The First Year Experience of Newborn Screening for Pompe Disease in California
Abstract
1. Introduction
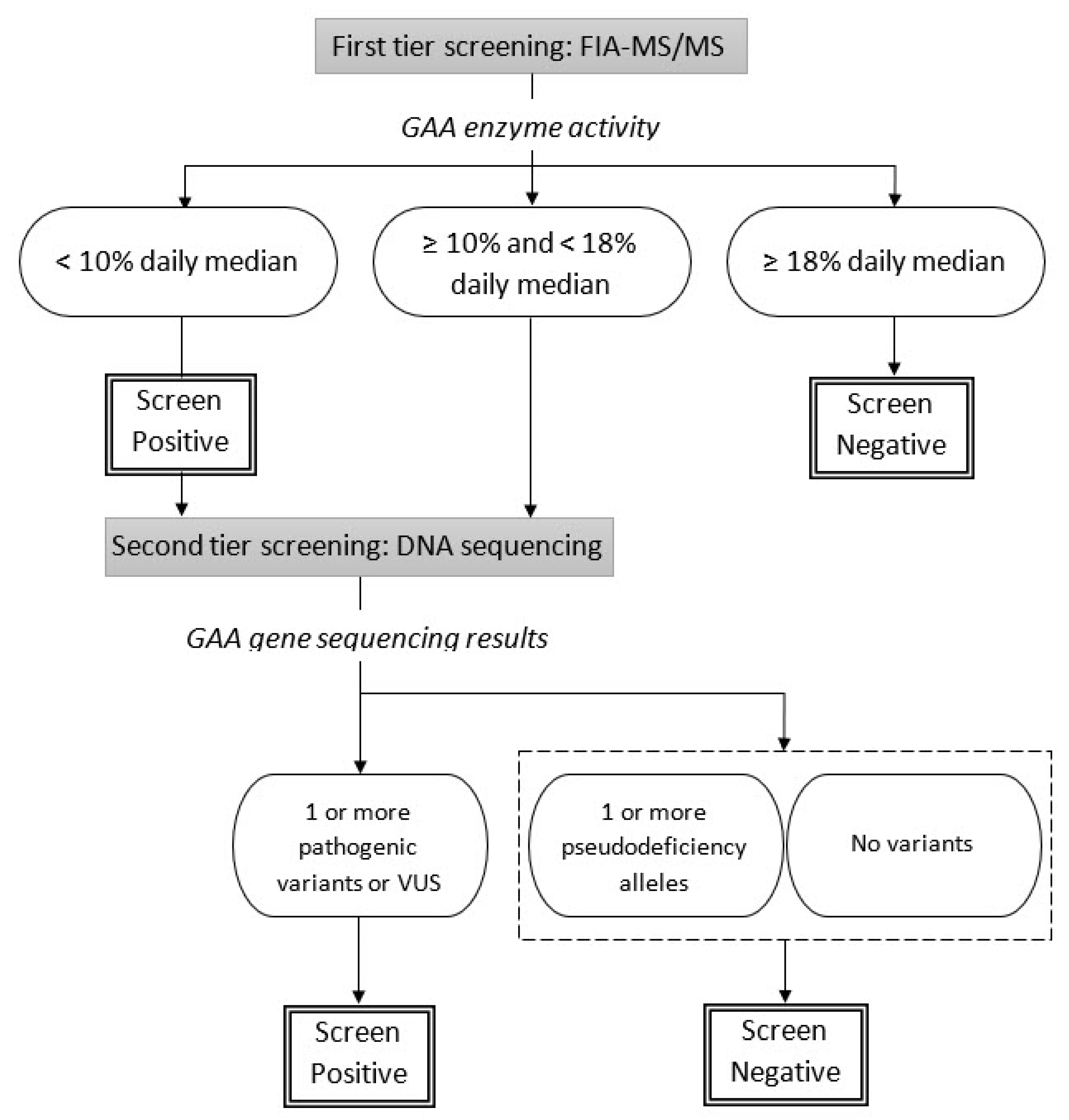
2. Materials and Methods
3. Results
3.1. Birth Prevalence
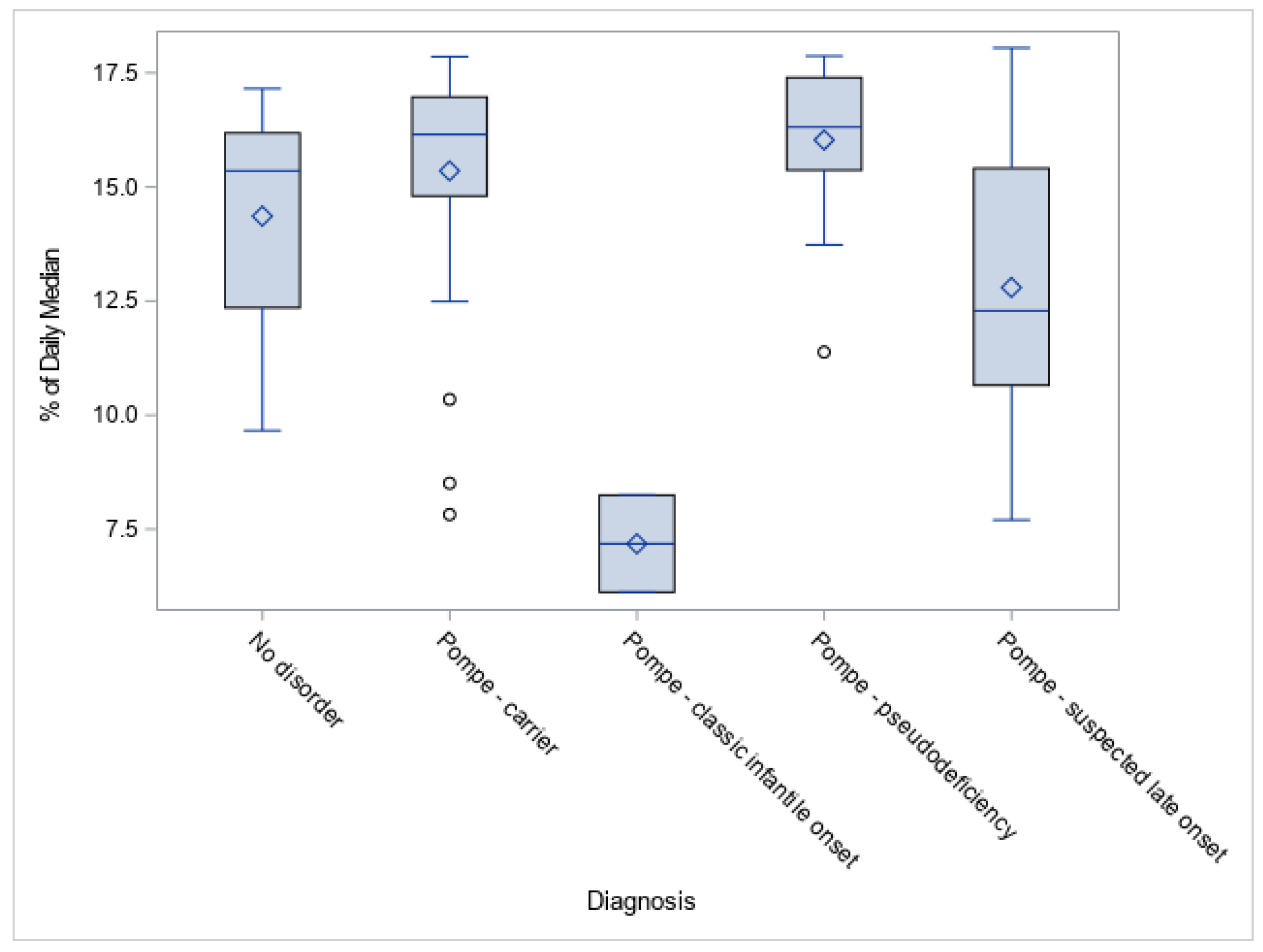
3.2. GAA Activities and Pompe Disease Diagnosis
3.3. GAA Gene Mutations and Allelic Frequency
3.4. Diagnosed Cases and Case Study of IOPD Patients
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reuser, A.J.J.; Hirschhorn, R.; Kroos, M.A. Pompe Disease: Glycogen storage disease type II: Acid α-glucosidase (acid maltase) deficiency. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D., Antonarakis, S., Ballabio, A., Eds.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Martiniuk, F.; Chen, A.; Mack, A.; Arvanitopoulos, E.; Chen, Y.; Rom, W.N.; Codd, W.J.; Hanna, B.; Alcabes, P.; Raben, N.; et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am. J. Med. Genet. 1998, 79, 69–72. [Google Scholar] [CrossRef]
- Bashan, N.; Potashnik, R.; Barash, V.; Gutman, A.; Moses, S.W. Glycogen storage disease type II in Israel. Isr. J. Med. Sci. 1988, 24, 224–227. [Google Scholar]
- Chiang, S.C.; Hwu, W.L.; Lee, N.C.; Hsu, L.W.; Chien, Y.H. Algorithm for Pompe disease newborn screening: Results from the Taiwan screening program. Mol. Genet. Metab. 2012, 106, 281–286. [Google Scholar] [CrossRef]
- Scott, C.R.; Elliott, S.; Buroker, N.; Thomas, L.I.; Keutzer, J.; Glass, M.; Gelb, M.H.; Turecek, F. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J. Pediatr. 2013, 163, 498–503. [Google Scholar] [CrossRef]
- Chien, Y.H.; Lee, N.C.; Huang, H.J.; Thurberg, B.L.; Tsai, F.J.; Hwu, W.L. Later-onset Pompe disease: Early detection and early treatment initiation enabled by newborn screening. J. Pediatr. 2011, 158, 1023–1027. [Google Scholar] [CrossRef]
- Dasouki, M.; Jawdat, O.; Almadhoun, O.; Pasnoor, M.; McVey, A.L.; Abuzinadah, A.; Herbelin, L.; Barohn, R.J.; Dimachkie, M.M. Pompe disease: Literature review and case series. Neurol. Clin. 2014, 32, 751–776. [Google Scholar] [CrossRef]
- Van den Hout, H.M.; Hop, W.; van Diggelen, O.P.; Smeitink, J.A.; Smit, G.P.; Poll-The, B.T.; Bakker, H.D.; Loonen, M.C.; de Klerk, J.B.; Reuser, A.J.; et al. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics 2003, 112, 332–340. [Google Scholar] [CrossRef]
- Umapathysivam, K.; Hopwood, J.J.; Meikle, P.J. Correlation of acid alpha-glucosidase and glycogen content in skin fibroblasts with age of onset in Pompe disease. Clin. Chim. Acta 2005, 361, 191–198. [Google Scholar] [CrossRef]
- Chien, Y.H.; Hwu, W.L.; Lee, N.C. Pompe disease: Early diagnosis and early treatment make a difference. Pediatr. Neonatol. 2013, 54, 219–227. [Google Scholar] [CrossRef]
- Lai, C.J.; Hsu, T.R.; Yang, C.F.; Chen, S.J.; Chuang, Y.C.; Niu, D.M. Cognitive development in infantile-Onset Pompe disease under very early enzyme replacement therapy. J. Child. Neurol. 2016, 31, 1617–1621. [Google Scholar] [CrossRef]
- Tarnopolsky, M.; Katzberg, H.; Petrof, B.J.; Sirrs, S.; Sarnat, H.B.; Myers, K.; Dupre, N.; Dodig, D.; Genge, A.; Venance, S.L.; et al. Pompe disease: Diagnosis and management. Evidence-based guidelines from a Canadian expert panel. Can. J. Neurol. Sci. 2016, 43, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Kronn, D.F.; Day-Salvatore, D.; Hwu, W.L.; Jones, S.A.; Nakamura, K.; Okuyama, T.; Swoboda, K.J.; Kishnani, P.S. Pompe Disease Newborn Screening Working Group. Management of confirmed newborn-Screened patients with Pompe disease across the disease spectrum. Pediatrics 2017, 140, S24–S45. [Google Scholar]
- Ortolano, R.; Baronio, F.; Masetti, R.; Prete, A.; Cassio, A.; Pession, A. Letter to the Editors: Concerning “Divergent clinical outcomes of alphaglucosidase enzyme replacement therapy in two siblings with infantile-onset Pompe disease treated in the symptomatic or pre-symptomatic state” by Takashi M et al. Mol. Genet. Metab. Rep. 2017, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Umapathysivam, K.; Whittle, A.M.; Ranieri, E.; Bindloss, C.; Ravenscroft, E.M.; van Diggelen, O.P.; Hopwood, J.J.; Meikle, P.J. Determination of acid alpha-Glucosidase protein: Evaluation as a screening marker for Pompe disease and other lysosomal storage disorders. Clin. Chem. 2000, 46, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.H.; Turecek, F.; Scott, C.R.; Chamoles, N.A. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J. Inherit. Metab. Dis. 2006, 29, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Spacil, Z.; Tatipaka, H.; Barcenas, M.; Scott, C.R.; Turecek, F.; Gelb, M.H. High-Throughput assay of 9 lysosomal enzymes for newborn screening. Clin. Chem. 2013, 59, 502–511. [Google Scholar] [CrossRef]
- Gucciardi, A.; Legnini, E.; Di Gangi, I.M.; Corbetta, C.; Tomanin, R.; Scarpa, M.; Giordano, G. A column-Switching HPLC-MS/MS method for mucopolysaccharidosis type I analysis in a multiplex assay for the simultaneous newborn screening of six lysosomal storage disorders. Biomed. Chromatogr. 2014, 28, 1131–1139. [Google Scholar] [CrossRef]
- Tortorelli, S.; Turgeon, C.T.; Gavrilov, D.K.; Oglesbee, D.; Raymond, K.M.; Rinaldo, P.; Matern, D. Simultaneous testing for 6 lysosomal storage disorders and x-Adrenoleukodystrophy in dried blood spots by tandem mass spectrometry. Clin. Chem. 2016, 62, 1248–1254. [Google Scholar] [CrossRef]
- Chien, Y.H.; Chiang, S.C.; Zhang, X.K.; Keutzer, J.; Lee, N.C.; Huang, A.C.; Chen, C.A.; Wu, M.H.; Huang, P.H.; Tsai, F.J.; et al. Early detection of Pompe disease by newborn screening is feasible: Results from the Taiwan screening program. Pediatrics 2008, 122, e39–e45. [Google Scholar] [CrossRef]
- Burton, B.K. Newborn screening for Pompe disease: An update, 2011. Am. J. Med. Genet. C. Semin. Med. Genet. 2012, 160C, 8–12. [Google Scholar] [CrossRef]
- Momosaki, K.; Kido, J.; Yoshida, S.; Sugawara, K.; Miyamoto, T.; Inoue, T.; Okumiya, T.; Matsumoto, S.; Endo, F.; Hirose, S.; et al. Newborn screening for Pompe disease in Japan: Report and literature review of mutations in the GAA gene in Japanese and Asian patients. J. Hum. Genet. 2019, 64, 741–755. [Google Scholar] [CrossRef]
- Wittmann, J.; Karg, E.; Turi, S.; Legnini, E.; Wittmann, G.; Giese, A.K.; Lukas, J.; Golnitz, U.; Klingenhager, M.; Bodamer, O.; et al. Newborn screening for lysosomal storage disorders in hungary. JIMD Rep. 2012, 6, 117–125. [Google Scholar] [PubMed]
- Kemper, A.R. Condition Review Workgroup Evidence Report: Newborn Screening for Pompe Disease. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/rusp/previous-nominations/pompe-external-evidence-review-report-2013.pdf (accessed on 14 November 2019).
- Advisory Committee on Heritable Disorders in Newborns and Children. Recommended Uniform Screening Panel [As of July 2018]. Available online: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendedpanel (accessed on 14 November 2019).
- The Newborn Screening Technical Assistance and Evaluation Program (NewSTEPs), Association of Public Health Laboratories. Disorders Screening Status Map: Pompe. Available online: https://www.newsteps.org/resources/newborn-screening-status-all-disorders (accessed on 14 November 2019).
- California State Legislature. Senate Bill No.1095: Newborn Screening Program. 2016. Available online: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201520160SB1095 (accessed on 14 November 2019).
- Califorina Health and Safety Code, Sections 125001, amended 2016. Available online: https://leginfo.legislature.ca.gov/faces/codes_displayText.xhtml?lawCode=HSC&division=106.&title=&part=5.&chapter=1.&article=2 (accessed on 19 December 2019).
- Bronstein, M.G.; Pan, R.J.; Dant, M.; Lubin, B. Leveraging evidence-Based public policy and advocacy to advance newborn screening in California. Pediatrics 2019, 143, e20181886. [Google Scholar] [CrossRef] [PubMed]
- The Newborn Screening Technical Assistance and Evaluation Program (NewSTEPs), Association of Public Health Laboratories. NewSTEPs Data Repository Case Definition. Available online: https://www.newsteps.org/case-definitions (accessed on 21 November 2019).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendationof the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Nino, M.Y.; in’t Groen, S.L.M.; Bergsma, A.J.; van der Beek, N.A.M.E.; Kroos, M.; Hoogeveen-Westerveld, M.; van der Ploeg, A.T.; Pijnappel, W.W.M.P. Extension of the Pompe mutation database by linking disease-Associated variants to clinical severity. Hum. Mutat. 2019, 40, 1954–1967. [Google Scholar] [CrossRef] [PubMed]
- Reuser, A.J.J.; van der Ploeg, A.T.; Chien, Y.H.; Llerena, J., Jr.; Abbott, M.A.; Clemens, P.R.; Kimonis, V.E.; Leslie, N.; Maruti, S.S.; Sanson, B.J.; et al. GAA variants and phenotypes among 1,079 patients with Pompe disease: Data from the Pompe Registry. Hum. Mut. 2019, 40, 2146–2164. [Google Scholar] [CrossRef]
- Herzog, A.; Hartung, R.; Reuser, A.J.; Hermanns, P.; Runz, H.; Karabul, N.; Gokce, S.; Pohlenz, J.; Kampmann, C.; Lampe, C.; et al. A cross-Sectional single-centre study on the spectrum of Pompe disease, German patients: Molecular analysis of the GAA gene, manifestation and genotype-phenotype correlations. Orphanet J. Rare Dis. 2012, 7, 35. [Google Scholar] [CrossRef]
- Burrow, T.A.; Bailey, L.A.; Kinnett, D.G.; Hopkin, R.J. Acute progression of neuromuscular findings in infantile Pompe disease. Pediatr. Neurol. 2010, 42, 455–458. [Google Scholar] [CrossRef]
- Nazari, F.; Sinaei, F.; Nilipour, Y.; Fatehi, F.; Streubel, B.; Ashrafi, M.R.; Aryani, O.; Nafissi, S. Late-Onset pompe disease in Iran: A clinical and genetic report. Muscle Nerve 2017, 55, 835–840. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-Coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Ausems, M.G.; Verbiest, J.; Hermans, M.P.; Kroos, M.A.; Beemer, F.A.; Wokke, J.H.; Sandkuijl, L.A.; Reuser, A.J.; van der Ploeg, A.T. Frequency of glycogen storage disease type II in The Netherlands: Implications for diagnosis and genetic counselling. Eur. J. Hum. Genet. 1999, 7, 713–716. [Google Scholar] [CrossRef]
- Elenga, N.; Verloes, A.; Mrsic, Y.; Basurko, C.; Schaub, R.; Cuadro-Alvarez, E.; Kom-Tchameni, R.; Carles, G.; Lambert, V.; Boukhari, R.; et al. Incidence of infantile Pompe disease in the Maroon population of French Guiana. BMJ Paediatr. Open 2018, 2, e000182. [Google Scholar] [CrossRef] [PubMed]
- Pruniski, B.; Lisi, E.; Ali, N. Newborn screening for Pompe disease: Impact on families. J. Inherit. Metab. Dis. 2018, 41, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Peruzzo, P.; Pavan, E.; Dardis, A. Molecular genetics of Pompe disease: A comprehensive overview. Ann. Transl. Med. 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Kroos, M.A.; Mullaart, R.A.; Van Vliet, L.; Pomponio, R.J.; Amartino, H.; Kolodny, E.H.; Pastores, G.M.; Wevers, R.A.; Van der Ploeg, A.T.; Halley, D.J.; et al. p.[G576S.; E689K]: Pathogenic combination or polymorphism in Pompe disease? Eur. J. Hum. Genet. 2008, 16, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, J.H.; Park, H.J.; Kim, S.Z.; Jeon, Y.M.; Kim, H.K.; Kim, D.S.; Choi, Y.C. Targeted population screening of late onset Pompe disease in unspecified myopathy patients for Korean population. Neuromuscul. Disord. 2017, 27, 550–556. [Google Scholar] [CrossRef]
- Becker, J.A.; Vlach, J.; Raben, N.; Nagaraju, K.; Adams, E.M.; Hermans, M.M.; Reuser, A.J.; Brooks, S.S.; Tifft, C.J.; Hirschhorn, R.; et al. The African origin of the common mutatoin in African American patients with glycogen-Storage disease type II. Am. J. Hum. Genet. 1998, 62, 991–994. [Google Scholar] [CrossRef]
- Liao, H.C.; Chan, M.J.; Yang, C.F.; Chiang, C.C.; Niu, D.M.; Huang, C.K.; Gelb, M.H. Mass spectrometry but not fluorimetry distinguisheds affected and pseudodeficiencies in newborn screening for Pompe disease. Clin. Chem. 2017, 63, 1271–1277. [Google Scholar] [CrossRef]
- Feuchtbaum, L.; Yang, J.; Currier, R. Follow-Up status during the first 5 years of life for metabolic disorders on the federal Recommended Uniform Screening Panel. Genet. Med. 2018, 20, 831–839. [Google Scholar] [CrossRef]
- Kemper, A.R.; Boyle, C.A.; Brosco, J.P.; Grosse, S.D. Ensuring the life-Span benefits of newborn screening. Pediatrics 2019, 144, e20190904. [Google Scholar] [CrossRef]
- Yang, C.F.; Yang, C.C.; Liao, H.C.; Huang, L.Y.; Chiang, C.C.; Ho, H.C.; Lai, C.J.; Chu, T.H.; Yang, T.F.; Hsu, T.R.; et al. Very early treatment for infantile-Onset Pompe disease contributes to better outcomes. J. Pediatr. 2016, 169, 174–180. [Google Scholar] [CrossRef]
| Diagnosis | Mutation Status | Symptoms | Long-Term Follow-Up |
|---|---|---|---|
| Pompe–classic infant onset (with cardiac involvement) * | Pathogenic/likely pathogenic/VUS alleles ** ≥ 2 | Yes, with positive cardiac involvement | Yes |
| Pompe–non-classic infant onset (without cardiac involvement) * | Pathogenic/likely pathogenic/VUS alleles ** ≥ 2 | Yes, without positive cardiac involvement | Yes |
| Pompe–late onset Pompe disease * | Pathogenic/likely pathogenic/VUS alleles ** ≥ 2 | No | Yes |
| Pompe–not otherwise specified * | Pathogenic/likely pathogenic/VUS alleles ** ≥ 2 | No | Yes |
| Pompe–carrier | Pathogenic/likely pathogenic/VUS alleles = 1 | No | No |
| Pompe–pseudodeficiency | Pseudodeficiency alleles | No | No |
| No disorder | No mutation found | No | No |
| Classic Infantile-Onset | Suspected Late-Onset | Carrier | Pseudo-Deficiency | No Disorder | Overall | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 1 | 5 | 14 | 11 | 6 | 37 |
| Male | 1 | 11 | 20 | 9 | 10 | 51 |
| Nursery | ||||||
| NICU | 2 | 1 | 4 | 11 | 14 | 32 |
| Non-NICU | 0 | 15 | 30 | 9 | 2 | 56 |
| Maturity | ||||||
| Premature | 1 | 2 | 4 | 8 | 2 | 17 |
| Full term | 1 | 14 | 30 | 12 | 14 | 71 |
| Total | 2 | 16 | 34 | 20 | 16 | 88 |
| Birth prevalence | 5/1,000,000 | 36/1,000,000 | 75/1,000,000 | 45/1,000,000 | ||
| (1 in 226,600) | (1 in 28,300) | (1 in 13,300) | (1 in 22,700) |
| Mutation Name | Count |
|---|---|
| Pathogenic variant | |
| c.-32-13T>G | 18 |
| c.[752C>T;761C>T] * | 8 |
| c.2238G>C, c.1099T>C, c.1799G>A, c.1437+1G>A, c.1548G>A, c.1579delA, c.1754+1_1754+12delinsCCA, c.1856G>A, c.1933G>A, c.1935C>A, c.1979G>A, c.2297A>C *, c.2408_2426del19, c.2560C>T, c.2646+2T>A, c.29delA, c.511del, c.546G>A, c.546G>C, c.573C>A, c.670C>T, c.925G>A | <5 |
| Subtotal | 52 |
| Pseudodeficiency allele | |
| c.[1726G>A;2065G>A] | 42 |
| c.2065G>A | 5 |
| c.271G>A | 5 |
| Subtotal | 52 |
| Variant of uncertain significance | |
| c.1048G>A, c.1019A>G, c.1357G>A, c.1375G>A, c.1392_1393delinsTT, c.1477C>T, c.1757C>T, c.2221G>A, c.2261C>T, c.265C>T, c.266G>A **, c.316C>T, c.546+5G>T, c.726G>A, c.868A>G | <3 |
| Subtotal | 16 |
| Total | 120 |
| Pathogenic | Pseudodeficiency Allele | Uncertain Significance | ||||
|---|---|---|---|---|---|---|
| Race/Ethnicity | Count | Allele Frequency | Count | Allele Frequency | Count | Allele Frequency |
| African American (n = 37,340) | 6 | 161/1,000,000 (1 in 6200) | 4 | 107/1,000,000 (1 in 9300) | 3 | 80/1,000,000 (1 in 12,500) |
| Asian/Pacific Islander (API, n = 69,510) | 15 | 216/1,000,000 (1 in 4600) | 30 | 432/1,000,000 (1 in 2300) | 6 | 86/1,000,000 (1 in 11,600) |
| Hispanic (n = 214,049) | 14 | 66/1,000,000 (1 in 15,300) | 7 | 33/1,000,000 (1 in 30,600) | 5 | 23/1,000,000 (1 in 42,800) |
| White (n = 115,281) | 17 | 148/1,000,000 (1 in 6800) | 11 | 95/1,000,000 (1 in 10,480) | 2 | 17/1,000,000 (1 in 57,600) |
| Diagnosis | Number of Cases | Mutation Status |
|---|---|---|
| Pompe—classic infantile onset | 1 | Pathogenic, homozygous |
| 1 | Pathogenic & pathogenic | |
| Pompe—suspected late onset | 3 | Pathogenic, homozygous |
| 7 | Pathogenic & Pathogenic/likely pathogenic | |
| 4 | Pathogenic & VUS | |
| 1 | VUS, homozygous | |
| 1 | VUS & VUS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Feuchtbaum, L.; Sciortino, S.; Matteson, J.; Mathur, D.; Bishop, T.; Olney, R.S. The First Year Experience of Newborn Screening for Pompe Disease in California. Int. J. Neonatal Screen. 2020, 6, 9. https://doi.org/10.3390/ijns6010009
Tang H, Feuchtbaum L, Sciortino S, Matteson J, Mathur D, Bishop T, Olney RS. The First Year Experience of Newborn Screening for Pompe Disease in California. International Journal of Neonatal Screening. 2020; 6(1):9. https://doi.org/10.3390/ijns6010009
Chicago/Turabian StyleTang, Hao, Lisa Feuchtbaum, Stanley Sciortino, Jamie Matteson, Deepika Mathur, Tracey Bishop, and Richard S. Olney. 2020. "The First Year Experience of Newborn Screening for Pompe Disease in California" International Journal of Neonatal Screening 6, no. 1: 9. https://doi.org/10.3390/ijns6010009
APA StyleTang, H., Feuchtbaum, L., Sciortino, S., Matteson, J., Mathur, D., Bishop, T., & Olney, R. S. (2020). The First Year Experience of Newborn Screening for Pompe Disease in California. International Journal of Neonatal Screening, 6(1), 9. https://doi.org/10.3390/ijns6010009





