Targeted Secondary Screening for Congenital Hypothyroidism in High-Risk Neonates: A 9 Year Review in a Large California Health Care System
Abstract
1. Introduction
2. Materials and Methods
- The familiarity of pediatricians and neonatologists with the primary screen in California based solely on TSH.
- The high prevalence of hypothyroxinemia without CH for the low-birth-weight population in the NICU.
- The current practice of the 15 pediatric endocrinologists in the medical group that relies almost entirely on abnormal TSH and not thyroxine or thyroid imaging to make a diagnosis of presumed primary hypothyroidism.
- CH by the California primary screen
- CH by the secondary screen per the clinical guidelines
- Infants started on levothyroxine who were screened or diagnosed due to incorrect or unanticipated use of the secondary screening guidelines
- Infants diagnosed with CH (often central) due to clinical suspicion (not by primary or secondary screens).
3. Results
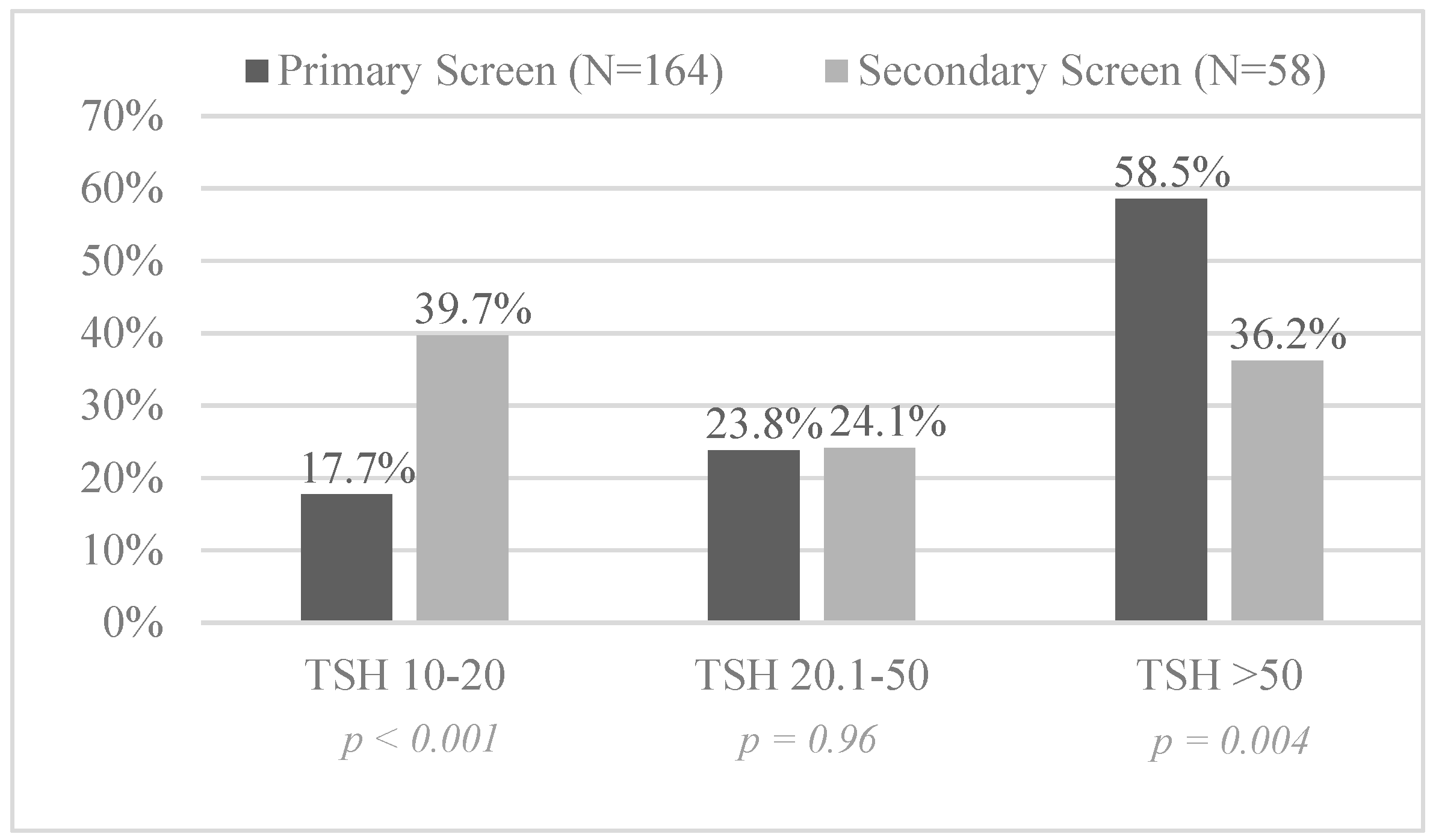
3.1. Secondary Screening Performance
3.2. Screening Results for Congenital Hypothyroidism
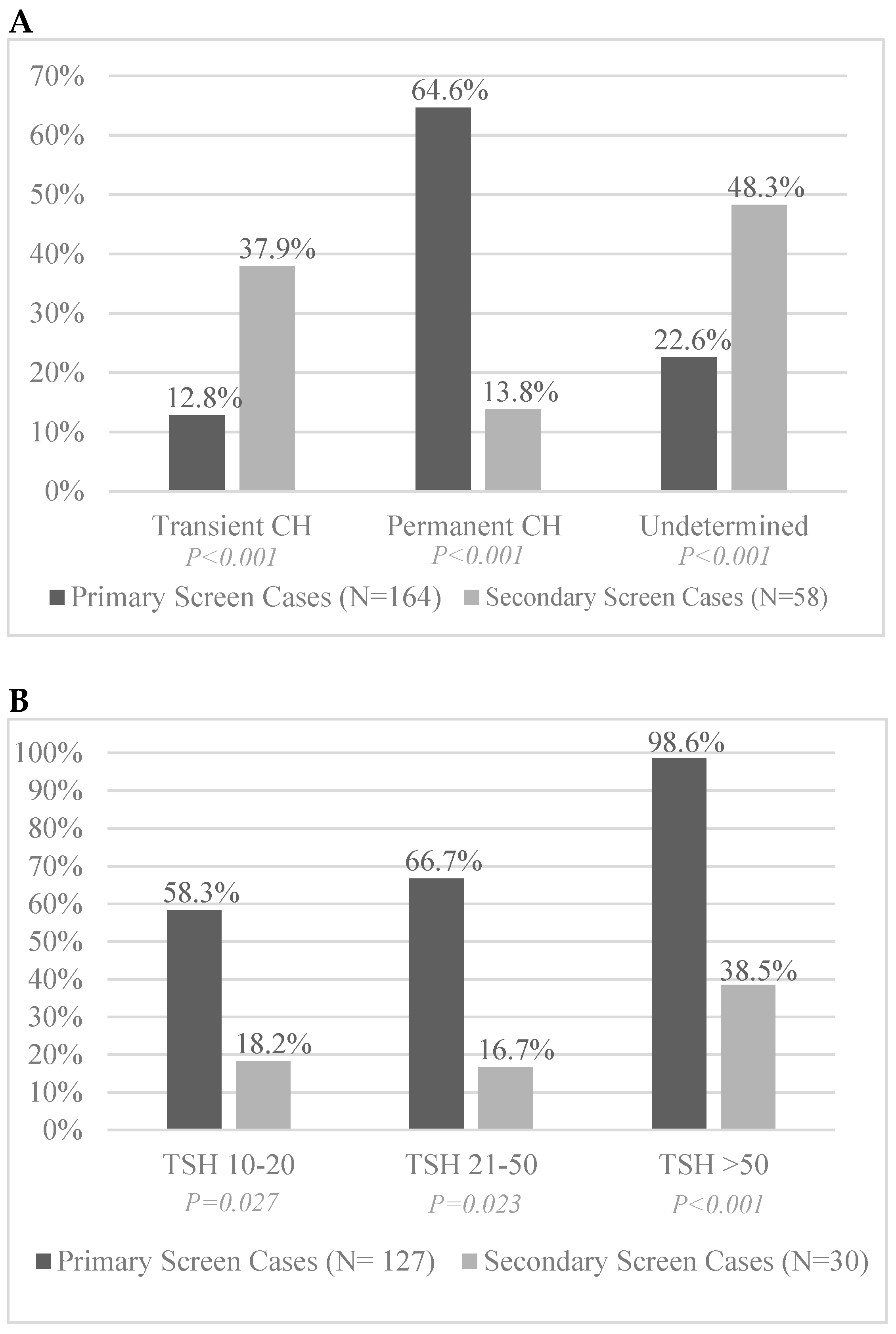
3.3. Permanence and Transience of Identified CH Infants
3.4. Levothyroxine Treatment Outside of the Screening Protocol
3.5. CH with Down Syndrome in the Screened Population
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | Congenital Hypothyroidism |
| CHD | Hospitalized congenital heart disease |
| DS | Down syndrome |
| NICU | Neonatal Intensive Care Unit |
| SSM | Same-sex multiple |
| TSH | Thyrotropin |
| VLBW | Very-low-birthweight |
References
- LaFranchi, S.H. Newborn screening strategies for congenital hypothyroidism: An update. J. Inherit. Metab. Dis. 2010, 33, S225–S233. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Rose, S.R.; Section on Endocrinology and Committee on Genetics; American Thyroid Association; Brown, R.S.; Public Health Committee; Lawson Wilkins Pediatric Endocrine Society. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006, 117, 2290–2303. [Google Scholar]
- Léger, J.; Olivieri, A.; Donaldson, M.; Torresani, T.; Krude, H.; van Vliet, G.; Polak, M.; Butler, G.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; The Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2014, 99, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.J.; Hermos, R.J.; Larson, C.A.; Prigozhin, A.B.; Rojas, D.A.; Mitchell, M.L. Atypical hypothyroidism and the very low birthweight infant. Thyroid 2000, 10, 693–695. [Google Scholar] [CrossRef]
- Larson, C.; Hermos, R.; Delaney, A.; Daley, D.; Mitchell, M. Risk factors associated with delayed thyrotropin elevations in congenital hypothyroidism. J. Pediatr. 2003, 143, 587–591. [Google Scholar] [CrossRef]
- Tylek-Lemanska, D.; Kumorowicz-Kopiec, M.; Starzyk, J. Screening for congenital hypothyroidism: The value of retesting after four weeks in neonates with low and very low birth weight. J. Med. Screen. 2005, 12, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Hinton, C.F.; Harris, K.B.; Borgfeld, L.; Drummond-Borg, M.; Eaton, R.; Lorey, F.; Therrell, B.L.; Wallace, J.; Pass, K.A. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: Data from the United States, California, Massachusetts, New York, and Texas. Pediatrics 2010, 125, S37–S47. [Google Scholar] [CrossRef]
- Silva, S.; Chagas, A.; Goulart, E.; Marçal, L.; Gomes, M.; Alves, V. Screening for congenital hypothyroidism in extreme premature and/or very low birth weight newborns: The importance of a specific protocol. J. Pediatr. Endocrinol. Metab. 2010, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bijarnia, S.; Wilcken, B.; Wiley, V.C. Newborn screening for congenital hypothyroidism in very-low-birth-weight babies: The need for a second test. J. Inherit. Metab. Dis. 2011, 34, 827–833. [Google Scholar] [CrossRef]
- Woo, H.C.; Lizarda, A.; Tucker, R.; Mitchell, M.L.; Vohr, B.; Oh, W.; Phornphutkul, C. Congenital hypothyroidism with a delayed thyroid-stimulating hormone (TSH) elevation in very premature infants: Incidence and growth and developmental outcomes. J. Pediatr. 2011, 158, 538–542. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.W.; Jeon, G.W.; Sin, J.B. Thyroid dysfunction in very low birth weight preterm infants. Korean J. Pediatr. 2015, 58, 224–229. [Google Scholar] [CrossRef][Green Version]
- Hashemipour, M.; Hovsepian, S.; Ansari, A.; Keikha, M.; Khalighinejad, P.; Niknamet, N. Screening of congenital hypothyroidism in preterm, low birth weight and very low birth weight neonates: A systematic review. Pediatr. Neonatol. 2018, 59, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Frigerio, M.; De Filippis, T.; Valaperta, R.; Capelli, P.; Costa, E.; Fugazzola, L.; Marelli, F.; Porazzi, P.; Arcidiacono, C.; et al. Increased Risk for Non-Autoimmune Hypothyroidism in Young Patients with Congenital Heart Defects. J. Clin. Endocrinol. Metab. 2011, 96, E1115–E1119. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, A.; Stazi, M.A.; Mastroiacovo, P.; Fazzini, C.; Medda, E.; Spagnolo, A. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: Data from the Italian Registry for Congenital Hypothyroidism, 1991–1998. J. Clin. Endocrinol. Metab. 2002, 87, 557–562. [Google Scholar]
- Tuysuz, B.; Beker, D.B. Thyroid dysfunction in children with Down syndrome. Acta Paediatr. 2001, 90, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Purdy, I.B.; Singh, N.; Brown, W.L.; Vangala, S.; Devaskar, U.P. Revisiting early hypothyroidism screening in infants with Down syndrome. J. Perinatol. 2014, 34, 936–940. [Google Scholar] [CrossRef]
- van Trotsenburg, A.S.P.; Vulsma, T.; van Santen, H.M.; Cheung, W.; de Vijlder, J.J.M. Lower Neonatal Screening Thyroxine Concentrations in Down Syndrome Newborns. J. Clin. Endocrinol. Metab. 2003, 88, 1512–1515. [Google Scholar] [CrossRef]
- Perry RHeinrichs, C.; Bourdoux, P.; Khoury, K.; Szöts, F.; Dussault, J.H.; Vassart, G.; van Vliet, G. Discordance of monozygotic twins for thyroid dysgenesis: Implications for screening and for molecular pathophysiology. J. Clin. Endocrinol. Metab. 2002, 87, 4072–4077. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, Y.-L.; Feng, Y.; Tang, F.; Jia, X.-F.; Chen, Q.-Y.; Tang, C.-F.; Liu, S.-C.; Li, B.; Zheng, R.-D.; et al. Same-sex twins have a high incidence of congenital hypothyroidism and a high probability to be missed at newborn screening. Clin. Chim. Acta 2020, 502, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.E.; Krainz, P.L.; Skeels, M.R.; Miyahira, R.S.; Sesser, D.E.; LaFranchi, S.H. Detection of congenital hypopituitary hypothyroidism: Ten-year experience in the Northwest Regional Screening Program. J. Pediatr. 1986, 109, 959–964. [Google Scholar] [CrossRef]
- Braslavsky, D.; Méndez, M.V.; Prieto, L.; Keselman, A.; Enacan, R.; Gruñeiro-Papendieck, L.; Jullien, N.; Savenau, A.; Reynaud, R.; Brue, T.; et al. Pilot Neonatal Screening Program for Central Congenital Hypothyroidism: Evidence of Significant Detection. Horm. Res. Paediatr. 2017, 88, 274–280. [Google Scholar] [CrossRef]
- Filippi, L.; Pezzati, M.; Cecchi, A.; Poggi, C. Dopamine infusion: A possible cause of undiagnosed congenital hypothyroidism in preterm infants. Pediatr. Crit. Care Med. 2006, 7, 249–251. [Google Scholar] [CrossRef] [PubMed]
- LaFranchi, S.H. Congenital hypothyroidism: Delayed detection after birth and monitoring treatment in the first year of life. J. Pediatr. 2011, 158, 525–527. [Google Scholar] [CrossRef]
- Ahmet, A.; Lawson, M.L.; Babyn, P.; Tricco, A.C. Hypothyroidism in neonates post-iodinated contrast media: A systematic review. Acta Paediatrica 2009, 98, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Thaker, V.V.; Leung, A.M.; Braverman, L.E.; Brown, R.S.; Levine, B. Iodine induced.hypothyroidism in full-term infants with congenital heart disease: More common than currently appreciated? J. Clin. Endocrinol. Metab. 2014, 99, 3521–3526. [Google Scholar] [CrossRef]
- Hemmati, F.; Pishva, N. Evaluation of thyroid status of infants in the intensive care setting. Singap. Med. J. 2009, 50, 875–878. [Google Scholar]
- Korada, S.M.; Pearce, M.; Platt, M.W.; Avis, E.; Turner, S.; Wastell, H.; Cheetham, T. Difficulties in selecting an appropriate neonatal thyroid stimulating hormone (TSH) screening threshold. Arch. Dis. Child. 2010, 95, 169–173. [Google Scholar] [CrossRef]
- Langham, S.; Hindmarsh, P.; Krywawych, S.; Peters, C. Screening for congenital hypothyroidism: Comparison of borderline screening cut-off points and the effect on the number of children treated with levothyroxine. Eur. Thyroid. J. 2013, 2, 180–186. [Google Scholar] [CrossRef]
- Kaluarachchi, D.C.; Colaizy, T.T.; Pesce, L.M.; Tansey, M.; Klein, J.M. Congenital hypothyroidism with delayed thyroid-stimulating hormone elevation in premature infants born at less than.30 weeks gestation. J. Perinatol. 2017, 37, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.K.; Hinton, C.F.; Held, P.K.; Jones, E.; Hannon, W.H.; Ojodu, J. Single newborn screen or routine second screening for primary congenital hypothyroidism. Mol. Genet. Metab. 2015, 116, 125–132. [Google Scholar] [CrossRef][Green Version]
- LaFranchi, S.H. Screening preterm infants for congenital hypothyroidism: Better the second time around. J. Pediatr. 2014, 164, 1259–1261. [Google Scholar] [CrossRef]
- United States Census Data (Estimates Last Updated July 1, 2019 Prior to Submission). Available online: www.census.gov/quickfacts/fact/table/CA (accessed on 1 August 2020).
- Feuchtbaum, L.; Carter, J.; Dowray, S.; Currier, R.; Lorey, F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet. Med. 2012, 14, 937–945. [Google Scholar] [CrossRef]
- Kanike, N.; Davis, A.; Shekhawat, P.S. Transient hypothyroidism in the newborn: To treat or not to treat. Transl. Pediatr. 2017, 6, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.A.; Rodd, C.; Dussault, J.H.; Van Vliet, G. Very low birth weight newborns do not need repeat screening for congenital hypothyroidism. J. Pediatr. 2002, 140, 311–314. [Google Scholar] [CrossRef]
- Olivieri, A.; Medda, E.; de Angelis, S.; Valensise, H.; De Felice, M.; Fazzini, C.; Cascino, I.; Cordeddu, V.; Sorcini, M.; Stazi, M.A. High Risk of Congenital Hypothyroidism in Multiple Pregnancies. J. Clin. Endocrinol. Metab. 2007, 92, 3141–3147. [Google Scholar] [CrossRef][Green Version]
- Cuestas, E.; Gaido, M.I.; Capra, R.H. Transient neonatal hyperthyrotropinemia is a risk factor for developing persistent hyperthyrotropinemia in childhood with repercussion on developmental status. Eur. J. Endocrinol. 2015, 172, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Fort, P.; Lifshitz, F.; Bellisario, R.; Davis, J.; Lanes, R.; Pugliese, M.; Richman, R.; Post, E.; David, R. Abnormalities of thyroid function in infants with Down syndrome. J. Pediatr. 1984, 104, 545–549. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Health Supervision for Children with Down Syndrome. Pediatrics 2011, 128, 393–406. [Google Scholar] [CrossRef] [PubMed]
- van Trotsenburg, A.S.; Vulsma, T.; van Rozenburg-Marres, S.L.; van Baar, A.L.; Ridder, J.C.; Heymans, H.S.; Tijssen, J.G.; de Vijlder, J.J. The effect of thyroxine treatment started in the neonatal period on development and growth of two-year-old Down syndrome children: A randomized clinical trial. J. Clin. Endocrinol. Metab. 2005, 90, 3304–3311. [Google Scholar] [CrossRef] [PubMed]
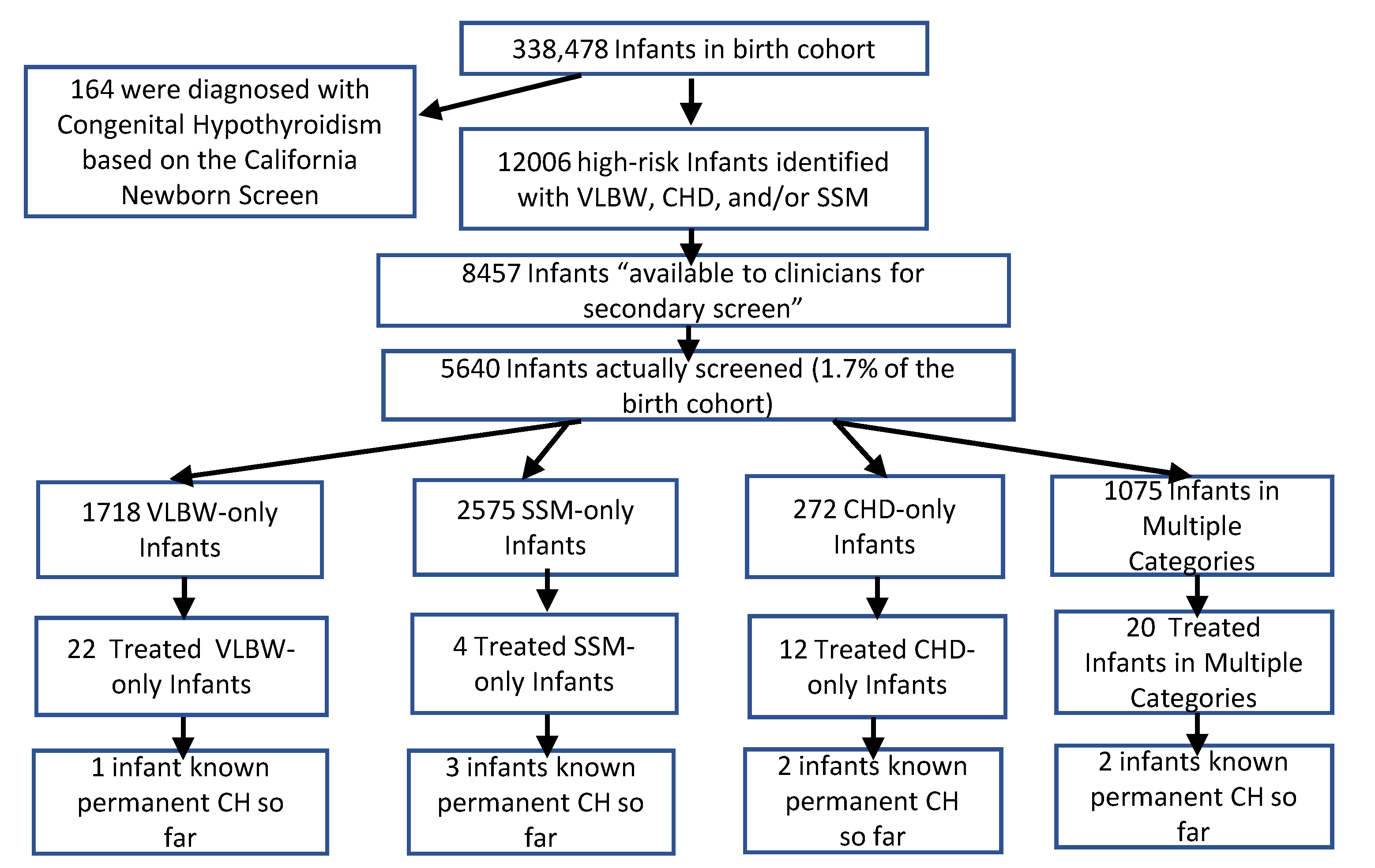
| Description | Total Cohort (%) | High Risk Cohort (%) |
|---|---|---|
| Births | 338,478 | 12,006 * |
| Female | 165,186 (48.8%) | 5827 (48.5%) |
| Race/Ethnicity | ||
| Non-Hispanic White | 88,683 (26.2%) | 3044 (25.3%) |
| Hispanic | 142,646 (42.1%) | 5593 (46.6%) |
| Non-Hispanic Black | 23,752 (7.0%) | 1328 (11.4%) |
| Asian | 38,736 (11.4%) | 1566 (13.0%) |
| Others | 44,661 (13.2%) | 475 (4.0%) |
| Gestational Age | ||
| Gestational Age < 28 Weeks | 2104 (0.6%) | 2116 (17.6) |
| Gestational Age 28–31 Weeks | 2849 (0.8%) | 2090 (17.4%) |
| Gestational Age 32–36 Weeks | 26,945 (8.0%) | 4684 (39.0%) |
| Gestational Age 37+ Weeks | 306,492 (90.6%) | 3116 (25.9%) |
| Maternal Age | ||
| Maternal Age ≤ 21 years | 27,105 (8.0%) | 697 (5.8%) |
| Maternal Age 22–34 years | 235,097 (69.5%) | 7578 (63.1%) |
| Maternal Age ≥ 35 years | 76,276 (22.5%) | 3731 (31.1%) |
| NICU ** Admission | 34,407 (10.2%) | 7273 (60.6%) |
| High Risk Category *** | ||
| VLBW | 4541 (1.3%) | 4541 (37.8%) |
| SSM | 7520 (2.2%) | 7520 (62.6%) |
| CHD | 1496 (0.4%) | 1496 (12.5%) |
| Down Syndrome (DS) | 704 (0.2%) | 183 (1.5%) |
| Categories of High-Risk Infants | Secondary Screening Criteria Met (N) | Available for Secondary Screening (N) | Number of Infants Screened (N) | Available Infants Screened (%) |
|---|---|---|---|---|
| All High-Risk Infants * | 12,006 | 8457 | 5640 | 66.7% |
| Very Low Birth Weight (VLBW) ** | 4541 | 2844 | 2698 | 94.9% |
| Birth weight: 1001–1500 g | 2355 | 1767 | 1643 | 93.0% |
| Birth weight: ≤1000 g | 2186 | 1077 | 1055 | 98.0% |
| Congenital Heart Disease in NICU (CHD) | 1496 | 1401 | 987 | 70.4% |
| Same-Sex Multiples (SSM) *** | 7520 | 5513 | 3208 | 58.2% |
| Subcategories of High-Risk Infants | ||||
| VLBW + CHD | 651 | 524 | 514 | 98.1% |
| VLBW + SSM | 816 | 588 | 560 | 95.2% |
| CHD + SSM | 195 | 195 | 189 | 96.9% |
| VLBW + CHD + SSM | 111 | 95 | 94 | 98.9% |
| VLBW only | 3185 | 1827 | 1718 | 94.0% |
| CHD only | 761 | 666 | 272 | 40.8% |
| SSM only | 6620 | 4847 | 2575 | 53.1% |
| (N = Total Primary Screened; N = Total Secondary Screened) | CH by Primary Screen | CH by Secondary Screen | CH Prevalence by Secondary Screening vs. Primary Screening in Total Birth Cohort (1:2064) | CH Prevalence by Secondary Screening vs. Primary Screening in Total Birth Cohort (1:2064) |
|---|---|---|---|---|
| N (Prevalence) | N (Prevalence) | p | Relative Risk (95%CI) | |
| Total birth cohort (338,478; 5640) | 164 (1:2064) | 58 (1:97) | <0.001 | 21.3 (16.0, 28.2) |
| Any high-risk category (12,006; 5640) | 5 (1:2401) | 58 (1:97) | <0.001 | 21.3 (16.0, 28.2) |
| High-risk without SSM-only (5386; 3065) * | 3 (1:1795) | 54 (1:57) | <0.001 | 36.2 (27.4, 48.1) |
| VLBW (≤1500 g) (4541; 2698) ** | 1 (1:4541) | 41 (1:66) | <0.001 | 31.3 (22.3, 44.1) |
| 1001–1500 g (2355; 1643) | 0 (n/a) | 11 (1:149) | <0.001 | 13.9 (7.5, 25.4) |
| ≤1000 g (2186; 1055) | 1 (1:2186) | 30 (1:35) | <0.001 | 59.0 (40.0, 86.2) |
| VLBW only (3185; 1718) | 1 (1:3185) | 22 (1:78) | <0.001 | 26.5 (17.0, 41.1) |
| VLBW + CHD (651; 514) | 0 (n/a) | 8 (1:64) | <0.001 | 32.3 (15.9, 65.0) |
| VLBW + SSM (816; 560) | 0 (n/a) | 12 (1:47) | <0.001 | 43.9 (24.8, 79.0) |
| VLBW + CHD + SSM (111; 94) | 0 (n/a) | 1 (1:94) | <0.001 | 22.0 (3.1, 155.2) |
| CHD *** (1496; 987) | 2 (1:748) | 21 (1:47) | <0.001 | 43.9 (28.0, 68.9) |
| CHD only (761; 272) | 2 (1:380) | 12 (1:23) | <0.001 | 89.7 (51.3, 161.6) |
| CHD + SSM (195; 189) | 0 (n/a) | 2 (1:95) | <0.001 | 21.7 (5.5, 87.4) |
| SSM (7520; 3208) | 2 (1:3760) | 17 (1:189) | <0.001 | 10.9 (6.6, 18.0) |
| SSM only (6620; 2575) | 2 (1:3310) | 4 (1:644) | 0.02 | 3.2 (1.2, 8.6) |
| Risk Factor(s) | CH Cases/Total Secondary Screen | Difference in Prevalence | p * |
|---|---|---|---|
| Ratio; %; Prevalence | %; (95% CI) | ||
| VLBW only | 22/1718 = 1.3% = 1:78 | Reference | Reference |
| VLBW/CHD/SSM | 1/94 = 1.1% = 1:94 | −0.2% (−2.5%, 2.1%) | 0.87 |
| VLBW/CHD | 8/514 = 1.6% = 1:64 | 0.3% (−0.9%, 1.5%) | 0.61 |
| VLBW/SSM | 12/560 = 2.1% = 1:47 | 0.8% (−0.4%, 2.0%) | 0.18 |
| CHD only | 12/272 = 4.4% = 1:23 | Reference | Reference |
| CHD/VLBW/SSM | 1/94 = 1.1% = 1:94 | −3.3% (−7.6%, 1.0%) | 0.14 |
| CHD/VLBW | 8/514 = 1.6% = 1:64 | −2.8% (−5.1%, −0.5%) | 0.02 |
| CHD/SSM | 2/189 = 1.1% = 1:95 | −3.3% (−6.5%, −0.1%) | 0.04 |
| SSM only | 4/2575 = 0.2% = 1:644 | Reference | Reference |
| SSM/VLBW/CHD | 1/94 = 1.1% = 1:94 | 0.9% (0.09%, 1.9%) | 0.07 |
| SSM/CHD | 2/189 = 1.1% = 1:95 | 0.9% (0.2%, 1.7%) | 0.02 |
| SSM/VLBW | 12/560 = 2.1% = 1:47 | 1.9% (1.2%, 2.6%) | <0.001 |
| Reasons for Treatment | Cases (N) | Known Permanent Cases (N) * |
|---|---|---|
| Secondary screening—unintended use | ||
| Screening out of guidelines (Down syndrome without risk category) | 7 | 3 |
| Screening out of guidelines (other reasons) | 3 | 0 |
| Diagnosis out of guidelines (TSH 6–10 mIU/L) | 11 | 0 |
| Diagnosis out of guidelines (hypothyroxinemia, normal TSH) | 8 | 0 |
| Diagnosis out of guidelines- other (unaffected twin) | 1 | 0 |
| Primary screening follow-up issues | ||
| Treatment for TSH >50 mIU/L but confirmatory test then ruled out CH | 4 | 0 |
| Maternal Graves Disease follow-up | 2 | 0 |
| Symptomatic screening | ||
| Hypopituitarism suspected | 11 | 7 |
| Other (neck surgery, hypotonia, hemangioma, genetic syndrome) | 4 | 0 |
| Total | 51 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortez, A.B.; Lin, B.; May, J.A. Targeted Secondary Screening for Congenital Hypothyroidism in High-Risk Neonates: A 9 Year Review in a Large California Health Care System. Int. J. Neonatal Screen. 2021, 7, 81. https://doi.org/10.3390/ijns7040081
Cortez AB, Lin B, May JA. Targeted Secondary Screening for Congenital Hypothyroidism in High-Risk Neonates: A 9 Year Review in a Large California Health Care System. International Journal of Neonatal Screening. 2021; 7(4):81. https://doi.org/10.3390/ijns7040081
Chicago/Turabian StyleCortez, Alan B., Bryan Lin, and Joshua A. May. 2021. "Targeted Secondary Screening for Congenital Hypothyroidism in High-Risk Neonates: A 9 Year Review in a Large California Health Care System" International Journal of Neonatal Screening 7, no. 4: 81. https://doi.org/10.3390/ijns7040081
APA StyleCortez, A. B., Lin, B., & May, J. A. (2021). Targeted Secondary Screening for Congenital Hypothyroidism in High-Risk Neonates: A 9 Year Review in a Large California Health Care System. International Journal of Neonatal Screening, 7(4), 81. https://doi.org/10.3390/ijns7040081






