The Impact of Seasonal Changes on Thyroxine and Thyroid-Stimulating Hormone in Newborns
Abstract
1. Introduction
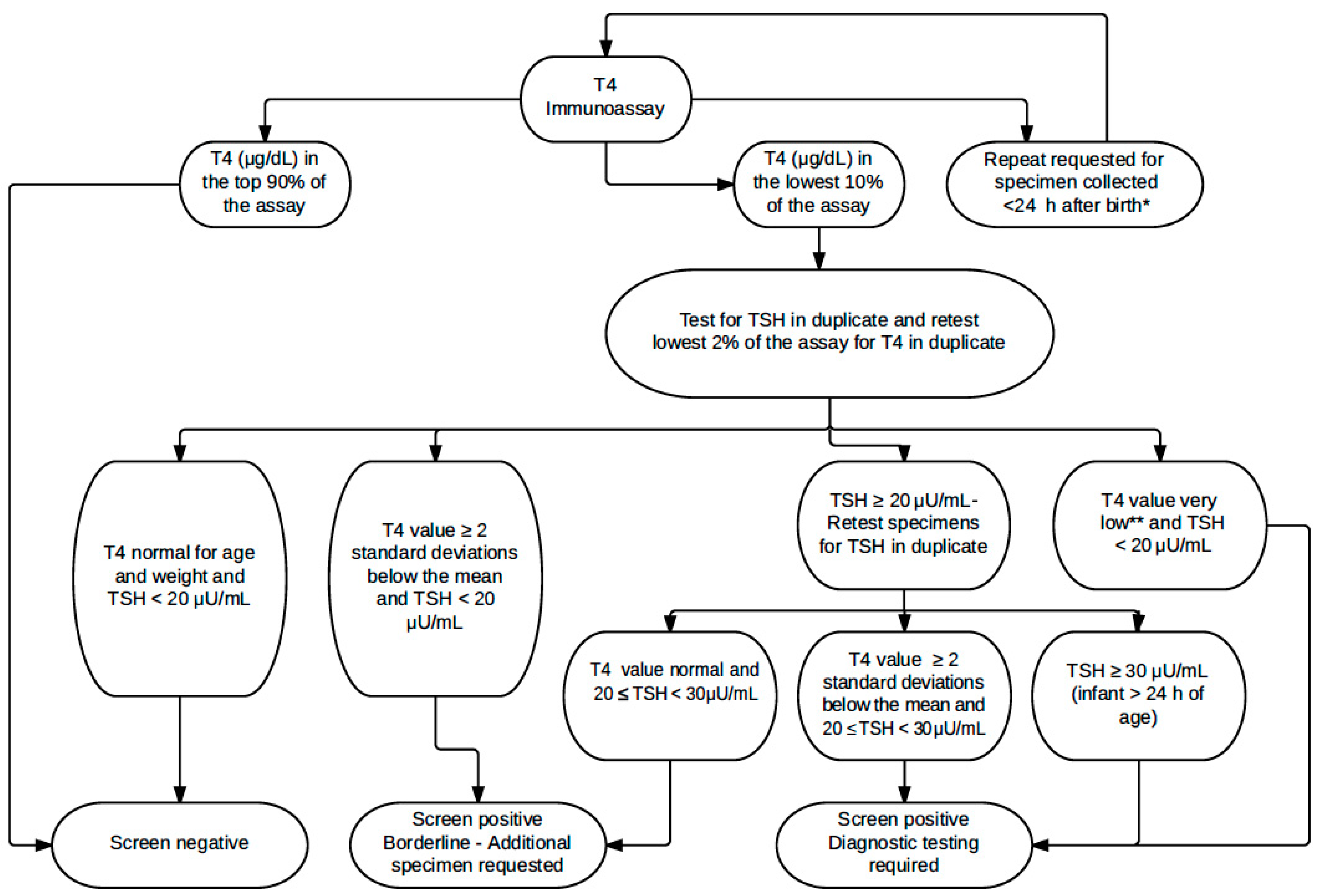
2. Materials and Methods
Statistical Analysis
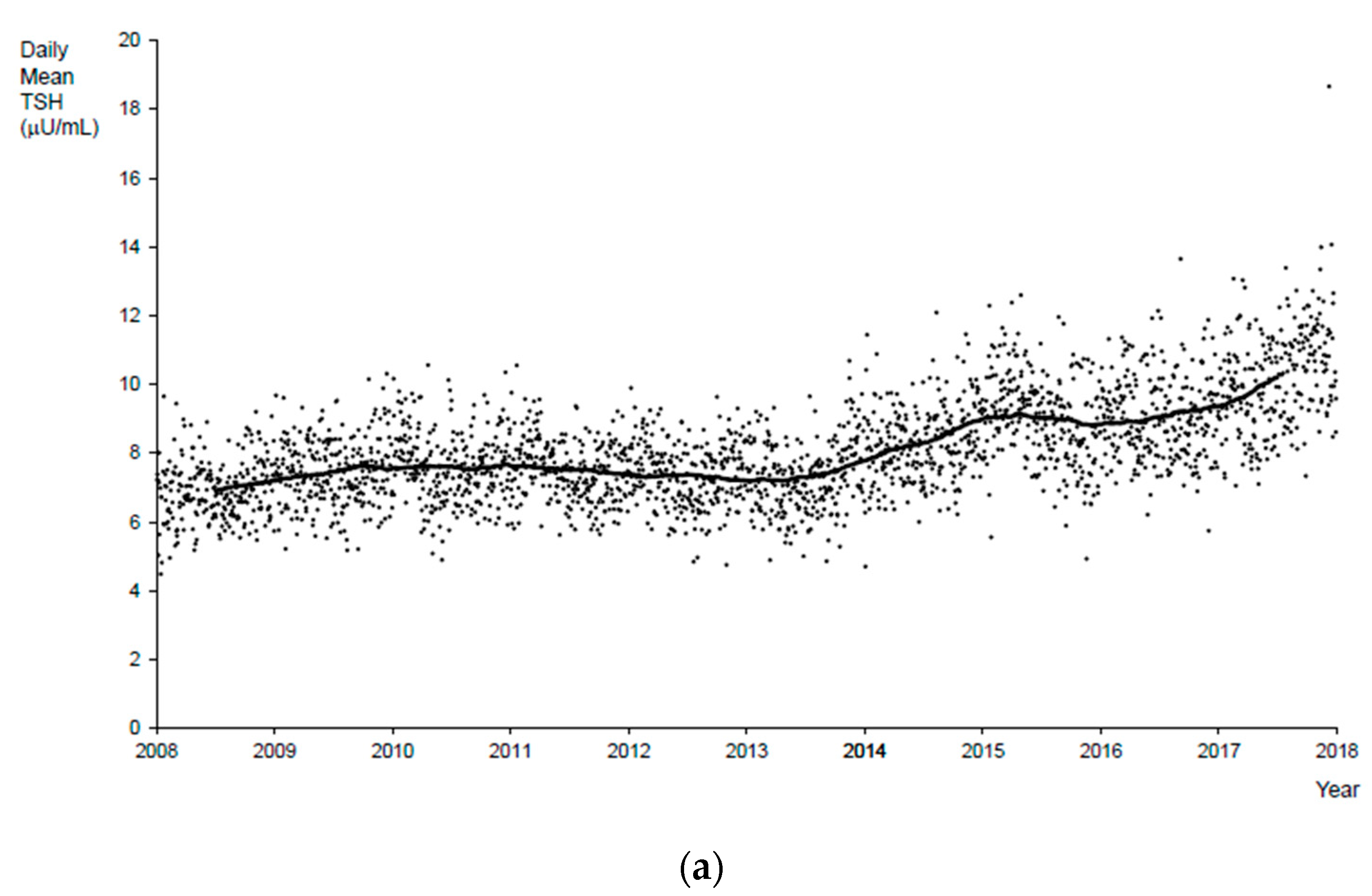
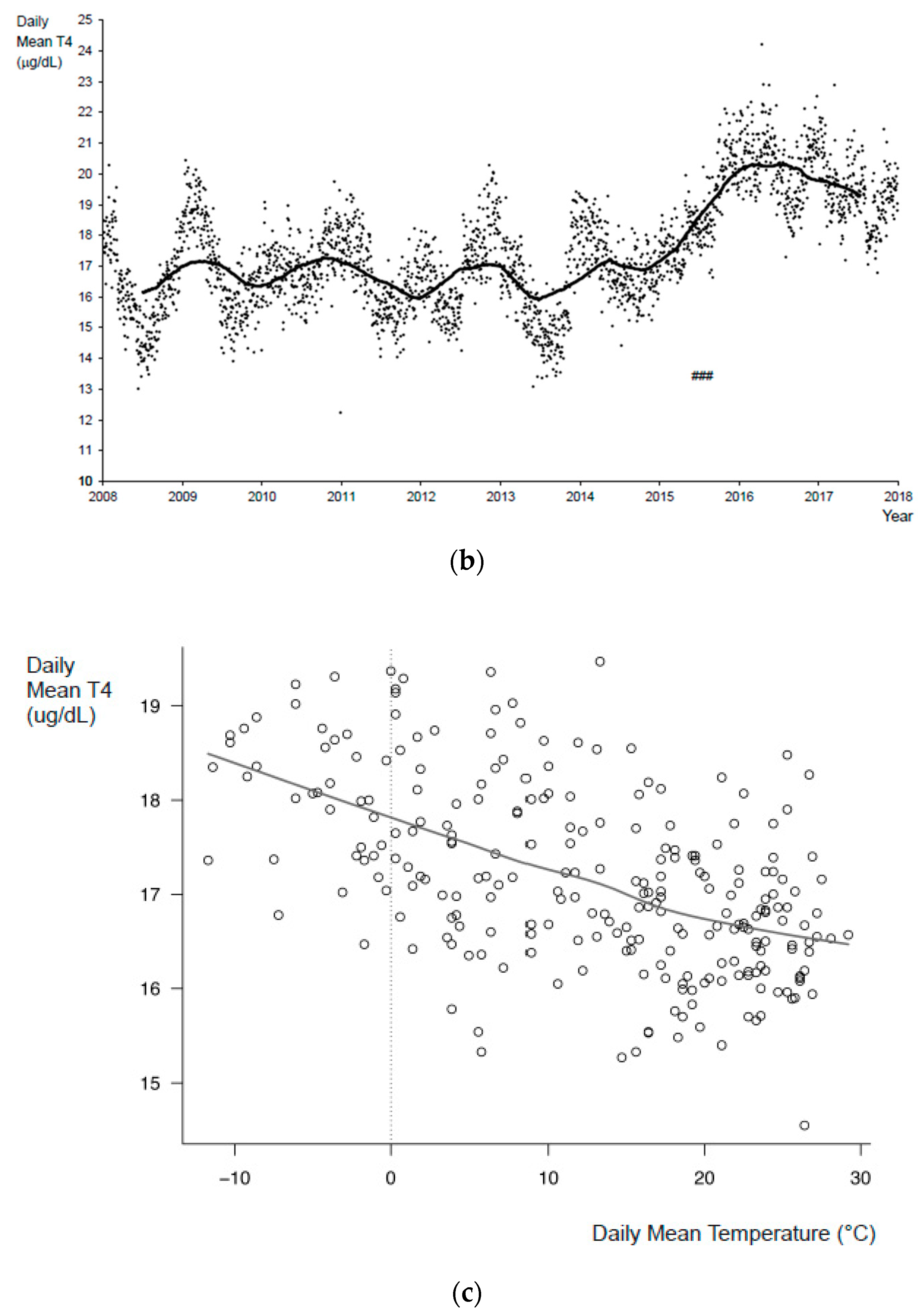
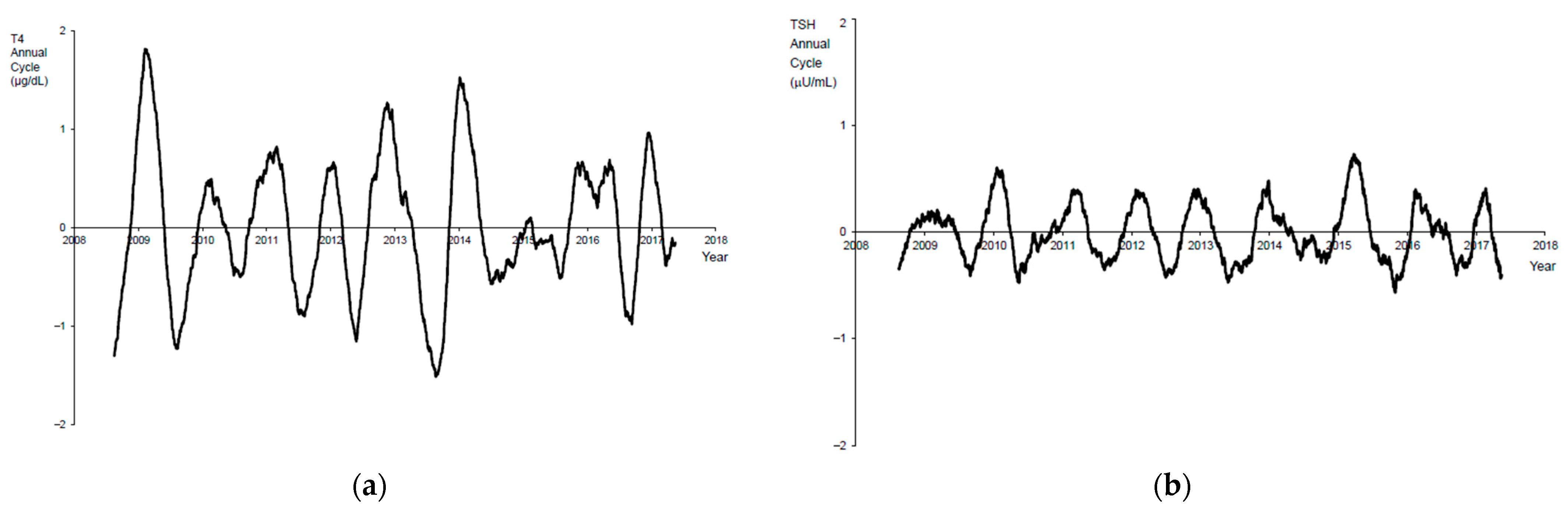
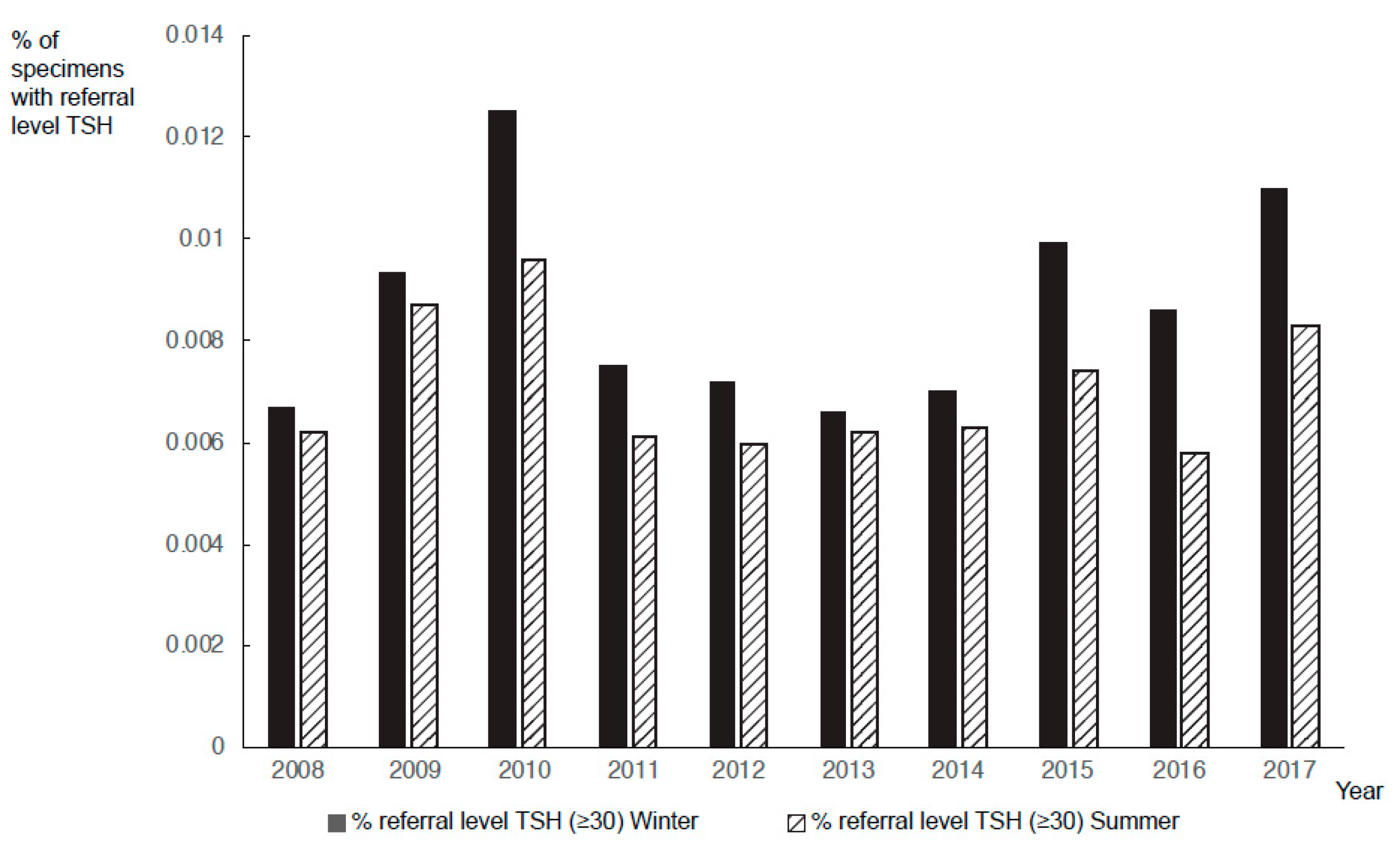
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Léger, J.; Olivieri, A.; Donaldson, M.; Torresani, T.; Krude, H.; Van Vliet, G.; Polak, M.; Butler, G.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology Consensus Guidelines on Screening, Diagnosis, and Management of Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2014, 99, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.; LaFranchi, S.H. Screening for congenital hypothyroidism: A worldwide view of strategies. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Odell, W.D. Acute release of thyrotropin in the newborn. J. Clin. Investig. 1969, 48, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.; Fisher, D.; Wang, C. Serum thyrotropin, prolactin, and growth hormone levels during the early neonatal period in the human infant. J. Pediatr. 1976, 89, 298–300. [Google Scholar] [CrossRef]
- La Franchi, S. Thyroid Function in the Preterm Infant. Thyroid 1999, 9, 71–78. [Google Scholar] [CrossRef]
- Fisher, D.A. Thyroid Function in Premature Infants: The Hypothyroxinemia of Prematurity. Clin. Perinatol. 1998, 25, 999–1014. [Google Scholar] [CrossRef]
- La Gamma, E.F.; Korzeniewski, S.J.; Ballabh, P.; Paneth, N. Transient Hypothyroxinemia of Prematurity. NeoReviews 2016, 17, e394–e402. [Google Scholar] [CrossRef]
- Van Wassenaer, A.G.; Kok, J.H.; Briët, J.M.; Pijning, A.M.; De Vijlder, J.J. Thyroid Function in Very Preterm Newborns: Possible Implications. Thyroid 1999, 9, 85–91. [Google Scholar] [CrossRef]
- Lezcano, A.C.; Ruiz-Cuevas, P.; Potau, N.; Almar, J.; Salcedo, S.; Clemente, M.; Fernández, D.Y. Thyroid Function in Seventy-Five Healthy Preterm Infants Thirty to Thirty-Five Weeks of Gestational Age: A Prospective and Longitudinal Study During the First Year of Life. Thyroid 2004, 14, 435–442. [Google Scholar] [CrossRef]
- Klein, R.; Carlton, E.L.; Faix, J.D.; Frank, J.E.; Hermos, R.J.; Mullaney, D.; Nelson, J.C.; Rojas, D.A.; Mitchell, M.L. Thyroid function in very low birth weight infants. Clin. Endocrinol. 1997, 47, 411–417. [Google Scholar] [CrossRef]
- Frank, J.E.; Faix, J.E.; Hermos, R.J.; Mullaney, D.M.; Rojan, D.A.; Mitchell, M.L.; Klein, R.Z. Thyroid function in very low birth weight infants: Effects on neonatal hypothyroidism screening. J. Pediatr. 1996, 128, 548–554. [Google Scholar] [CrossRef]
- Allen, D.B.; Sieger, J.E.; Litsheim, T.; Duck, S.C. Age-adjusted thyrotropin criteria for neonatal screening for hypothyroidism. J. Pediatr. 1990, 117, 309–312. [Google Scholar] [CrossRef]
- Kilberg, M.J.; Rasooly, I.R.; LaFranchi, S.H.; Bauer, A.J.; Hawkes, C.P. Newborn Screening in the US May Miss Mild Persistent Hypothyroidism. J. Pediatr. 2018, 192, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Bijarnia-Mahay, S.; Wilcken, B.; Wiley, V.C. Newborn screening for congenital hypothyroidism in very-low-birth-weight babies: The need for a second test. J. Inherit. Metab. Dis. 2011, 34, 827–833. [Google Scholar] [CrossRef]
- Cavarzere, P.; Camilot, M.; Popa, F.I.; Lauriola, S.; Teofoli, F.; Gaudino, R.; Vincenzi, M.; Antoniazzi, F. Congenital hypothyroidism with delayed TSH elevation in low-birth-weight infants: Incidence, diagnosis and management. Eur. J. Endocrinol. 2016, 175, 395–402. [Google Scholar] [CrossRef]
- De Martino, L.; McMahon, R.; Caggana, M.; Tavakoli, N.P. Gender disparities in screening for congenital hypothyroidism using thyroxine as a primary screen. Eur. J. Endocrinol. 2018, 179, 161–167. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Berberich, S.L.; Shchelochkov, O.A.; Cook, D.E.; Murray, J.C. Clinical and environmental influences on metabolic biomarkers collected for newborn screening. Clin. Biochem. 2013, 46, 133–138. [Google Scholar] [CrossRef]
- Kay, D.M.; Maloney, B.; Hamel, R.; Pearce, M.; De Martino, L.; McMahon, R.; McGrath, E.; Krein, L.M.; Vogel, B.H.; Saavedra-Matiz, C.A.; et al. Screening for cystic fibrosis in New York State: Considerations for algorithm improvements. Eur. J. Pediatr. 2016, 175, 181–193. [Google Scholar] [CrossRef]
- Pearce, M.; Dauerer, E.; Di Rienzo, A.G.; Caggana, M.; Tavakoli, N.P. The influence of seasonality and manufacturer kit lot changes on 17α-hydroxyprogesterone measurements and referral rates of congenital adrenal hyperplasia in newborns. Eur. J. Pediatr. 2016, 176, 121–129. [Google Scholar] [CrossRef]
- Rezaeian, S.; Moghimbeigi, A.; Nasab, N.E. Gender Differences in Risk Factors of Congenital Hypothyroidism: An Interaction Hypothesis Examination. Int. J. Endocrinol. Metab. 2014, 12, e13946. [Google Scholar] [CrossRef]
- Gu, Y.-H.; Kato, T.; Harada, S.; Inomata, H.; Saito, T.; Aoki, K. Seasonality in the Incidence of Congenital Hypothyroidism in Japan: Gender-Specific Patterns and Correlation with Temperature. Thyroid 2007, 17, 869–874. [Google Scholar] [CrossRef]
- Hall, S.; Hutchesson, A.; Kirk, J. Congenital hypothyroidism, seasonality and consanguinity in the West Midlands, England. Acta Paediatr. 1999, 88, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; Korada, M.; Day, J.; Turner, S.; Allison, D.; Kibirige, M.; Cheetham, T.D. Increasing Incidence, but Lack of Seasonality, of Elevated TSH Levels, on Newborn Screening, in the North of England. J. Thyroid Res. 2010, 28, 101948. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, M.B.; Perlini, C.; Ciatti, R.; Burroni, M. Is the birthdate a risk factor for congenital hypothyroidism? A statistical an-swer based on personal experience. Minerva Pediatr. 2001, 53, 531–536. [Google Scholar]
- Deladoëy, J.; Bélanger, N.; Van Vliet, G. Random Variability in Congenital Hypothyroidism from Thyroid Dysgenesis over 16 Years in Québec. J. Clin. Endocrinol. Metab. 2007, 92, 3158–3161. [Google Scholar] [CrossRef] [PubMed]
- Mehran, L.; Khalili, D.; Yarahmadi, S.; Amouzegar, A.; Mojarrad, M.; Ajang, N.; Azizi, F. Worldwide Recall Rate in Newborn Screening Programs for Congenital Hypothyroidism. Int. J. Endocrinol. Metab. 2017, 15, e55451. [Google Scholar] [CrossRef]
- McGrath, N.; Hawkes, C.P.; Mayne, P.; Murphy, N.P. Optimal Timing of Repeat Newborn Screening for Congenital Hypothyroidism in Preterm Infants to Detect Delayed Thyroid-Stimulating Hormone Elevation. J. Pediatr. 2019, 205, 77–82. [Google Scholar] [CrossRef]
- La Franchi, S.H. Screening Preterm Infants for Congenital Hypothyroidism: Better the Second Time Around. J. Pediatr. 2014, 164, 1259–1261. [Google Scholar] [CrossRef]
- Coombes, E.J.; Gamlen, T.R.; Batstone, G.F. Effect of Temperature on the Stability of Thyroid Stimulating Hormone in Dried Blood Spots. Ann. Clin. Biochem. 1983, 20, 252–253. [Google Scholar] [CrossRef]
- Waite, K.V.; Maberly, G.F.; Eastman, C.J. Storage conditions and stability of thyrotropin and thyroid hormones on filter paper. Clin. Chem. 1987, 33, 853–855. [Google Scholar] [CrossRef]
- Adam, B.; Hall, E.; Sternberg, M.; Lim, T.; Flores, S.; O’Brien, S.; Simms, D.; Li, L.; De Jesus, V.; Hannon, W. The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin. Biochem. 2011, 44, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Lando, V.S.; Batista, M.C.; Nakamura, I.T.; Mazi, C.R.; De Mendonça, B.B.; Brito, V.N. Effects of long-term storage of filter paper blood samples on neonatal thyroid stimulating hormone, thyroxin and 17-alpha-hydroxyprogesterone measurements. J. Med. Screen. 2008, 15, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Baroni, M.G.; Leonetti, F.; De Bernardinis, M.; Bertoccini, L.; Fontana, M.; Mazzei, E.; Fraioli, A.; Cavallo, M.G. TSH levels are associated with vitamin D status and seasonality in an adult population of euthyroid adults. Clin. Exp. Med. 2014, 15, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Carlucci, M.A.; Semeraro, D.; Giuliani, C.; Napolitano, G.; Caturegli, P.; Bucci, I. A Detailed Analysis of the Factors Influencing Neonatal TSH: Results from a 6-Year Congenital Hypothyroidism Screening Program. Front. Endocrinol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Çaylan, N.; Tezel, B.; Özbaş, S.; Şahin, N.; Aydın, Ş.; Acıcan, D.; Keskinkılıç, B. Neonatal Thyroid-Stimulating Hormone Screening as a Monitoring Tool for Iodine Deficiency in Turkey. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 187–191. [Google Scholar] [CrossRef]
- Newborn Screening Timeliness Goals. 2015. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/newborn-screening-timeliness.html (accessed on 28 November 2020).
- Kok, J.H.; Hart, G.; Endert, E.; Koppe, J.G.; De Vijlder, J.J. Normal ranges of T4 screening values in low birthweight infants. Arch. Dis. Child. 1983, 58, 190–194. [Google Scholar] [CrossRef]
- Fisher, D.A. Physiological variations in thyroid hormones: Physiological and pathophysiological considerations. Clin. Chem. 1996, 42, 135–139. [Google Scholar] [CrossRef]
| Winter (January–March) | Summer (July–September) | p-Value | |
|---|---|---|---|
| Total Babies Tested | 590,553 | 640,863 | <0.0001 |
| Total Referrals | 2033 | 1758 | <0.0001 |
| Total Confirmed CH | 236 | 282 | 0.2663 |
| Total False-Positive | 1368 | 1088 | <0.0001 |
| Cases Closed as “Other” * | 429 | 388 | - |
| Incidence of CH | 1 in 2502 | 1 in 2273 | - |
| Referral Rate | 1 in 290 | 1 in 365 | - |
| False-Positive Rate | 1 in 432 | 1 in 589 | - |
| Positive Predictive Value (PPV) | 14.7% | 20.6% | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMahon, R.; DeMartino, L.; Sowizral, M.; Powers, D.; Tracy, M.; Caggana, M.; Tavakoli, N.P. The Impact of Seasonal Changes on Thyroxine and Thyroid-Stimulating Hormone in Newborns. Int. J. Neonatal Screen. 2021, 7, 8. https://doi.org/10.3390/ijns7010008
McMahon R, DeMartino L, Sowizral M, Powers D, Tracy M, Caggana M, Tavakoli NP. The Impact of Seasonal Changes on Thyroxine and Thyroid-Stimulating Hormone in Newborns. International Journal of Neonatal Screening. 2021; 7(1):8. https://doi.org/10.3390/ijns7010008
Chicago/Turabian StyleMcMahon, Rebecca, Lenore DeMartino, Mycroft Sowizral, Diana Powers, Melissa Tracy, Michele Caggana, and Norma P. Tavakoli. 2021. "The Impact of Seasonal Changes on Thyroxine and Thyroid-Stimulating Hormone in Newborns" International Journal of Neonatal Screening 7, no. 1: 8. https://doi.org/10.3390/ijns7010008
APA StyleMcMahon, R., DeMartino, L., Sowizral, M., Powers, D., Tracy, M., Caggana, M., & Tavakoli, N. P. (2021). The Impact of Seasonal Changes on Thyroxine and Thyroid-Stimulating Hormone in Newborns. International Journal of Neonatal Screening, 7(1), 8. https://doi.org/10.3390/ijns7010008






