Newborn Screen for X-Linked Adrenoleukodystrophy Using Flow Injection Tandem Mass Spectrometry in Negative Ion Mode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of C26:0-LPC
2.3. FIA-MS/MS
2.4. Linearity, Accuracy, and Precision
2.5. Ion Suppression, Filter Paper Matrix, and Carryover
2.6. Population Distribution and Cutoff Establishment
3. Results
3.1. Linearity, Accuracy, and Precision
3.2. Ion Suppression, Filter Paper Matrix, and Carryover
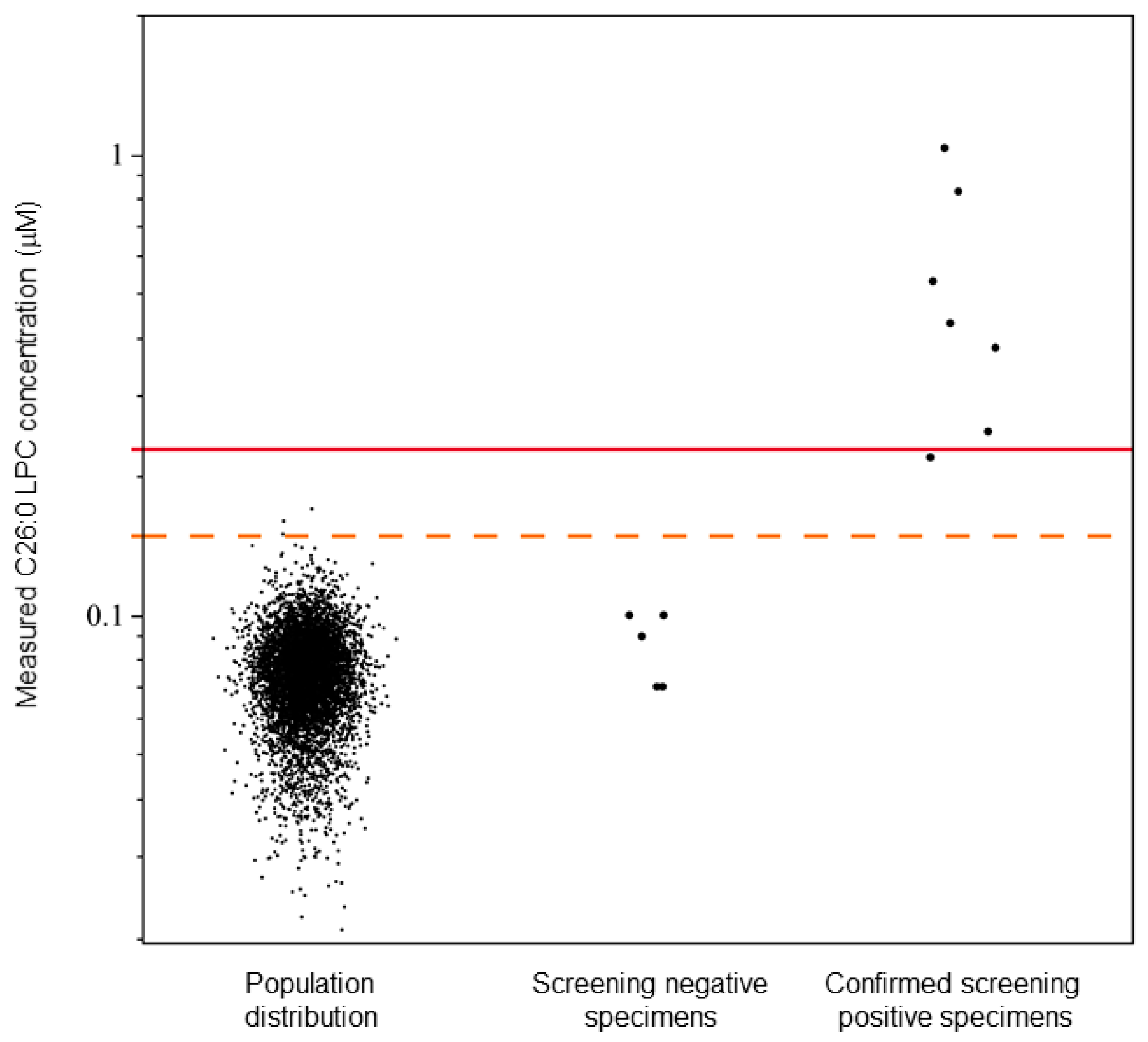
3.3. Population Distribution and Cutoff Establishment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Engelen, M.; Kemp, S.; De Visser, M.; Van Geel, B.M.; Wanders, R.J.A.; Aubourg, P.; Poll-The, B.T. X-linked adrenoleukodystrophy (X-ALD): Clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J. Rare Dis. 2012, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.B.; Jones, R.O.; Hubbard, W.C.; Tortorelli, S.; Orsini, J.J.; Caggana, M.; Vogel, B.H.; Raymond, G.V. Newborn Screening for X-Linked Adrenoleukodystrophy. Int. J. Neonatal Screen. 2016, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, S.; Berger, J.; Aubourg, P. X-linked adrenoleukodystrophy: Clinical, metabolic, genetic and pathophysiological aspects. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 1465–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezman, L.; Moser, A.B.; Raymond, G.V.; Rinaldo, P.; Watkins, P.A.; Smith, K.D.; Kass, N.E.; Moser, H.W. Adrenoleukodystrophy: Incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 2001, 49, 512–517. [Google Scholar] [CrossRef]
- Engelen, M.; Kemp, S.; Poll-The, B.-T. X-Linked Adrenoleukodystrophy: Pathogenesis and Treatment. Curr. Neurol. Neurosci. Rep. 2014, 14, 486. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, I.; Aubourg, P.; Pujol, A. General Aspects and Neuropathology of X-Linked Adrenoleukodystrophy. Brain Pathol. 2009, 20, 817–830. [Google Scholar] [CrossRef]
- Powers, J.M.; Liu, Y.; Moser, A.B.; Moser, H.W. The inflammatory myelinopathy of adreno-leukodystrophy: Cells, effector molecules, and pathogenetic implications. J. Neuropathol. Exp. Neurol. 1992, 51, 630–643. [Google Scholar] [CrossRef]
- Turk, B.R.; Theda, C.; Fatemi, A.; Moser, A.B. 1X-linked adrenoleukodystrophy: Pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int. J. Dev. Neurosci. 2020, 80, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Aubourg, P.; Blanche, S.; Jambaqué, I.; Rocchiccioli, F.; Kalifa, G.; Naud-Saudreau, C.; Rolland, M.-O.; Debré, M.; Chaussain, J.-L.; Griscelli, C.; et al. Reversal of Early Neurologic and Neuroradiologic Manifestations of X-Linked Adrenoleukodystrophy by Bone Marrow Transplantation. N. Engl. J. Med. 1990, 322, 1860–1866. [Google Scholar] [CrossRef]
- Cartier, N.; Aubourg, P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleu-kodystrophy. Brain Pathol. 2010, 20, 857–862. [Google Scholar] [CrossRef]
- Cartier, N.; Hacein-Bey-Abina, S.; Bartholomae, C.C.; Veres, G.; Schmidt, M.; Kutschera, I.; Vidaud, M.; Abel, U.; Dal-Cortivo, L.; Caccavelli, L.; et al. Hematopoietic Stem Cell Gene Therapy with a Lentiviral Vector in X-Linked Adrenoleukodystrophy. Science 2009, 326, 818–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichler, F.; Duncan, C.; Musolino, P.L.; Orchard, P.J.; De Oliveira, S.; Thrasher, A.; Armant, M.; Dansereau, C.; Lund, T.C.; Miller, W.P.; et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med. 2017, 377, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichler, F.; Mahmood, A.; Loes, D.; Bezman, L.; Lin, D.; Moser, H.W.; Raymond, G.V. Magnetic Resonance Imaging Detection of Lesion Progression in Adult Patients With X-linked Adrenoleukodystrophy. Arch. Neurol. 2007, 64, 659–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korenke, G.; Pouwels, P.J.; Frahm, J.; Hunneman, D.H.; Stoeckler, S.; Krasemann, E.; Jost, W.; Hanefeld, F. Arrested cerebral adrenoleukodystrophy: A clinical and proton magnetic resonance spectroscopy study in three patients. Pediatr. Neurol. 1996, 15, 103–107. [Google Scholar] [CrossRef]
- Loes, D.J.; Fatemi, A.; Melhem, E.R.; Gupte, N.; Bezman, L.; Moser, H.W.; Raymond, G.V. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology 2003, 61, 369–374. [Google Scholar] [CrossRef]
- Melhem, E.R.; Loes, D.J.; Georgiades, C.S.; Raymond, G.V.; Moser, H.W. X-linked Adrenoleukodystrophy: The Role of Contrast-enhanced MR Imaging in Predicting Disease Progression. Am. J. Neuroradiol. 2000, 21, 839–844. [Google Scholar]
- Rajanayagam, V.; Grad, J.; Krivit, W.; Loes, D.J.; Lockman, L.; Shapiro, E.; Balthazor, M.; Aeppli, D.; Stillman, A.E. Proton MR spectroscopy of childhood adrenoleukodystrophy. AJNR Am. J. Neuroradiol. 1996, 17, 1013–1024. [Google Scholar]
- Health Resources and Services Administration. Recommended Uniform Screening Panel. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html (accessed on 28 December 2021).
- Hubbard, W.C.; Moser, A.B.; Tortorelli, S.; Liu, A.; Jones, D.; Moser, H. Combined liquid chromatography–Tandem mass spectrometry as an analytical method for high throughput screening for X-linked adrenoleukodystrophy and other peroxisomal disorders: Preliminary findings. Mol. Genet. Metab. 2006, 89, 185–187. [Google Scholar] [CrossRef]
- Moser, H.W.; Moser, A.B.; Frayer, K.K.; Chen, W.; Schulman, J.D.; O’Neill, B.P.; Kishimoto, Y. Adrenoleukodystrophy: Increased plasma content of saturated very long chain fatty acids. Neurology 1981, 31, 1241. [Google Scholar] [CrossRef] [Green Version]
- Theda, C.; Moser, A.B.; Powers, J.M.; Moser, H.W. Phospholipids in X-linked adrenoleukodystrophy white matter: Fatty acid abnormalities before the onset of demyelination. J. Neurol. Sci. 1992, 110, 195–204. [Google Scholar] [CrossRef]
- Wilson, R.; Sargent, J.R. Lipid and Fatty Acid Composition of Brain Tissue from Adrenoleukodystrophy Patients. J. Neurochem. 1993, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Brosco, J.; Comeau, A.M.; Green, N.S.; Grosse, S.D.; Jones, E.; Kwon, J.M.; Lam, W.K.K.; Ojodu, J.; Prosser, L.A.; et al. Newborn screening for X-linked adrenoleukodystrophy: Evidence summary and advisory committee recommenda-tion. Genet. Med. 2017, 19, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matteson, J.; Sciortino, S.; Feuchtbaum, L.; Bishop, T.; Olney, R.; Tang, H. Adrenoleukodystrophy Newborn Screening in California Since 2016: Programmatic Outcomes and Follow-Up. Int. J. Neonatal Screen. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Bradley, S.; Adams, D.; D’Aco, K.; Erbe, R.; Fong, C.; Iglesias, A.; Kronn, D.; Levy, P.; Morrissey, M.; et al. Newborn screening for X-linked adrenoleukodystrophy in New York State: Diagnostic protocol, surveillance protocol and treatment guidelines. Mol. Genet. Metab. 2015, 114, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, W.C.; Moser, A.B.; Liu, A.C.; Jones, R.O.; Steinberg, S.J.; Lorey, F.; Panny, S.R.; Vogt, R.F., Jr.; Macaya, D.; Turgeon, C.T. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): Validation of a combined liquid chromatog-raphy–tandem mass spectrometric (LC–MS/MS) method. Mol. Genet. Metab. 2009, 97, 212–220. [Google Scholar] [CrossRef]
- Haynes, C.A.; De Jesus, V.R. Improved analysis of C26:0-lysophosphatidylcholine in dried-blood spots via negative ion mode HPLC-ESI-MS/MS for X-linked adrenoleukodystrophy newborn screening. Clin. Chim. Acta 2012, 413, 1217–1221. [Google Scholar] [CrossRef]
- Lee, S.; Clinard, K.; Young, S.P.; Rehder, C.W.; Fan, Z.; Calikoglu, A.S.; Bali, D.S.; Bailey, D.B.; Gehtland, L.M.; Millington, D.S.; et al. Evaluation of X-Linked Adrenoleukodystrophy Newborn Screening in North Carolina. JAMA Netw. Open 2020, 3, e1920356. [Google Scholar] [CrossRef]
- Wiens, K.; Berry, S.; Choi, H.; Gaviglio, A.; Gupta, A.; Hietala, A.; Kenney-Jung, D.; Lund, T.; Miller, W.; Pierpont, E.; et al. A report on state-wide implementation of newborn screening for X-linked Adrenoleukodystrophy. Am. J. Med. Genet. Part A 2019, 179, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Shimozawa, N.; Takashima, S.; Kawai, H.; Kubota, K.; Sasai, H.; Orii, K.; Ogawa, M.; Ohnishi, H. Advanced Diagnostic System and Introduction of Newborn Screening of Adrenoleukodystrophy and Peroxiso-mal Disorders in Japan. Int. J. Neonatal Screen. 2021, 7, 58. [Google Scholar] [CrossRef]
- Theda, C.; Gibbons, K.; DeFor, T.E.; Donohue, P.K.; Golden, W.; Kline, A.D.; Gulamali-Majid, F.; Panny, S.R.; Hubbard, W.C.; Jones, R.O.; et al. Newborn screening for X-linked adrenoleukodystrophy: Further evidence high throughput screening is feasible. Mol. Genet. Metab. 2014, 111, 55–57. [Google Scholar] [CrossRef] [Green Version]
- Adam, B.; Hall, E.; Sternberg, M.; Lim, T.; Flores, S.; O’Brien, S.; Simms, D.; Li, L.; De Jesus, V.; Hannon, W. The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin. Biochem. 2011, 44, 1445–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesús, V.R.; Mei, J.V.; Bell, C.J.; Hannon, H.W. Improving and assuring newborn screening laboratory quality worldwide: 30-year experience at the Centers for Disease Control and Prevention. Semin. Perinatol. 2010, 34, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Newborn Screening Quality Assurance Program 2019 Quality Control Report. Available online: https://www.cdc.gov/labstandards/nsqap.html (accessed on 28 December 2021).
- Adrienne, M.; (Connecticut Department of Public Health). Personal Communication, 2019.
- Clinical and Laboratory Standards Institute. Newborn Screening by Tandem Mass Spectrometry; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Lynch, K.L. CLSI C62-A: A New Standard for Clinical Mass Spectrometry. Clin. Chem. 2016, 62, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, C.A.; De Jesús, V.R. Simultaneous quantitation of hexacosanoyl lysophosphatidylcholine, amino acids, acylcarnitines, and succinylacetone during FIA–ESI–MS/MS analysis of dried blood spot extracts for newborn screening. Clin. Biochem. 2016, 49, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Jaspers, Y.R.; Sacha, F.; Dijkstra, I.M.E.; Willem, B.R.; van Lenthe, H.; Wim, K.; Marc, E.; Goorden, S.M.I.; Vaz, F.M.; Stephan, K. Comparison of the diagnostic performance of C26: 0-lysophosphatidylcholine and very long-chain fatty acids anal-ysis for peroxisomal disorders. Front. Cell Dev. Biol. 2020, 8, 690. [Google Scholar] [CrossRef]
- Harrison, K.A.; Murphy, R.C. Negative electrospray ionization of glycerophosphocholine lipids: Formation of [M—15]−ions occurs via collisional decomposition of adduct anions. Biol. Mass Spectrom. 1995, 30, 1772–1773. [Google Scholar] [CrossRef]
- Pulfer, M.; Murphy, R.C. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 2003, 22, 332–364. [Google Scholar] [CrossRef]

| MRM | Q1 Mass (Da) | Q3 Mass (Da) | DP(V) | EP(V) | CE(V) | CXP(V) |
|---|---|---|---|---|---|---|
| C26:0-LPC | 620.5 | 395.25 | −189 | −10 | −45 | −16 |
| d4-C26:0-LPC | 624.5 | 399.3 | −189 | −10 | −45 | −16 |
| Curtain gas (psi) | 28 |
| Ion spray voltage (V) | −4500 |
| Temperature (°C) | 650 |
| Gas1 (psi) | 30 |
| Gas2 (psi) | 50 |
| Collision assisted dissociation (psi) | 7 |
| Panel # | Measured C26:0-LPC (µM) | X-ALD Screening Outcome | ||
|---|---|---|---|---|
| Expected | Obtained | Expected | Obtained | |
| 1 | 1.06 | 1.03 | Positive | Positive |
| 2 | 0.06 | 0.07 | Negative | Negative |
| 3 | 0.07 | 0.09 | Negative | Negative |
| 4 | 0.26 | 0.25 | Positive | Positive |
| 5 | 0.18 | 0.22 | Borderline | Borderline |
| 6 | 0.10 | 0.10 | Negative | Negative |
| 7 | 0.10 | 0.10 | Negative | Negative |
| 8 | 0.06 | 0.07 | Negative | Negative |
| 9 | 0.44 | 0.43 | Positive | Positive |
| 10 | 0.74 | 0.83 | Positive | Positive |
| 11 | 0.49 | 0.53 | Positive | Positive |
| 12 | 0.33 | 0.38 | Positive | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teber, T.A.; Conti, B.J.; Haynes, C.A.; Hietala, A.; Baker, M.W. Newborn Screen for X-Linked Adrenoleukodystrophy Using Flow Injection Tandem Mass Spectrometry in Negative Ion Mode. Int. J. Neonatal Screen. 2022, 8, 27. https://doi.org/10.3390/ijns8020027
Teber TA, Conti BJ, Haynes CA, Hietala A, Baker MW. Newborn Screen for X-Linked Adrenoleukodystrophy Using Flow Injection Tandem Mass Spectrometry in Negative Ion Mode. International Journal of Neonatal Screening. 2022; 8(2):27. https://doi.org/10.3390/ijns8020027
Chicago/Turabian StyleTeber, Tarek A., Brian J. Conti, Christopher A. Haynes, Amy Hietala, and Mei W. Baker. 2022. "Newborn Screen for X-Linked Adrenoleukodystrophy Using Flow Injection Tandem Mass Spectrometry in Negative Ion Mode" International Journal of Neonatal Screening 8, no. 2: 27. https://doi.org/10.3390/ijns8020027
APA StyleTeber, T. A., Conti, B. J., Haynes, C. A., Hietala, A., & Baker, M. W. (2022). Newborn Screen for X-Linked Adrenoleukodystrophy Using Flow Injection Tandem Mass Spectrometry in Negative Ion Mode. International Journal of Neonatal Screening, 8(2), 27. https://doi.org/10.3390/ijns8020027







