppermann, M.; Stark, L.; Goldenberg, A.; Wong, B. Prenatal education of parents about newborn screening and residual dried blood spots: A randomized clinical trial. JAMA Pediatr. 2016, 170, 543–549. [Google Scholar] [CrossRef]
- Tluczek, A.; Zaleski, C.; Stachiw-Hietpas, D.; Modaff, P.; Adamski, C.R.; Nelson, M.R.; Reiser, C.A.; Ghate, S.; Josephson, K.D. A Tailored Approach to Family-Centered Genetic Counseling for Cystic Fibrosis Newborn Screening: The Wisconsin Model. J. Genet. Couns. 2010, 20, 115–128. [Google Scholar] [CrossRef]
- Farrell, P.M.; Langfelder-Schwind, E.; Farrell, M.H. Challenging the dogma of the healthy heterozygote: Implications for newborn screening policies and practices. Mol. Genet. Metab. 2021, 134, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Bush, L.; Davidson, H.; Gelles, S.; Lea, D.; Koehly, L.M. Experiences of Families Caring for Children with Newborn Screening-Related Conditions: Implications for the Expansion of Genomics in Population-Based Neonatal Public Health Programs. Int. J. Neonatal Screen. 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Harmon, J.; Gropman, A.L. Select Ethical Aspects of Next-Generation Sequencing Tests for Newborn Screening and Diagnostic Evaluation of Critically Ill Newborns. Int. J. Neonatal Screen. 2021, 7, 76. [Google Scholar] [CrossRef]
- Clark, C.C.; Boardman, F.K. Expanding the notion of “benefit”: Comparing public, parent, and professional attitudes towards whole genome sequencing in newborns. New Genet. Soc. 2022, 41, 96–115. [Google Scholar] [CrossRef]
- Woerner, A.C.; Gallagher, R.C.; Vockley, J.; Adhikari, A.N. The Use of Whole Genome and Exome Sequencing for Newborn Screening: Challenges and Opportunities for Population Health. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).