Newborn Screening for Duchenne Muscular Dystrophy: First Year Results of a Population-Based Pilot
Abstract
1. Introduction
1.1. Newborn Screening
1.2. DMD Pilot
2. Materials and Methods
2.1. Protocol Development
- Validate a high-throughput first-tier immunoassay screen for Duchenne in a high-birth-number state and determine the utility of the Collaborative Laboratory Integrated Reports (CLIR) tool and possible other biochemical markers for interpreting results.
- Optimize a second-tier molecular testing strategy for confirming diagnosis of DMD and other muscular disorders, after positive CM-MM first-tier screening.
- Using the first- and second-tier testing algorithm, identify infants who will develop DMD before clinically detectable symptom onset and enable parents the opportunity to see a subspecialist to confirm the diagnosis, identify the DMD genotype, and determine treatment course, including participation in clinical trials.
- Use the results of the pilot testing to provide evidence required for state and federal assessments of the benefits and risks of NBS for Duchenne and develop the infrastructure to educate parents and health care providers about Duchenne NBS.
- Nomination of DMD to the RUSP.
2.2. Enrollment
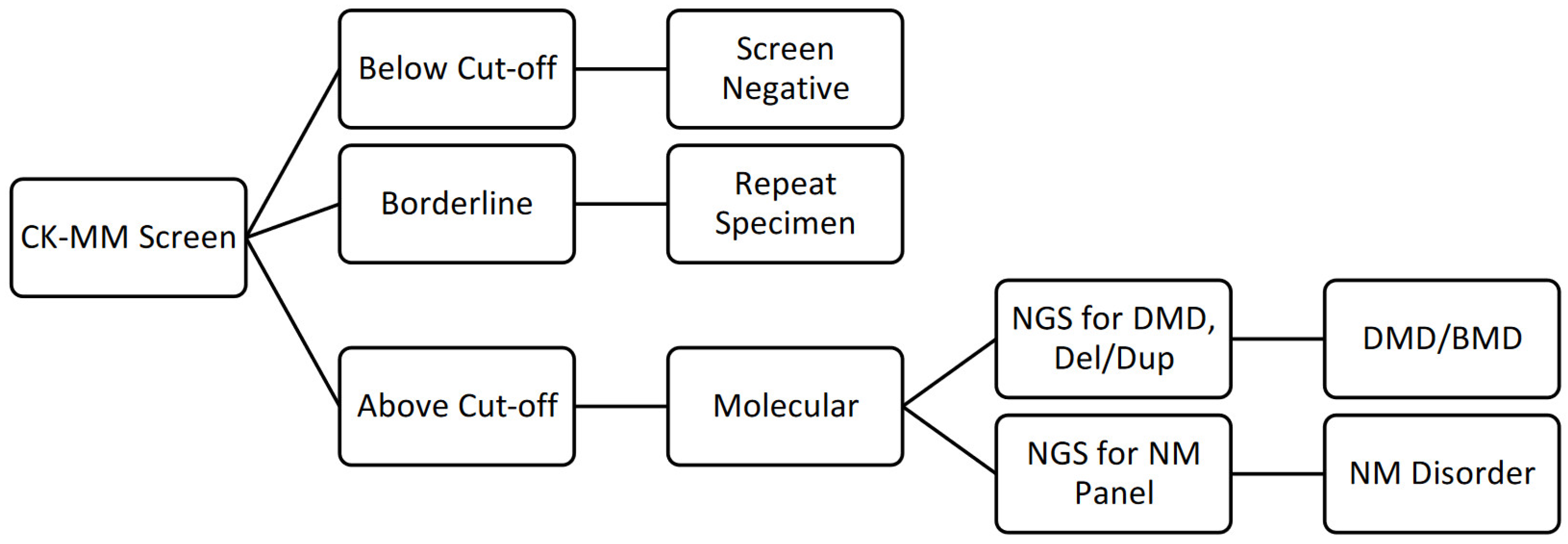
2.3. Screening
- DMD Sequencing and Microarray-based Comparative Genomic Hybridization (aCGH) Analysis: In solution hybridization of the 79 coding exons, the muscle promoter as well as the region surrounding several known deep intronic pathogenic variants, within the DMD gene. Direct sequencing of the amplified captured regions performed using next generation short base pair read sequencing. A custom aCGH for the DMD gene was used to detect deletions and/or duplications.
- If needed, perform neuromuscular disorders panel (47 genes): In solution hybridization of the targeted coding exons within the genes tested.* The genes on this panel were chosen through evidence-based analysis and direct sequencing of the amplified captured regions was performed using next generation short base pair read sequencing.
- If needed, perform additional analysis (90 to 104 genes): These gene panels include sequencing and deletion/duplication testing by NGS of up to 103 additional genes associated with neuromuscular disorders and related neurological disorders.**
2.4. Diagnosis and Referral to Clinical Care
2.5. Data Collection
3. Results
3.1. Enrolled
3.2. Borderline
3.3. Referred
3.4. DMD Diagnosed
3.5. Non-DMD Screen Positive Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Parsons, E.P.; Clarke, A.J.; Bradley, D.M. Developmental progress in Duchenne muscular dystrophy: Lessons for earlier detection. Eur. J. Paediatr. Neurol. 2004, 8, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M. Breakthrough to Bedside: Bringing Gene Therapy to Neuromuscular Diseases. Hum. Gene Ther. Clin. Dev. 2019, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Shilling, C.; Leslie, N.D.; Flanigan, K.M.; al-Dahhak, R.; Gastier-Foster, J.; Kneile, K.; Dunn, D.M.; Duval, B.; Aoyagi, A.; et al. Evidence based path to newborn screening for Duchenne muscular dystrophy. Ann. Neurol. 2012, 71, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Lloyd-Puryear, M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2013, 48, 21–26. [Google Scholar] [CrossRef]
- Al-Zaidy, S.A.; Lloyd-Puryear, M.; Kennedy, A.; Lopez, V.; Mendell, J.R. A Roadmap to Newborn Screening for Duchenne Muscular Dystrophy. Int. J. Neonatal Screen. 2017, 3, 8. [Google Scholar] [CrossRef]
- Project Parent Muscular Dystrophy Duchenne Drug Development Pipeline. Available online: https://www.parentprojectmd.org/duchenne-drug-development-pipeline/ (accessed on 25 July 2022).
- Advisory Committee on Heritable Disorders in Newborns and Children. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/index.html (accessed on 18 January 2022).
- Advisory Committee on Heritable Disorders in Newborns and Children: Nominating a Condition. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/nominate.html#:~:text=Conditions%20for%20consideration%20by%20the,for%20inclusion%20on%20the%20RUSP (accessed on 18 January 2022).
- Bradley, D.M.; Parsons, E. Newborn screening for Duchenne muscular dystrophy. Semin. Neonatol. 1998, 3, 27–34. [Google Scholar] [CrossRef]
- Gatheridge, M.A.; Kwon, J.M.; Mendell, J.M.; Scheuerbrandt, G.; Moat, S.J.; Eyskens, F.; Rockman-Greenberg, C.; Drousiotou, A.; Griggs, R.C. Identifying Non-Duchenne Muscular Dystrophy-Positive and False Negative Results in Prior Duchenne Muscular Dystrophy Newborn Screening Programs: A Review. JAMA Neurol. 2016, 73, 111–116. [Google Scholar] [CrossRef]
- Lloyd-Puryear, M.; Brower, A.; Berry, S.A.; Brosco, J.P.; Bowdish, B.; Watson, M.S. Foundation of the Newborn Screening Translational Research Network and its tools for research. Genet. Med. 2019, 21, 1271–1279. [Google Scholar] [CrossRef]
- New York State Health Profiles. Available online: https://profiles.health.ny.gov/measures/all_state/16511 (accessed on 22 October 2021).
- Wynn, J.; Tavakoli, N.P.; Armstrong, N.; Gomez, J.; Koval, C.; Lai, C.; Tang, S.; Quevedo Prince, A.; Quevedo, Y.; Rufino, K.; et al. Improving recruitment for a newborn screening pilot study with adaptations in response to the COVID-19 pandemic. Int. J. Neonatal Screen. 2022, 8, 23. [Google Scholar] [CrossRef]
- Timonen, A.; Lloyd-Puryear, M.; Hougaard, D.M.; Meriö, L.; Mäkinen, P.; Laitala, V.; Pölönen, T.; Skogstrand, K.; Kennedy, A.; Airenne, S.; et al. Duchenne Muscular Dystrophy Newborn Screening: Evaluation of a New GSP Neonatal Creatine Kinase-MM Kit in a US and Danish Population. Int. J. Neonatal Screen. 2019, 5, 27. [Google Scholar] [CrossRef]
- Newborn Screening Technical Assistance and Evaluation Program (NewSTEPs) Data Resources Reports. Available online: https://www.newsteps.org/data-resources/reports/screening-methodologies-and-targets-report (accessed on 2 December 2021).
- Park, S.; Maloney, B.; Caggana, M.; Tavakoli, N.P. Creatine kinase-MM concentration in dried blood spots from newborns and implications for newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2022, 65, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.L.; Hardisty, E.; Coviello, E.; Stuebe, A. Telehealth to provide prenatal genetics services: Feasibility and importance revealed during global pandemic. Prenat. Diagn. 2020, 40, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- American College of Medical Genetics and Genomics. n.d. ACT Sheets and Algorithms. Available online: https://www.acmg.net/ACMG/Medical-Genetics-Practice-Resources/ACT_Sheets_and_Algorithms.aspx (accessed on 27 January 2021).
- American College of Medical Genetics and Genomics. n.d. Pathogenic Variant in Dystrophin (DMD Gene) and Elevated Creatine Kinase Muscle Isoform (CK-MM) Duchenne and Becker Muscular Dystrophy. Available online: https://www.acmg.net/PDFLibrary/DMD_Pathogenic_Variants.pdf (accessed on 22 May 2022).
- American College of Medical Genetics and Genomics. n.d. Elevated Creatine Kinase Muscle Isoform (CK-MM) Genetic Neuromuscular Disease. Available online: https://www.acmg.net/PDFLibrary/DMD_CKMM.pdf (accessed on 22 May 2022).
- American College of Medical Genetics and Genomics. n.d. No Pathogenic Variant in Dystrophin (DMD) Gene after Elevated Creatine Kinase Muscle Isoform (CK-MM) Genetic Neuromuscular Disease. Available online: https://www.acmg.net/PDFLibrary/DMD_No_pathogenic_Variant.pdf (accessed on 22 May 2022).
- Ishizaki, M.; Kobayashi, M.; Adachi, K.; Matsumura, T.; Kimura, E. Female dystrophinopathy: Review of current literature. Neuromuscul. Disord. 2018, 28, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Xie, Y.; Bhandari, V.; Chen, G.; Dang, Y.; Liao, H.; Zhang, J.; Lan, D. Clinical and genetic characteristics of female dystrophinopathy carriers. Mol. Med. Rep. 2019, 19, 3035–3044. [Google Scholar] [CrossRef]
- Mah, M.L.; Cripe, L.; Slawinski, M.K.; Al-Zaidy, S.A.; Camino, E.; Lehman, K.J.; Jackson, J.L.; Iammarino, M.; Miller, N.; Mendell, J.R.; et al. Duchenne and Becker muscular dystrophy carriers: Evidence of cardiomyopathy by exercise and cardiac MRI testing. Int. J. Cardiol. 2020, 316, 257–265. [Google Scholar] [CrossRef]
- Lloyd-Puryear, M.A.; Crawford, T.O.; Brower, A.; Stephenson, K.; Trotter, T.; Goldman, E.; Goldenberg, A.; Howell, R.R.; Kennedy, A.; Watson, M. Duchenne Muscular Dystrophy Newborn Screening, a Case Study for Examining Ethical and Legal Issues for Pilots for Emerging Disorders: Considerations and Recommendations. Int. J. Neonatal Screen. 2018, 4, 6. [Google Scholar] [CrossRef]
- Brower, A.; Chan, K.; Hartnett, M.; Taylor, J. The Longitudinal Pediatric Data Resource: Facilitating Longitudinal Collection of Health Information to Inform Clinical Care and Guide Newborn Screening Efforts. Int. J. Neonatal Screen. 2021, 7, 37. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- National Institutes of Health: Common Data Elements. Available online: https://cde.nlm.nih.gov/home (accessed on 8 January 2021).
- Gill, S.V.; May-Benson, T.A.; Teasdale, A.; Munsell, E.G. Birth and development Birth and developmental correlates of birth weight in a sample of children with potential sensory processing disorder. BMC Pediatrics 2013, 13, 29. [Google Scholar] [CrossRef]
- The American College of Obstetrics and Gynecology Definition of Term Pregnancy. Available online: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2013/11/definition-of-term-pregnancy (accessed on 29 April 2021).
- Cho, Y.; Choi, Y. Female Carriers of Duchenne Muscular Dystrophy. J. Genet. Med. 2013, 10, 94–98. [Google Scholar] [CrossRef]
- Thomas, S.; Conway, K.M.; Fapo, O.; Street, N.; Mathews, K.D.; Mann, J.R.; Romitti, P.A.; Soim, A.; Westfield, C.; Fox, D.J.; et al. Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). Time to diagnosis of Duchenne muscular dystrophy remains unchanged: Findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000-2015. Muscle Nerve 2022, 66, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Parent Project Muscular Dystrophy Duchenne Drug Development Pipeline: AMONDYS 45™. Available online: https://www.parentprojectmd.org/drug-development-pipeline/casimersen-srp-4045/ (accessed on 12 August 2022).
- Parent Project Muscular Dystrophy Duchenne Drug Development Pipeline: EXONDYS 51®. Available online: https://www.parentprojectmd.org/drug-development-pipeline/exondys-51/ (accessed on 12 August 2022).
- Parent Project Muscular Dystrophy Duchenne Drug Development Pipeline: VILTEPSO™. Available online: https://www.parentprojectmd.org/drug-development-pipeline/viltolarsen-ns-065-ncnp-01/ (accessed on 12 August 2022).
- Parent Project Muscular Dystrophy Duchenne Drug Development Pipeline: VYONDYS 53®. Available online: https://www.parentprojectmd.org/drug-development-pipeline/golodirsen-srp-4053/ (accessed on 12 August 2022).
- Parent Project Muscular Dystrophy Duchenne Drug Development Pipeline: ATALUREN (TRANSLARNA®). Available online: https://www.parentprojectmd.org/drug-development-pipeline/ataluren-translarna/ (accessed on 12 August 2022).
- Parent Project Muscular Dystrophy Duchenne Drug Development Pipeline: PF-06939926. Available online: https://www.parentprojectmd.org/drug-development-pipeline/pf-06939926-mini-dystrophin-gene-therapy/ (accessed on 12 August 2022).
- Vita, G.L.; Vita, G. Is it the right time for an infant screening for Duchenne muscular dystrophy? Neurol. Sci. 2020, 41, 1677–1683. [Google Scholar] [CrossRef]
- Chung, W.K.; Berg, J.S.; Botkin, J.R.; Brenner, S.E.; Brosco, J.P.; Brothers, K.B.; Currier, R.J.; Gaviglio, A.; Kowtoniuk, W.E.; Olson, C.; et al. Newborn screening for neurodevelopmental diseases: Are we there yet? Am. J. Med. Genet. Part C Semin. Med. Genet. 2022. [Google Scholar] [CrossRef] [PubMed]

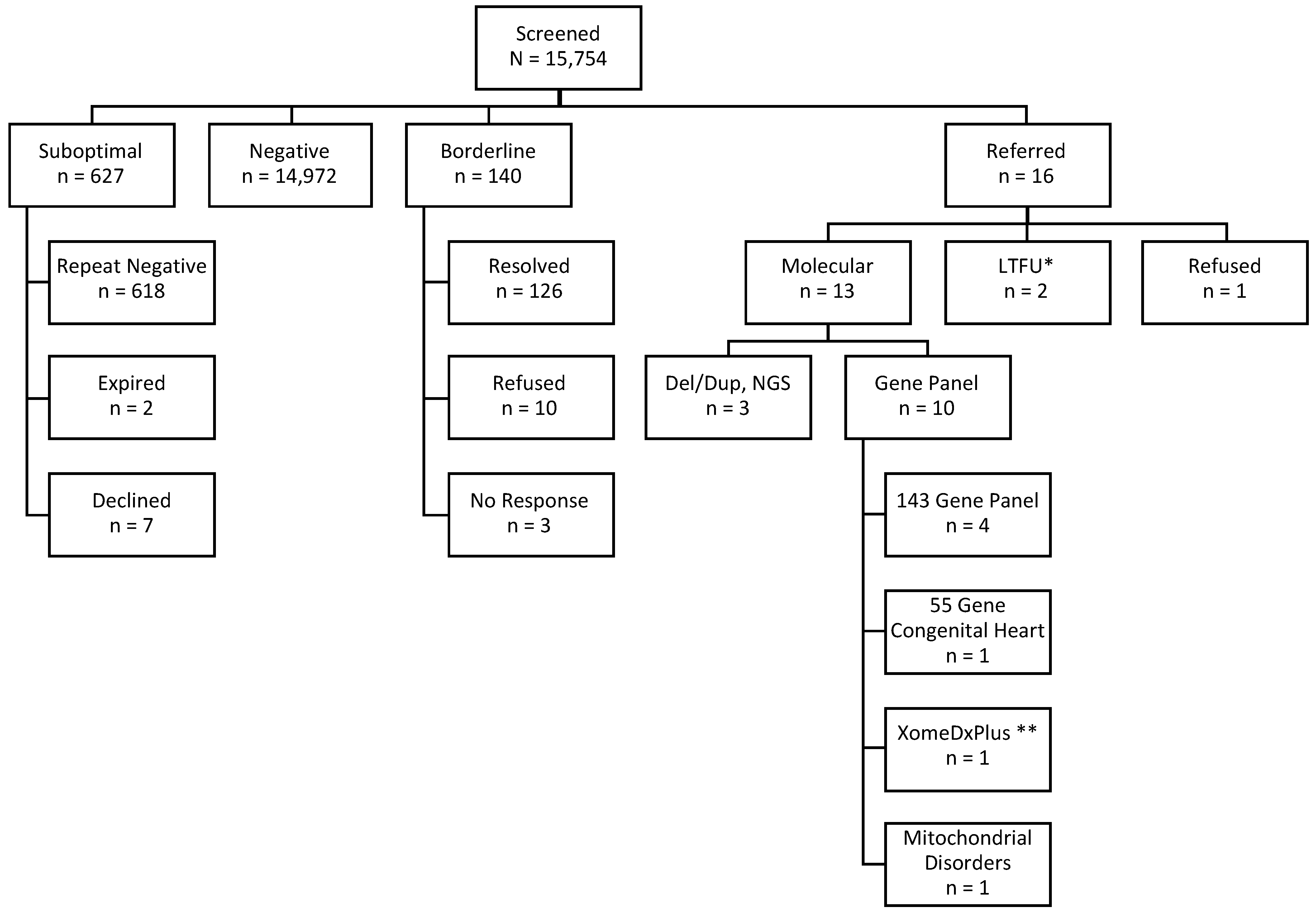
| Total Hospital Births | 35,570 | ||
|---|---|---|---|
| Missed | 16,980 (48%) | ||
| Approached | 18,590 (52%) | Declined to Participate | 2797 (15%) |
| Enrolled | 15,793 (85%) |
| Case * | Diagnosis | Sex | Screen Result CK-MM (ng/mL) | Molecular Result | Case Summary |
|---|---|---|---|---|---|
| R-5 | DMD | M | 7809 | DMD dup ex18 (LP) | Molecular diagnosis of Duchenne/Becker muscular dystrophy. Followed in MDA clinic. Last visit at 17 months: out-toes but no toe walking. With support can raise self-off floor. Cannot assess if Gower sign is present. Appears to have trouble raising body with use of one leg. Physical therapy 2x per week and development therapy 1x per week. No medications. |
| R-6 | BMD | M | 6384 | DMD del ex48-49 (LP) | Followed in MDA clinic. No cognitive or motor delays identified at 7 months. Sequencing result is consistent with a deletion of this region of the gene and predicted to result in in-frame deletion in the DMD mRNA. This is consistent with a diagnosis of DMD/BMD. The subject’s maternal grandfather was diagnosed with molecularly confirmed BMD. His symptoms started in mid-life. |
| R-15 | DMD | M | 18,574 | DMD del ex3-43 (LP) | Not screened as a newborn. Referred to genetics and enrolled in study because of family history of DMD, markedly elevated CK, and troponin T suggestive of congenital myopathy and hydronephrosis. DMD and gene panel ordered concurrently. |
| R-8 | Alagille Syndrome | F | 4370 | JAG1 (P) | CK normalized at 4 months. Baby had a congenital heart defect and very high liver enzyme. Clinical genetics evaluation revealed JAG1 pathogenic variant consistent with Alagille syndrome. |
| R-12 | None | F | 4150 | SGCA, (P), het; TTN (VUS), het | Normalized CK and no evidence of muscle weakness at 1 month. Family received genetic counseling regarding AR inheritance of limb girdle muscular dystrophy and was offered parental testing. |
| R-9 | None | F | 6007 | GNE c.218G>A, (P), het; POMT1 (VUS), het; TTN (VUS) x 3, het | CK not repeated. Clinical evaluation showed no evidence of sialuria. |
| R-3 | None | F | 4895 | DMD 17 kb del intron 55 (VUS), het; RYR1 (VUS), het | Normalized CK and no evidence of weakness at 9 months. |
| R-10 | None | F | 5365 | LAMA2, (VUS), het | CK not repeated. Declined further follow-up care. |
| R-11 | None | M | 4154 | TTN, (VUS), het | Normalized CK and no evidence of weakness at 1 month. |
| R-13 | None | M | 5128 | SIL1, (VUS), het | CK not repeated. Declined further follow-up care. |
| R-14 | None | F | 12,002 | AMPD1, (VUS), het | CK was not repeated. Family moved out of state. |
| R-16 | None | F | 4507 | RYR2, (VUS), het; TTN, (VUS), het | CK normalized at 9 days. Declined further follow-up care. |
| R-4 | None | M | 4850 | DYSP, (VUS), het PLEC, (VUS), het RYR2, (VUS), het | Normalized CK at 10 days. At birth there was a concern for inborn error of metabolism because of the standard newborn screening panel. Complete metabolic workup and exome sequencing with mitochondrial genome seq/del were negative. |
| R-7 | None | F | 5054 | Declined testing | Parents report normal development at 7 months. Declined molecular testing. |
| R-1 | LTFU | M | 4593 | NA | Unknown. |
| R-2 | LTFU | F | 8399 | NA | Unknown. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartnett, M.J.; Lloyd-Puryear, M.A.; Tavakoli, N.P.; Wynn, J.; Koval-Burt, C.L.; Gruber, D.; Trotter, T.; Caggana, M.; Chung, W.K.; Armstrong, N.; et al. Newborn Screening for Duchenne Muscular Dystrophy: First Year Results of a Population-Based Pilot. Int. J. Neonatal Screen. 2022, 8, 50. https://doi.org/10.3390/ijns8040050
Hartnett MJ, Lloyd-Puryear MA, Tavakoli NP, Wynn J, Koval-Burt CL, Gruber D, Trotter T, Caggana M, Chung WK, Armstrong N, et al. Newborn Screening for Duchenne Muscular Dystrophy: First Year Results of a Population-Based Pilot. International Journal of Neonatal Screening. 2022; 8(4):50. https://doi.org/10.3390/ijns8040050
Chicago/Turabian StyleHartnett, Michael J., Michele A. Lloyd-Puryear, Norma P. Tavakoli, Julia Wynn, Carrie L. Koval-Burt, Dorota Gruber, Tracy Trotter, Michele Caggana, Wendy K. Chung, Niki Armstrong, and et al. 2022. "Newborn Screening for Duchenne Muscular Dystrophy: First Year Results of a Population-Based Pilot" International Journal of Neonatal Screening 8, no. 4: 50. https://doi.org/10.3390/ijns8040050
APA StyleHartnett, M. J., Lloyd-Puryear, M. A., Tavakoli, N. P., Wynn, J., Koval-Burt, C. L., Gruber, D., Trotter, T., Caggana, M., Chung, W. K., Armstrong, N., & Brower, A. M. (2022). Newborn Screening for Duchenne Muscular Dystrophy: First Year Results of a Population-Based Pilot. International Journal of Neonatal Screening, 8(4), 50. https://doi.org/10.3390/ijns8040050








