Newborn Screening for 5q Spinal Muscular Atrophy: Comparisons between Real-Time PCR Methodologies and Cost Estimations for Future Implementation Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and DNA Samples
2.2. Real-Time PCR
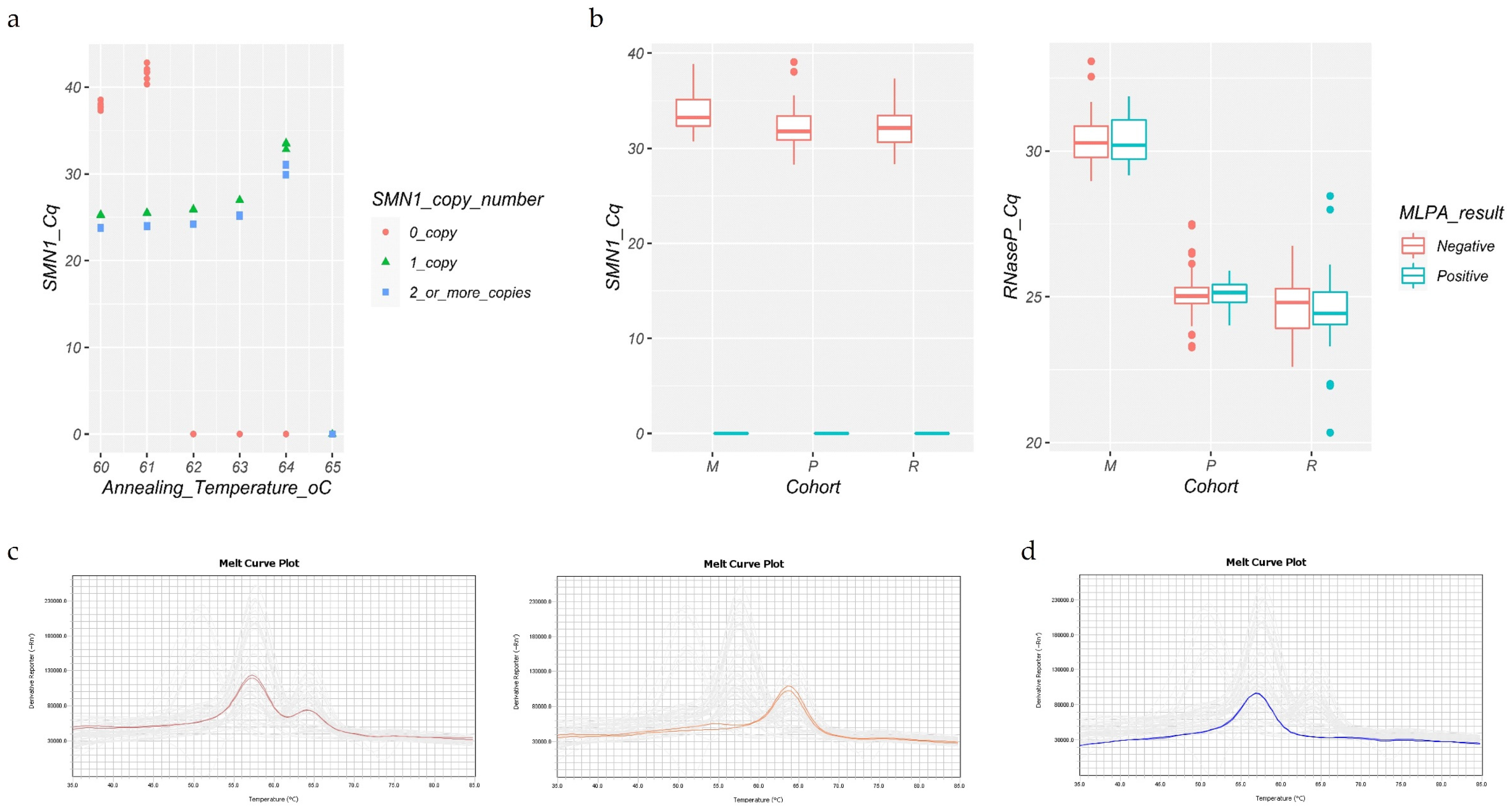
2.2.1. Presence and Absence (P/A) Assay—In-House SMN1 Standardized Test
2.2.2. Melt Curve Assay—Commercial Simplex SMN1 Test
2.3. Multiplex Ligation-Dependent Probe Amplification (MLPA)—Standard Test
2.4. Statistical Analysis
3. Results
3.1. Presence and Absence (P/A) Protocol
3.2. Melt Curve Protocol
3.3. Cost Estimation and Test Workflow
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arkblad, E.; Tulinius, M.; Kroksmark, A.K.; Henricsson, M.; Darin, N. A population-based study of genotypic and phenotypic variability in children with spinal muscular atrophy. Acta Paediatr. Int. J. Paediatr. 2009, 98, 865–872. [Google Scholar] [CrossRef]
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012, 20, 27–32. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy-A literature review. Orphanet J. Rare Dis. 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jędrzejowska, M. Advances in Newborn Screening and Presymptomatic Diagnosis of Spinal Muscular Atrophy. Degener. Neurol. Neuromuscul. Dis. 2020, 10, 39–47. [Google Scholar] [CrossRef]
- Lunn, M.R.; Wang, C.H. Spinal muscular atrophy. Lancet 2008, 371, 2120–2133. [Google Scholar] [CrossRef]
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 2000, 15, 228–237. [Google Scholar] [CrossRef]
- Prior, T.W. Spinal muscular atrophy: A time for screening. Curr. Opin. Pediatr. 2010, 22, 696–702. [Google Scholar] [CrossRef]
- Maretina, M.A.; Zheleznyakova, G.Y.; Lanko, K.M.; Egorova, A.A.; Baranov, V.S.; Kiselev, A.V. Molecular Factors Involved in Spinal Muscular Atrophy Pathways as Possible Disease-modifying Candidates. Curr. Genom. 2018, 19, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, S.; Servais, L. New treatments in spinal muscular atrophy: An overview of currently available data. Expert Opin. Pharmacother. 2020, 21, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Neil, E.E.; Bisaccia, E.K. Nusinersen: A novel antisense oligonucleotide for the treatment of spinal muscular atrophy. J. Pediatr. Pharmacol. Ther. 2019, 24, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.; Weetall, M.; Heinig, K.; Bucheli, F.; Schoenlein, K.; Alsenz, J.; Bassett, S.; Ullah, M.; Senn, C.; Ratni, H.; et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Claborn, M.K.; Gildon, B.L.; Kessler, T.L.; Walker, C. Onasemnogene Abeparvovec-xioi: Gene Therapy for Spinal Muscular Atrophy. Ann. Pharmacother. 2020, 54, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, K.J.; Prior, T.W.; Scott, C.B.; McNaught, T.P.; Wride, M.C.; Reyna, S.P.; Bromberg, M.B. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann. Neurol. 2005, 57, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govoni, A.; Gagliardi, D.; Comi, G.P.; Corti, S. Time Is Motor Neuron: Therapeutic Window and Its Correlation with Pathogenetic Mechanisms in Spinal Muscular Atrophy. Mol. Neurobiol. 2018, 55, 6307–6318. [Google Scholar] [CrossRef]
- Chien, Y.H.; Chiang, S.C.; Weng, W.C.; Lee, N.C.; Lin, C.J.; Hsieh, W.S.; Lee, W.T.; Jong, Y.J.; Ko, T.M.; Hwu, W.L. Presymptomatic Diagnosis of Spinal Muscular Atrophy Through Newborn Screening. J. Pediatr. 2017, 190, 124–129.e1. [Google Scholar] [CrossRef]
- Boemer, F.; Caberg, J.H.; Dideberg, V.; Dardenne, D.; Bours, V.; Hiligsmann, M.; Dangouloff, T.; Servais, L. Newborn screening for SMA in Southern Belgium. Neuromuscul. Disord. 2019, 29, 343–349. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, C.H.; Yin, X.; Zhu, L.; Yang, J.; Shen, Y.; Yang, C.; Chen, X.; Hu, H.; Ma, Q.; et al. Newborn Screening for Spinal Muscular Atrophy in China Using DNA Mass Spectrometry. Front. Genet. 2019, 10, 1255. [Google Scholar] [CrossRef]
- Shinohara, M.; Niba, E.T.E.; Wijaya, Y.O.S.; Takayama, I.; Mitsuishi, C.; Kumasaka, S.; Kondo, Y.; Takatera, A.; Hokuto, I.; Morioka, I.; et al. A novel system for spinal muscular atrophy screening in newborns: Japanese pilot study. Int. J. Neonatal Screen. 2019, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Vill, K.; Kölbel, H.; Schwartz, O.; Blaschek, A.; Olgemöller, B.; Harms, E.; Burggraf, S.; Röschinger, W.; Durner, J.; Gläser, D.; et al. One year of newborn screening for SMA–Results of a German pilot project. J. Neuromuscul. Dis. 2019, 6, 503–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czibere, L.; Burggraf, S.; Fleige, T.; Glück, B.; Keitel, L.M.; Landt, O.; Durner, J.; Röschinger, W.; Hohenfellner, K.; Wirth, B.; et al. High-throughput genetic newborn screening for spinal muscular atrophy by rapid nucleic acid extraction from dried blood spots and 384-well qPCR. Eur. J. Hum. Genet. 2020, 28, 23–30. [Google Scholar] [CrossRef]
- Kay, D.M.; Stevens, C.F.; Parker, A.; Saavedra-Matiz, C.A.; Sack, V.; Chung, W.K.; Chiriboga, C.A.; Engelstad, K.; Laureta, E.; Farooq, O.; et al. Implementation of population-based newborn screening reveals low incidence of spinal muscular atrophy. Genet. Med. 2020, 22, 1296–1302. [Google Scholar] [CrossRef]
- Strom, C.M.; Anderson, B.; Peng, M.; Patel, U.; Braastad, C.D.; Sun, W. 1000 sample comparison of MLPA and RT-PCR for carrier detection and diagnostic testing for Spinal Muscular Atrophy Type 1. Open J. Genet. 2013, 3, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Zhang, C.; Teng, Y.; Zeng, S.; Chen, S.; Liang, D.; Li, Z.; Wu, L. Detection of Spinal Muscular Atrophy Using a Duplexed Real-Time PCR Approach With Locked Nucleic Acid-Modified Primers. Ann. Lab. Med. 2021, 41, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pyatt, R.E.; Prior, T.W. A feasibility study for the newborn screening of spinal muscular atrophy. Genet. Med. 2006, 8, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anhuf, D.; Eggermann, T.; Rudnik-Schöneborn, S.; Zerres, K. Determination of SMN1 and SMN2 copy number using TaqManTM technology. Hum. Mutat. 2003, 22, 74–78. [Google Scholar] [CrossRef]
- Passon, N.; Pozzo, F.; Molinis, C.; Bregant, E.; Gellera, C.; Damante, G.; Lonigro, R.I. A Simple Multiplex Real-Time PCR Methodology for the SMN1 Gene Copy Number Quantification. Genet. Test. Mol. Biomark. 2009, 13, 37–42. [Google Scholar] [CrossRef] [Green Version]
- McMillan, H.J.; Kernohan, K.D.; Yeh, E.; Amburgey, K.; Boyd, J.; Campbell, C.; Dowling, J.J.; Gonorazky, H.; Marcadier, J.; Tarnopolsky, M.A.; et al. Newborn Screening for Spinal Muscular Atrophy: Ontario Testing and Follow-up Recommendations. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2020, 48, 504–511. [Google Scholar] [CrossRef]
- Wirth, B.; Herz, M.; Wetter, A.; Moskau, S.; Hahnen, E.; Rudnik-Schöneborn, S.; Wienker, T.; Zerres, K. Quantitative analysis of survival motor neuron copies: Identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype- phenotype correlation, and implications for genetic counseling. Am. J. Hum. Genet. 1999, 64, 1340–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior, T.W.; Snyder, P.J.; Rink, B.D.; Pearl, D.K.; Pyatt, R.E.; Mihal, D.C.; Conlan, T.; Schmalz, B.; Montgomery, L.; Ziegler, K.; et al. Newborn and carrier screening for spinal muscular atrophy. Am. J. Med. Genet. Part A 2010, 152, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Strunk, A.; Abbes, A.; Stuitje, A.R.; Hettinga, C.; Sepers, E.M.; Snetselaar, R.; Schouten, J.; Asselman, F.L.; Cuppen, I.; Lemmink, H.; et al. Validation of a fast, robust, inexpensive, two-tiered neonatal screening test algorithm on dried blood spots for spinal muscular atrophy. Int. J. Neonatal Screen. 2019, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Kraszewski, J.N.; Kay, D.M.; Stevens, C.F.; Koval, C.; Haser, B.; Ortiz, V.; Albertorio, A.; Cohen, L.L.; Jain, R.; Andrew, S.P.; et al. Pilot study of population-based newborn screening for spinal muscular atrophy in New York state. Genet. Med. 2018, 20, 608–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 3 July 2021).
- Gutierrez-Mateo, C.; Timonen, A.; Vaahtera, K.; Jaakkola, M.; Hougaard, D.M.; Bybjerg-Grauholm, J.; Baekvad-Hansen, M.; Adamsen, D.; Filippov, G.; Dallaire, S.; et al. Development of a multiplex real-time PCR assay for the newborn screening of SCID, SMA, and XLA. Int. J. Neonatal Screen. 2019, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Cavdarli, B.; Ozturk, F.N.; Guntekin Ergun, S.; Ergun, M.A.; Dogan, O.; Percin, E.F. Intelligent Ratio: A New Method for Carrier and Newborn Screening in Spinal Muscular Atrophy. Genet. Test. Mol. Biomark. 2020, 24, 569–577. [Google Scholar] [CrossRef]
- Phan, H.C.; Taylor, J.L.; Hannon, H.; Howell, R. Newborn screening for spinal muscular atrophy: Anticipating an imminent need. Semin. Perinatol. 2015, 39, 217–229. [Google Scholar] [CrossRef]
- Vidal-Folch, N.; Gavrilov, D.; Raymond, K.; Rinaldo, P.; Tortorelli, S.; Matern, D.; Oglesbee, D. Multiplex droplet digital PCR method applicable to newborn screening, carrier status, and assessment of spinal muscular atrophy. Clin. Chem. 2018, 64, 1753–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellar-Guenther, Y.; McKasson, S.; Hale, K.; Singh, S.; Sontag, M.K.; Ojodu, J. Implementing statewide newborn screening for new disorders: U.S. Program experiences. Int. J. Neonatal Screen. 2020, 6, 35. [Google Scholar] [CrossRef]
- Glascock, J.; Sampson, J.; Connolly, A.M.; Darras, B.T.; Day, J.W.; Finkel, R.; Howell, R.R.; Klinger, K.W.; Kuntz, N.; Prior, T.; et al. Revised Recommendations for the Treatment of Infants Diagnosed with Spinal Muscular Atrophy Via Newborn Screening Who Have 4 Copies of SMN2. J. Neuromuscul. Dis. 2020, 7, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Müller-Felber, W.; Vill, K.; Schwartz, O.; Gläser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; Durner, J.; et al. Infants Diagnosed with Spinal Muscular Atrophy and 4 SMN2 Copies through Newborn Screening-Opportunity or Burden? J. Neuromuscul. Dis. 2020, 7, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Kemper, A.R.; Lam, K.; Comeau, A.M.; Kwon, J.; Green, N.S.; Ojodu, J.; Grosse, S.; Prosser, L.A.; Jones, E.; Tanksley, S.; et al. Evidence-Based Review of Newborn Screening for Spinal Muscular Atrophy (SMA): Final Report (v5.2); United States Secretary of Health and Human Services Advisory Committee on Heritable Disorders in Newborns and Children, Maternal Child Health Bureau: Rockville, MA, USA, 2018. [Google Scholar]
- Dangouloff, T.; Servais, L. Clinical evidence supporting early treatment of patients with spinal muscular atrophy: Current perspectives. Ther. Clin. Risk Manag. 2019, 15, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, W.D.; Kassar, D.; Kissel, J.T. Spinal muscular atrophy: Diagnosis and management in a new therapeutic era. Muscle Nerve 2015, 51, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal muscular atrophy: Mutations, testing, and clinical relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef] [PubMed]

| Real-Time PCR Assay Cost Per Reaction | ||||
|---|---|---|---|---|
| Simplex Test | Multiplex Test | |||
| In-House Standardized | Commercial Tests | In-House Standardized | Commercial Tests | |
| Consumables | USD 1.68 | USD 4.42 | USD 2.04 | USD 5.99–12.76 |
| (R$ 9.64) | (R$ 25.35) | (R$ 11.68) | (R$ 34.40–73.23) | |
| Laboratory Status | ||
|---|---|---|
| Runs Genetic/Molecular Tests Other than Real-Time PCR | Does Not Run Any Genetic/ Molecular Test | |
| Without automation investment | USD 44,817.07 | USD 75,503.13 |
| (R$ 257,250.00) * | (R$ 433,387.98) | |
| Automation investment† | USD 391,749.97 | |
| (R$ 2,248,644.80) | ||
| Total estimative | USD 436,567.04 | USD 467,253.10 |
| (R$ 2,505,894.80) | (R$ 2,682,032.78) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanelli Tavares, V.L.; Monfardini, F.; Lourenço, N.C.V.; da Rocha, K.M.; Weinmann, K.; Pavanello, R.; Zatz, M. Newborn Screening for 5q Spinal Muscular Atrophy: Comparisons between Real-Time PCR Methodologies and Cost Estimations for Future Implementation Programs. Int. J. Neonatal Screen. 2021, 7, 53. https://doi.org/10.3390/ijns7030053
Romanelli Tavares VL, Monfardini F, Lourenço NCV, da Rocha KM, Weinmann K, Pavanello R, Zatz M. Newborn Screening for 5q Spinal Muscular Atrophy: Comparisons between Real-Time PCR Methodologies and Cost Estimations for Future Implementation Programs. International Journal of Neonatal Screening. 2021; 7(3):53. https://doi.org/10.3390/ijns7030053
Chicago/Turabian StyleRomanelli Tavares, Vanessa Luiza, Frederico Monfardini, Naila Cristina Vilaça Lourenço, Katia Maria da Rocha, Karina Weinmann, Rita Pavanello, and Mayana Zatz. 2021. "Newborn Screening for 5q Spinal Muscular Atrophy: Comparisons between Real-Time PCR Methodologies and Cost Estimations for Future Implementation Programs" International Journal of Neonatal Screening 7, no. 3: 53. https://doi.org/10.3390/ijns7030053
APA StyleRomanelli Tavares, V. L., Monfardini, F., Lourenço, N. C. V., da Rocha, K. M., Weinmann, K., Pavanello, R., & Zatz, M. (2021). Newborn Screening for 5q Spinal Muscular Atrophy: Comparisons between Real-Time PCR Methodologies and Cost Estimations for Future Implementation Programs. International Journal of Neonatal Screening, 7(3), 53. https://doi.org/10.3390/ijns7030053






