Report of Five Years of Experience in Neonatal Screening for Mucopolysaccharidosis Type I and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening Population
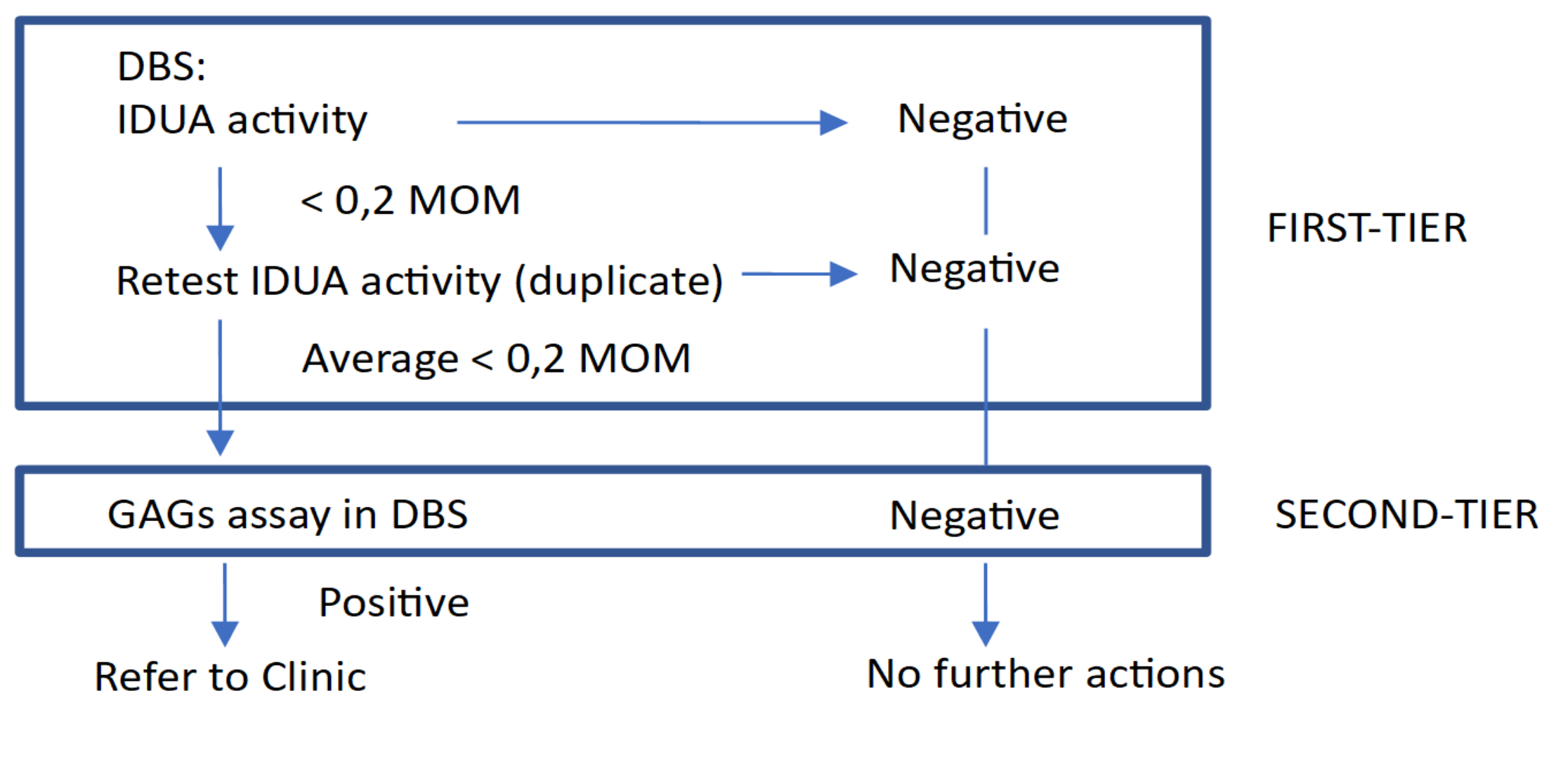
2.2. Clinical and Biochemical Assessment
3. Results
3.1. MPS I Screening Results
3.2. Clinical Follow-Up
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neufeld, E.F.; Muenzer, J. The mucopolysaccharidosis. In The Metabolic and Molecular Basis of Inherited Disease; Scriver, C., Beaudet, A., Sly, W., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Wraith, J.E. The mucopolysaccharidoses: A clinical review and guide to management. Arch. Dis. Child. 1995, 72, 263–267. [Google Scholar] [CrossRef]
- Martins, A.M.; Dualibi, A.P.; Norato, D.; Takata, E.T.; Santos, E.S.; Valadares, E.R.; Porta, G.; de Luca, G.; Moreira, G.; Pimentel, H.; et al. Guidelines for the management of mucopolysaccharidosis type 1. J. Pediatr. 2009, 155 (Suppl. 4), S32–S46. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Connock, M.J.; Wraith, E.; Lavery, C. The prevalence of and survival in Mucopolysaccharidosis I: Hurler, Hurler-Scheie and Scheie syndromes in the UK. Orphanet J. Rare Dis. 2008, 3, 24. [Google Scholar] [CrossRef]
- Sawamoto, K.; Chen, H.H.; Alméciga-Díaz, C.J.; Mason, R.W.; Tomatsu, S. Gene therapy for mucopolysaccharidoses. Mol. Genet. Metab. 2018, 123, 59–68. [Google Scholar] [CrossRef]
- Giugliani, R.; Federhen, A.; Vairo, F.; Vanzella, C.; Pasqualim, G.; da Silva, L.M.R. Emerging drugs for the treatment of mucopolysaccharidoses. Expert Opin. Emerg. Drugs 2016, 21, 9–26. [Google Scholar] [CrossRef]
- Chamoles, N.A.; Blanco, B.; Gaggioli, D.; Casentini, C. Hurler-like phenotype: Enzymatic diagnosis in dried blood spots on filter paper. Clin. Chem. 2001, 47, 2098–2102. [Google Scholar] [CrossRef]
- Taylor, J.L.; Clinard, K.; Powell, C.M.; Rehder, C.; Young, S.P.; Bali, D.; Beckloff, S.E.; Gehtland, L.M.; Kemper, A.R.; Lee, S.; et al. The North Carolina experience with Mucopolysaccharidosis type I Newborn Screening. J. Pediatr. 2019, 211, 193–200.e2. [Google Scholar] [CrossRef]
- Lin, S.-P.; Lin, H.-Y.; Wang, T.-J.; Chang, C.-Y.; Lin, K.-J.; Huang, S.-F.; Tsai, C.-C.; Liu, H.-L.; Keutzer, J.; Chuang, C.-K. A pilot newborn screening program for mucopolysaccharidosis type 1 in Taiwan. Orphanet J. Rare Dis. 2013, 8, 147. [Google Scholar] [CrossRef]
- Sista, R.S.; Wang, T.; Wu, N.; Graham, C.; Eckhardt, A.; Winger, T.; Srinivasan, V.; Bali, D.; Millington, D.S.; Pamula, V.K. Multiplex newborn screening for Pompe, Fabry, Hunter, Gaucher and Hurler diseases using a digital microfluidic platform. Clin. Chim. Acta 2013, 424, 12–18. [Google Scholar] [CrossRef]
- Paciotti, S.; Persichetti, E.; Pagliardini, S.; Deganuto, M.; Rosano, C.; Balducci, C.; Codini, M.; Filocamo, M.; Menghini, A.R.; Pagliardini, V.; et al. First pilot newborn screening for four lysosomal storage disease in an Italian region: Identification and analysis of a putative causative mutation in the GBA gene. Clin. Chim. Acta 2012, 413, 1827–1831. [Google Scholar] [CrossRef]
- Navarrete-Martínez, J.I.; Limón-Rojas, A.E.; Gaytán-García, M.D.J.; Reyna-Figueroa, J.; Wakida-Kusunoki, G.; Delgado-Calvillo, M.D.R.; Cantú-Reyna, C.; Cruz-Camino, H.; Cervantes-Barragan, D.E. Newborn screening for six lysosomal storage disorders in a cohort of Mexican patients: Three year findings from a screening program in a closed Mexican Health System. Mol. Genet. Metab. 2017, 121, 16–21. [Google Scholar]
- Hopkins, P.V.; Klug, T.; Vermette, L.; Raburn-Miller, J.; Kiesling, J.; Rogers, S. Incidence of 4 lysosomal storage disorders from 4 years of newborn screening. JAMA Pediatr. 2018, 172, 696–697. [Google Scholar] [CrossRef]
- Scott, C.R.; Elliott, S.; Buroker, N.; Thomas, L.I.; Keutzer, J.; Glass, M.; Gelb, M.H.; Turecek, F. Identification of infants at risk for developing Fabry, Pompe or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J. Pediatr. 2013, 163, 498–503. [Google Scholar] [CrossRef]
- Wasserstein, M.P.; Caggana, M.; Bailey, S.M.; Desnick, R.J.; Edelmann, L.; Estrella, L.; Holzman, I.; Kelly, N.R.; Kornreich, R.; Kupchik, S.G.; et al. The New York pilot newborn screening program for lysosomal storage diseases: Report of the first 65,000 infants. Genet. Med. 2019, 21, 631–640. [Google Scholar] [CrossRef]
- Burton, B.K.; Charrow, J.; Hoganson, G.E.; Waggoner, D.; Tinkle, B.; Braddock, S.R.; Schneider, M.; Grange, D.K.; Nash, C.; Shryock, H.; et al. Newborn screening for lysosomal storage disorders in Illinois: The initial 15-month experience. J. Pediatr. 2017, 190, 130–135. [Google Scholar] [CrossRef]
- Elliott, S.; Buroker, N.; Cournoyer, J.J.; Potier, A.M.; Trometer, J.D.; Elbin, C.; Schermer, M.J.; Kantola, J.; Boyce, A.; Turecek, F.; et al. Pilot study of newborn screening for six lysosomal storage diseases using tandem mass spectrometry. Mol. Genet. Metab. 2016, 118, 304–309. [Google Scholar] [CrossRef]
- Burlina, A.B.; Polo, G.; Salviati, L.; Duro, G.; Zizzo, C.; Dardis, A.; Bembi, B.; Cazzorla, C.; Rubert, L.; Zordan, R.; et al. Newborn screening for lysosomal storage disorders by tandem mass spectrometry in North East Italy. J. Inherit. Metab. Dis. 2018, 41, 209–219. [Google Scholar] [CrossRef]
- Lin, S.-P.; Lin, H.-Y.; Wang, T.-J.; Huang, Y.-H.; Chan, M.-J.; Liao, H.-C.; Lo, Y.-T.; Wang, L.-Y.; Tu, R.-Y.; Fang, Y.-Y.; et al. Status of newborn screening and follow up investigations for mucopolysaccharidoses I and II in Taiwan. Orphanet J. Rare Dis. 2018, 13, 84. [Google Scholar]
- Donati, M.A.; Pasquini, E.; Spada, M.; Polo, G.; Burlina, A. Newborn screening in mucopolysaccharidosis. Ital. J. Pediatr. 2018, 44 (Suppl. 2), 126. [Google Scholar] [CrossRef]
- Baerg, M.M.M.; Stoway, S.D.; Hart, J.; Mott, L.; Peck, D.S.; Nett, S.L.; Eckerman, J.S.; Lacey, J.M.; Turgeon, C.T.; Gavrilov, D.; et al. Precision newborn screening for lysosomal disorders. Genet. Med. 2018, 20, 847–854. [Google Scholar] [CrossRef]
- Bravo, H.; Neto, E.C.; Schulte, J.; Pereira, J.; Filho, C.S.; Bittencourt, F.; Sebastião, F.; Bender, F.; De Magalhães, A.P.S.; Guidobono, R.; et al. Investigation of newborns with abnormal results in a newborn screening program for 4 lysosomal storage disease in Brazil. Mol. Genet. Metab. Rep. 2017, 12, 92–97. [Google Scholar] [CrossRef]
- Hall, P.L.; Sanchez, R.; Hagar, A.F.; Jerris, S.C.; Wittenauer, A.; Wilcox, W.R. Two-Tiered Newborn Screening with Post-Analytical Tools for Pompe Disease and Mucopolysaccharidosis Type I Results in Performance Improvement and Future Direction. Int. J. Neonatal Screen. 2020, 6, 2. [Google Scholar] [CrossRef]
- Stapleton, M.; Kubaski, F.; Mason, R.W.; Shintaku, H.; Kobayashi, H.; Yamaguchi, S.; Taketani, T.; Suzuki, Y.; Orii, K.; Orii, T.; et al. Newborn screening for mucopolysaccharidoses: Measurement of glycosaminoglycans by LC-MS/MS. Mol. Genet. Metab. Rep. 2020, 22, 100563. [Google Scholar] [CrossRef]
- Bender, F.; Burin, M.G.; Tirelli, K.M.; Medeiros, F.; De Bitencourt, F.H.; Civallero, G.; Kubaski, F.; Bravo, H.; Daher, A.; Carnier, V.; et al. Newborn screening for lysosomal disorders in Brazil: A pilot study using customized fluorimetric assays. Genet. Mol. Biol 2020, 43, e20180334. [Google Scholar] [CrossRef]
- Polo, G.; Gueraldi, D.; Giuliani, A.; Rubert, L.; Cazzorla, C.; Salviati, L.; Marzollo, A.; Biffi, A.; Burlina, A.P.; Burlina, A.B. The combined use of enzyme activity and metabolite assays as a strategy for newborn screening of MPS type I. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Fenzi, C.R.; Teramoto, K.; Moshirfar, M. Ocular manifestations and management recommendation of lysosomal storage disorders I: Mucopolysaccharidoses. Clin. Ophthalmol. 2015, 9, 1633–1644. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Lee, N.-C.; Thurberg, B.L.; Chiang, S.-C.; Zhang, X.K.; Keutzer, J.; Huang, A.-C.; Wu, M.-H.; Huang, P.-H.; Tsai, F.-J.; et al. Pompe disease in infants: Improving the prognosis by newborn screening and early treatment. Pediatrics 2009, 124, e1116–e1125. [Google Scholar] [CrossRef]
- Clarke, L.A.; Atherton, A.M.; Burton, B.K.; Day-Salvatore, D.L.; Kaplan, P.; Leslie, N.D.; Scott, C.R.; Stockton, D.W.; Thomas, J.A.; Muenzer, J. Mucopolysaccharidosis type I newborn screening: Best practices for diagnosis and management. J. Pediatr. 2017, 182, 363–370. [Google Scholar] [CrossRef]
- Burlina, A.B.; Polo, G.; Rubert, L.; Gueraldi, D.; Cazzorla, C.; Duro, G.; Salviati, L.; Burlina, A.P. Implementation of second-tier tests in newborn screening for lysosomal disorders in North Easten Italy. Int. J. Neonatal Screen. 2019, 5, 24. [Google Scholar] [CrossRef]
- Tomatsu, S.; Azario, I.; Sawamoto, K.; Pievani, A.S.; Biondi, A.; Serafini, M. Neonatal cellular and gene therapies for mucopolysaccharidoses: The earlier the better? J. Inherit. Metab. Dis. 2016, 39, 189–202. [Google Scholar] [CrossRef]
- Martin, J.J.; Ceuterick, C. Prenatal pathology in mucopolysaccharidoses: A comparison with postnatal cases. Clin. Neuropathol. 1983, 2, 122–127. [Google Scholar] [PubMed]
- Boelens, J.J.; Erocha, V.; Aldenhoven, M.; Wynn, R.; O’Meara, A.; Michel, G.; Ionescu, I.; Parikh, S.; Prasad, V.K.; Szabolcs, P.; et al. Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with Hurler syndrome. Biol. Blood Marrow Transplant. 2009, 15, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Aldenhoven, M.; Jones, S.A.; Bonney, D.; Borrill, R.E.; Coussons, M.; Mercer, J.; Bierings, M.B.; Versluys, B.; Van Hasselt, P.M.; Wijburg, F.A.; et al. Hematopoietic cell transplantation for mucopolysaccharidosis patients safe and effective: Results after implementation of international guidelines. Biol. Blood Marrow Transplant. 2015, 21, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Parini, R.; Deodato, F.; Di Rocco, M.; Lanino, E.; Locatelli, F.; Messina, C.; Rovelli, A.; Scarpa, M. Open issues in Mucopolysaccharidosis type I-Hurler. Orphanet J. Rare Dis. 2017, 12, 112. [Google Scholar] [CrossRef]
- Gabrielli, O.; Clarke, L.A.; Ficcadenti, A.; Santoro, L.; Zampini, L.; Volpi, N.; Coppa, G.V. 12 years follow up of enzyme-replacement therapy in two siblings with attenuated mucopolysaccharidosis I: The important role of early treatment. BMC Med. Genet. 2016, 17, 19. [Google Scholar] [CrossRef]
- Laraway, S.; Breen, C.; Mercer, J.; Jones, S.; Wraith, J.E. Does early use of enzyme replacement therapy alter the natural history of mucopolysaccharidosis I? Experience in three siblings. Mol. Genet. Metab. 2013, 109, 315–316. [Google Scholar] [CrossRef]
- Al-Sannaa, N.A.; Bay, L.; Barbouth, D.S.; Benhayoun, Y.; Goizet, C.; Guelbert, N.; Jones, S.A.; Kyosen, S.O.; Martins, A.M.; Phornphutkul, C.; et al. Early treatment with laronidase improves clinical outcomes in patients with attenuated MPS I: A retrospective case series analysis of nine sibships. Orphanet J. Rare Dis. 2015, 10, 131. [Google Scholar] [CrossRef]
- Yamazaki, N.; Kosuga, M.; Kida, K.; Takei, G.; Fukuhara, Y.; Matsumoto, H.; Senda, M.; Honda, A.; Ishiguro, A.; Koike, T.; et al. Early enzyme replacement therapy enables a successful hematopoietic stem cell transplantation in mucopolysaccharidosis type IH: Divergent clinical outcomes in two Japanese siblings. Brain Dev. 2019, 41, 546–550. [Google Scholar] [CrossRef]
- Dionisi-Vici, C.; Rizzo, C.; Burlina, A.; Caruso, U.; Sabetta, G.; Uziel, G.; Abeni, D. Inborn errors of metabolism in the Italian pediatric population: A national retrospective survey. J. Pediatr. 2002, 140, 321–327. [Google Scholar] [CrossRef]
- Scott, C.R.; Elliott, S.; Hong, X.; Huang, J.-Y.; Kumar, A.B.; Yi, F.; Pendem, N.; Chennamaneni, N.K.; Gelb, M.H. Newborn screening for mucopolysaccharidoses: Results of a pilot study with 100,000 dried blood spots. J. Pediatr. 2020, 216, 204–207. [Google Scholar] [CrossRef]
- De Ruijter, J.; De Ru, M.H.; Wagemans, T.; Ijlst, L.; Lund, A.M.; Orchard, P.J.; Schaefer, G.B.; Wijburg, F.A.; Van Vlies, N. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses type I, II and III. Mol. Genet. Metab. 2012, 107, 705–710. [Google Scholar] [CrossRef]
| Years | Region | Methodology | Screened Disorders Other Than MPS I | Screened Newborns | False-Positive Rate | Incidence |
|---|---|---|---|---|---|---|
| 2008–2013 [9] | Taiwan | Fluorometric enzymatic assay | / | 35,285 | 0.048% | 1/17,643 |
| 2010–2012 [11] | Italy | Fluorometric enzymatic assay | Pompe disease, Fabry disease, Gaucher disease | 3403 | 0.088% | / |
| 2012–2016 [12] | Mexico 1 | MS/MS enzymatic assay | Pompe disease, Fabry disease, Gaucher disease, Niemann Pick type A/B, Krabbe disease | 20,018 | 0.009% | / |
| Since 2013 [13] | Missouri | Digital microfluidic fluorometric enzymatic assay | Pompe disease, Fabry disease, Gaucher disease | 308,000 | 0.040% | 1/154,000 |
| 2013 [14] | Washington 2 | MS/MS enzymatic assay | Fabry disease, Pompe disease | 106,526 | 0.006% | 1/35,700 |
| 2015–2016 [15] | New York | MS/MS enzymatic assay | Pompe disease, Fabry disease, Gaucher disease, Niemann Pick type A/B | 35,816 | 0.036% | / |
| 2014-2016 [16] | Illinois | MS/MS enzymatic assay | Pompe disease, Fabry disease, Gaucher disease, Niemann Pick type A/B | 219,973 | 0.068% | 1/219,973 |
| 2016 [17] | Washington 2 | MS/MS enzymatic assay | Pompe disease, Fabry disease, Gaucher disease, Niemann Pick type A/B, Krabbe disease | About 43,000 | 0.014% | / |
| 2015–2017 [18] | Italy | MS/MS enzymatic assay | Pompe disease, Fabry disease, Gaucher disease | 44,411 | 0.027% | 1/44,411 |
| 2015–2017 [19] | Taiwan | MS/MS enzymatic assay | MPS II | 294,196 | 0.004% | 1/73,549 |
| 2015–2017 [20] | Italy | MS/MS enzymatic assay | Pompe disease, Fabry disease | 64,907 | 0.012% | / |
| 2016–2017 [8] | North Carolina | MS/MS enzymatic assay | / | 62,734 | 0.028% | 1/62,734 |
| 2016 [21] | Kentucky | MS/MS enzymatic assay | Pompe disease, Krabbe disease | 55,161 | 0.002% | 1/55,161 |
| 2017 [22] | Brazil 3 | Digital microfluidic fluorometric enzymatic assay | Pompe disease, Fabry disease, Gaucher disease | 10,567 | 0.019% | / |
| 2017 [23] | Georgia | MS/MS enzymatic assay | Pompe disease | 59,332 | 0.018% | / |
| 2017 [24] | Japan | GAGs on DBS by MS/MS assay | MPS II | 18,222 | / | / |
| 2018 [25] | Brazil2 | Fluorometric enzymatic assay | MPS VI, Pompe disease, Fabry disease, Gaucher disease | 834 | 1.3% | / |
| Every 3 Months 1 | Every 6 Months 1 | Annually 1 | |
|---|---|---|---|
| Medical history | X | ||
| Physical examination, including neurological assessment | X | ||
| Intellectual function (IQ) | X | ||
| Routine biochemical tests | X | ||
| Urinary GAGs | X | ||
| Enzyme activity 2 and chimerism | Only after HSCT (2/month during the first 6 months, 1/month during the following year) | ||
| Anti-ERT antibodies | X | ||
| Abdominal ultrasound | X | ||
| Cardiac assessment, including electrocardiogram and echocardiogram | X | ||
| Audiometry | X | ||
| Ophthalmologic assessment | X | ||
| Full-body X-ray | X | ||
| Brain and spine MRN | X | ||
| Patient No. | Year of Birth | Sex | Ethnic Origin | IDUA Activity I DBS (µM/h) 1 | IDUA Activity II DBS (µM/h) 1 | DBS GAGs DS/HS mg/L | Genotype | Phenotype | Urinary GAGs DS/HS (mg/mmol Creatinine) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2016 | M | West Africa | 0.10 | 0.13 | 1.52/1.13 | p.A79T/p.A79T (pseudoMPS I) | Not affected | NP | No | Dismissed |
| 2 | 2017 | F | European | 0.17 | 0.34 | 8.84/10.42 | p.S16_A19del/p.Y201X | MPS I Hurler | 80.4/121.9 | ERT+HSCT | Progressive improvement, very mild features of MPS I |
| 3 | 2018 | M | South Asia | 0.20 | 0.23 | 2.86/2.35 | NP 2 | Not affected | 37.9/4.1 | No | Failed to follow up |
| 4 | 2017 | F | North Africa | 0.22 | 0.20 | 7.38/4.88 | p.P533R/p.P533R | MPS I H/S | 172.0/148.9 | ERT | Progressive improvement, asymptomatic, except for corneal clouding |
| 5 | 2017 | F | North Africa | 0.38 | 0.73 | 2.85/3.1 | p.R628G/p.R628G (VUS) | Uncertain significance | 6.8/2.0 | No | 12-month follow-up appointment (refused) |
| 6 | 2018 | M | West Africa | 0.40 | 0.67 | 1.87/2.03 | p.A79T/p.D223N (pseudoMPS I) | Not affected | 29.1/3.4 | No | Dismissed |
| 7 | 2016 | M | North Africa | 0.41 | 0.62 | 1.54/1.05 | p.A79T+p.A361T/p.Y581X (carrier- Pseudo MPS I) | Not affected | 14.4/2.1 | No | Dismissed |
| 8 | 2019 (January) | F | West Africa | 0.47 | 0.53 | NA | NP 2 | Not affected | 27.6/3.6 | No | Dismissed |
| 9 | 2018 | F | West Africa | 0.49 | 0.47 | 2.02/1.91 | p.A79T/p.A79T (Pseudo MPS I) | Not affected | 19.6/3.4 | No | Dismissed |
| 10 | 2017 | F | West Africa | 0.53 | 0.57 | 1.75/2.03 | p.A79T/p.D223N (Pseudo MPS I) | Not affected | NP | No | Dismissed |
| 11 | 2016 | M | West Africa | 0.54 | 0.61 | 1.52/1.52 | p.A79T+p.T99I/p.D223N (Pseudo MPS I) | Not affected | NP | No | Dismissed |
| 12 | 2017 | F | European | 0.55 | 0.56 | 1.62/1.72 | p.L526P/p.L526P (VUS) | Uncertain significance | 9.1/1.8 | No | 12-month follow-up appointment (refused) |
| 13 | 2016 | F | West Africa | 0.58 | 0.56 | 2.06/1.63 | p.A79T/p.A361T (Pseudo MPS I) | Not affected | NP | No | Dismissed |
| 14 | 2017 | F | North Africa | 0.59 | 1.02 | 1.56/1.25 | p.R263W/p.P650L (Pseudo MPS I) | Not affected | 19.8/1.4 | No | Dismissed |
| 15 | 2017 | F | West Africa | 0.66 | 0.70 | 1.75/1.53 | p.A79T/p.A79T (Pseudo MPS I) | Not affected | 35.0/5.3 | No | Dismissed |
| 16 | 2016 | F | European | 0.71 | 0.55 | NA | p.S16_A19del/p.H82Q (Carrier- Pseudo MPS I) | Not affected | NP | No | Dismissed |
| 17 | 2016 | M | West Africa | 0.72 | 0.84 | 1.71/1.58 | p.A79T/p.A79T (Pseudo MPS I) | Not affected | NP | No | Dismissed |
| 18 | 2018 | M | North Africa | 0.85 | 0.98 | 2.09/2.06 | p.A79T/p.R263W (Pseudo MPS I) | Not affected | 13.8/1.7 | No | Dismissed |
| 19 | 2018 | M | NA | 0.88 | 0.79 | NA | NP 2 | Not affected | 21.0/1.8 | No | Dismissed |
| 20 | 2018 | F | West Africa | 0.92 | 1.01 | 2.01/1.77 | p.A79T/p.V322E (Pseudo MPS I) | Not affected | 16.8/0.8 | No | Dismissed |
| 21 | 2017 | M | West Africa | 0.97 | 1.51 | 1.86/1.48 | p.A79T/p.F501L (Pseudo MPS I) | Not affected | 9.9/1.7 | No | Dismissed |
| 22 | 2018 | M | NA | 1.07 | 1.21 | 1.6/1.67 | p.A79T/p.R263W (Pseudo MPS I) | Not affected | 23.2/2.5 | No | Dismissed |
| 23 | 2018 | M | West Africa | 1.1 | 1.36 | 1.93/1.55 | p.A79T/p.S586F (Pseudo MPS I) | Not affected | 13.6/1.3 | No | Dismissed |
| 24 | 2017 | F | West Africa | 1.14 | 1.39 | 2.75/2.37 | p.A79T/p.A79T (Pseudo MPS I) | Not affected | 13.7/1.6 | No | Dismissed |
| 25 | 2015 | M | Nord Africa | 1.16 | 1.19 | 2.07/1.1 | P.R263W/p.S586F (Pseudo MPS I) | Not affected | NP | No | Dismissed |
| 26 | 2018 | M | European | 1.26 | 1.56 | 1.89/1.53 | NP 2 | Not affected | 19.4/1.6 | No | Dismissed |
| 27 | 2017 | F | West Africa | 2.21 | 2.30 | 1.46/1.67 | p.A79T/wt (Pseudo MPS I) | Not affected | NP | No | Dismissed |
| A. Patient A Follow-Up | ||
| Pre-ERT | Last Follow-Up | |
| Physical examination, including neurological assessment | Recurrent respiratory infections in the first year, no neurological signs | Regular growth, no neurological signs |
| Intellectual function (IQ)–Bayley scale–III Ed. | Not performed | Developmental Quotient 100 |
| Routine biochemical tests | Normal | Normal |
| Urinary GAGs | HS 148.9 mg/mMol creatinine (nv < 4.6) DS 172.0 mg/mMol creatinine (nv < 38.1) | HS 1.8 mg/mMol creatinine (nv < 1.2) DS 6.7 mg/mMol creatinine (nv < 11.4) |
| Abdominal ultrasound | Normal | Normal |
| Cardiac assessment: -Electrocardiogram -Echocardiogram | Normal Patent foramen ovale (normal for age) and mild mitral insufficiency | Normal Normal |
| Audiometry/auditory brainstem response | Mild bilateral conductive hearing loss | Normal |
| Ophthalmological assessment | Diffuse corneal clouding | Diffuse corneal clouding, strabismus |
| Full-body X-Ray | Oval shaped lumbar vertebral bodies | Normal |
| Brain and spine MRI | Normal | Normal |
| B. Patient B Follow-Up | ||
| Pre-HSCT | Last Follow-Up | |
| Physical examination, including neurological assessment | Coarse facial features, poor growth | Coarse facial features, small umbilical ernia, normal growth, subtle flexion contractures of distal interphalangeal joints (reducible) |
| Intellectual function (IQ)–Bayley scale–III Ed. | Developmental Quotient 65 | Developmental Quotient 90 |
| Routine biochemical tests | Normal | Normal |
| Urinary GAGs | Initial values: HS 121.9 mg/mMol creatinine (nv < 4.6) DS 80.4 mg/mMol creatinine (nv < 38.1) Post-ERT, pre-HSCT: HS 21.7 mg/mMol creatinine (nv < 27.9) DS 29.2 mg/mMol creatinine (nv < 14.7) | HS 4.2 mg/mMol creatinine (nv < 1.2) DS 11.9 mg/mMol creatinine (nv < 11.4) |
| Enzyme activity | 0.17 uM/h (nv 1.9–15) | 6.70 uM/h (nv 1.9–15), max value 18 uM/h |
| Chimerism | 3% recipient, 97% donor | |
| Abdominal ultrasound | Normal | Normal |
| Cardiac assessment: -Electrocardiogram -Echocardiogram | Normal Patent foramen ovale (normal for age) | Normal Normal |
| Audiometry/auditorybrainstem response | Mild-moderate bilateral sensorineural and conductive hearing loss | Normal |
| Ophthalmological assessment | Diffuse corneal clouding | Diffuse corneal clouding |
| Full body X-Ray | Rounding of the iliac wings | Rounded thoracic and lumbar vertebral bodies |
| Brain and spine MRI | Large cisterna magna | Mild thickening of the tissues around the odontoid process, normal spinal canal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gragnaniello, V.; Gueraldi, D.; Rubert, L.; Manzoni, F.; Cazzorla, C.; Giuliani, A.; Polo, G.; Salviati, L.; Burlina, A. Report of Five Years of Experience in Neonatal Screening for Mucopolysaccharidosis Type I and Review of the Literature. Int. J. Neonatal Screen. 2020, 6, 85. https://doi.org/10.3390/ijns6040085
Gragnaniello V, Gueraldi D, Rubert L, Manzoni F, Cazzorla C, Giuliani A, Polo G, Salviati L, Burlina A. Report of Five Years of Experience in Neonatal Screening for Mucopolysaccharidosis Type I and Review of the Literature. International Journal of Neonatal Screening. 2020; 6(4):85. https://doi.org/10.3390/ijns6040085
Chicago/Turabian StyleGragnaniello, Vincenza, Daniela Gueraldi, Laura Rubert, Francesca Manzoni, Chiara Cazzorla, Antonella Giuliani, Giulia Polo, Leonardo Salviati, and Alberto Burlina. 2020. "Report of Five Years of Experience in Neonatal Screening for Mucopolysaccharidosis Type I and Review of the Literature" International Journal of Neonatal Screening 6, no. 4: 85. https://doi.org/10.3390/ijns6040085
APA StyleGragnaniello, V., Gueraldi, D., Rubert, L., Manzoni, F., Cazzorla, C., Giuliani, A., Polo, G., Salviati, L., & Burlina, A. (2020). Report of Five Years of Experience in Neonatal Screening for Mucopolysaccharidosis Type I and Review of the Literature. International Journal of Neonatal Screening, 6(4), 85. https://doi.org/10.3390/ijns6040085







