Benzene Derivatives from Ink Lead to False Positive Results in Neonatal Hyperphenylalaninemia Screening with Ninhydrin Fluorometric Method
Abstract
1. Introduction
- (a) if there were changes in transport and storage conditions of specimen collection devices;
- (b) if there were changes in the care of neonates before blood sampling, for example, feeding special dietary supplements, intravenous infusion of nutritional ingredients, medication, etc.;
- (c) if there were changes in the environment in which blood specimens were dried, for example, using of chemical reagents for sanitization or air freshener;
- (d) if there were changes in the conditions for DBS storage before and during transport to the screening laboratory.
2. Samples and Methods
2.1. Samples
2.2. Screening Procedures
2.3. Fluorometric Tests of DBS Collection Devices for Contaminants
2.4. Tandem Mass Spectrometry
2.5. UV–Visible Spectrometry
2.6. HPLC
2.7. Gas Chromatography–Mass Spectrometry (GC-MS)
3. Results
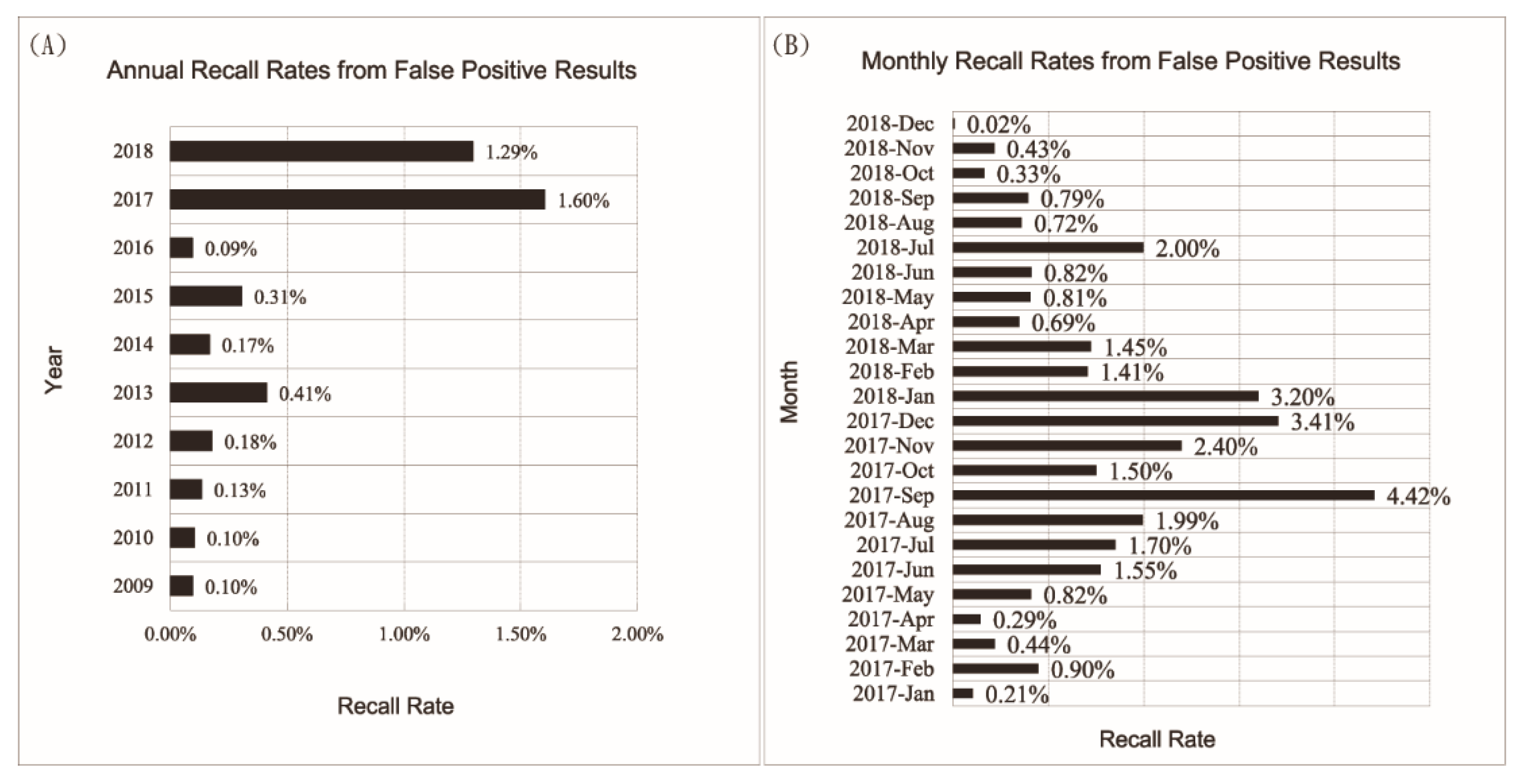
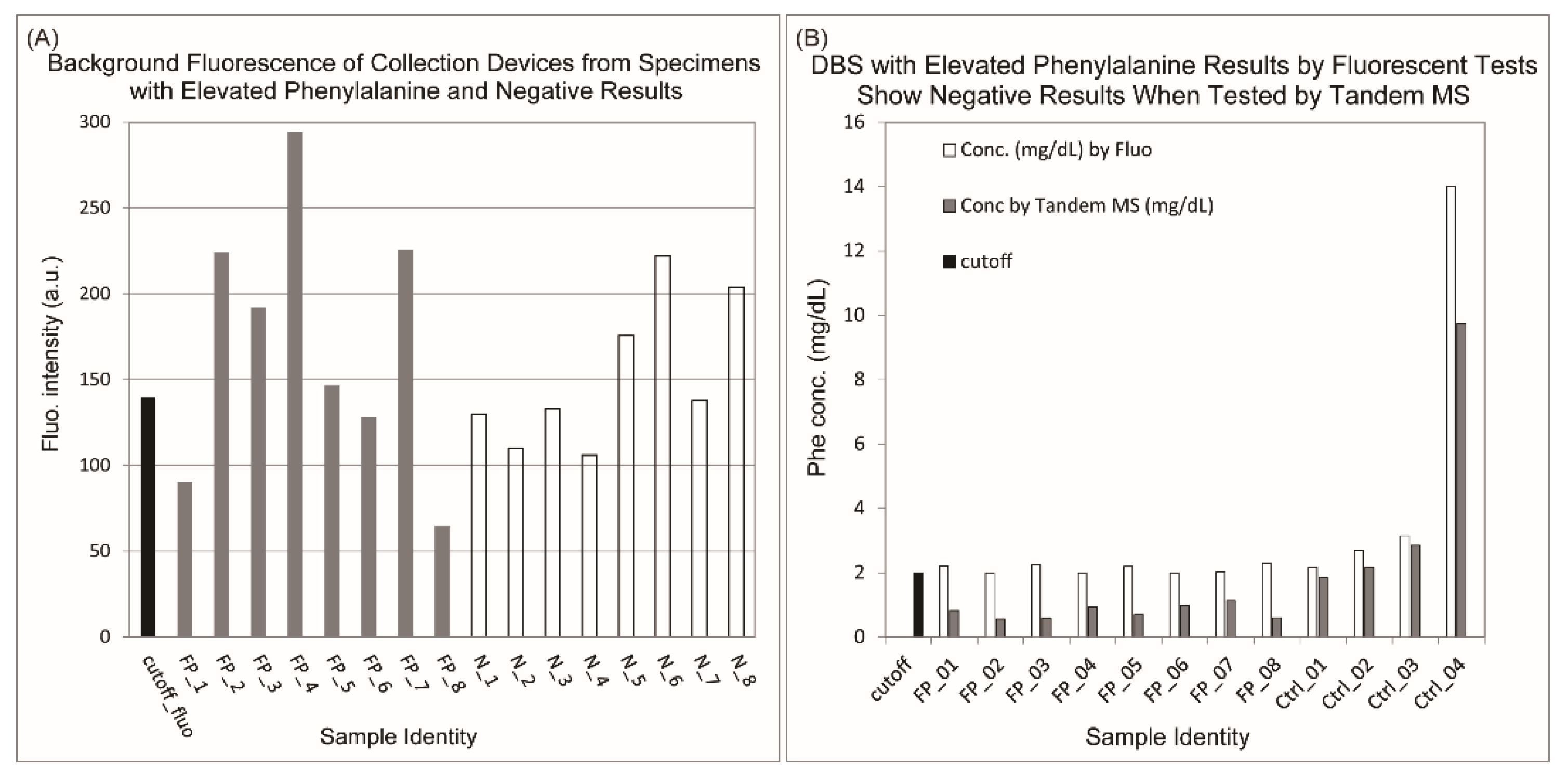
3.1. Increased False Positive Cases Were Due to Contamination of Specimen Collection Devices by Chemicals Other Than Phenylalanine
3.2. Higher Concentration of Contaminants Was Observed from Ink Circles on the Collection Devices
3.3. Absorption Peak around 245 nm Was Only Observed on Samples from Contaminated Collection Devices, Which May Be Represented by Two Elution Peaks Specific for Contaminated Collection Devices on HPLC Chromatograms
3.4. Major Contaminants from Ink Circles Were Identified by GC-MS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blau, N. Genetics of Phenylketonuria: Then and Now. Hum. Mutat. 2016, 37, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.; Wolfgart, E.; Weglage, J.; Rutsch, F. Sapropterin treatment does not enhance the health-related quality of life of patients with phenylketonuria and their parents. Acta Paediatr. 2017, 106, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Jahja, R.; Huijbregts, S.C.J.; de Sonneville, L.M.J.; van der Meere, J.J.; Legemaat, A.M.; Bosch, A.M.; Hollak, C.E.M.; Rubio-Gozalbo, M.; Estela Brouwers, M.C.G.J.; Hofstede, F.C.; et al. Cognitive profile and mental health in adult phenylketonuria: A PKU-COBESO study. Neuropsychology 2017, 31, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.C.; MacDonald, A. Dietary intervention in the management of phenylketonuria: Current perspectives. Pediatric Health Med. Ther. 2016, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, G.; Scholz, R.; Ullrich, K.; Santer, R.; Rune, G.M. Phenylketonuria: Direct and indirect effects of phenylalanine. Exp. Neurol. 2016, 281, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chace, D.H.; De Jesús, V.R.; Haynes, C.A. Analytical Perspectives on the Use of Dried Blood Spots and Mass Spectrometry in Newborn Screening. Encycl. Anal. Chem. 2015, 1–26. [Google Scholar] [CrossRef]
- CLSI. Blood Collection on Filter Papers for Neonatal Screening Programs: Approved Standard, 6th ed.; NBS01-A6; Clinical and Laboratory Standard Institute: Wayne, NY, USA, 2013. [Google Scholar]
- Mei, L.; Song, P.; Xu, L. Newborn screening and related policy against Phenylketonuria in China. Intractable Rare Dis. Res. 2013, 2, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Cai, J.; Shi, X. Newborn screening for inborn errors of metabolism in Beijing, China: 22 years of experience. J. Med. Screen. 2011, 18, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Song, P.; Kokudo, N.; Xu, L.; Tang, W. Current situation and prospects of newborn screening and treatment for Phenylketonuria in China—Compared with the current situation in the United States, UK and Japan. Intractable Rare Dis. Res. 2013, 2, 106–114. [Google Scholar] [CrossRef] [PubMed]
- McCaman, M.W.; Robins, E. Fluorimetric method for the determination of phenylalanine in serum. J. Lab. Clin. Med. 1962, 59, 885–890. [Google Scholar]
- Gerasimova, N.S.; Steklova, I.V.; Tuuminen, T. Fluorometric method for phenylalanine microplate assay adapted for phenylketonuria screening. Clin. Chem. 1989, 35, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, N.S.; Samutin, A.A.; Steklova, I.V.; Tuuminen, T. Phenylketonuria screening in Moscow using a microplate fluorometric method. Screening 1992, 1, 27–35. [Google Scholar] [CrossRef]
- Tuuminen, T. Changing of critical variables in the fluorometric microplate-based method for phenylketonuria screening. Clin. Chem. 1992, 38, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.R. The NIST/EPA/MSDC mass spectral database, personal computer versions 1.0 and 2.0. Chemom. Intell. Lab. Syst. 1990, 8, 3–5. [Google Scholar] [CrossRef]
- Udenfriend, S. Development of a new fluorescent reagent and its application to the automated assay of amino acids and peptides at the picomole level. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1972, 76A, 637. [Google Scholar] [CrossRef]
- Zobel, S. Annual Summary Report. Centers for Disease Control and Prevention. e. 2017. Available online: https://www.cdc.gov/labstandards/nsqap_reports.html (accessed on 5 August 2019).


| Background (80% Ethanol) | Extracted Contaminants | ||
|---|---|---|---|
| CAS# | Portion(%) | CAS# | Portion(%) |
| 538-24-9 | 36.56 | 606-28-0 | 38.6 |
| 6114-18-7 | 32.42 | 106-90-1 | 11.94 |
| 111-61-5 | 8.75 | 57-10-3 | 8.34 |
| 77-90-7 | 8.53 | 57-11-1 | 6.87 |
| 628-97-7 | 3.37 | 2156-97-0 | 6.7 |
| 6848-50-0 | 2.89 | 77-90-7 | 6.61 |
| 7098-22-8 | 2.35 | 13048-33-4 | 4.52 |
| 7459-33-8 | 1.82 | 629-96-9 | 4.06 |
| 1560-84-5 | 1.51 | 111-61-5 | 3.97 |
| 1560-96-9 | 1.42 | 96-76-4 | 3.45 |
| 1560-88-9 | 1.38 | 628-97-7 | 2.33 |
| -- -- | -- -- | 112-80-1 | 1.24 |
| -- -- | -- -- | 119-61-9 | 0.72 |
| -- -- | -- -- | 137-89-3 | 0.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Mei, J.; Yang, L.; Luo, P.; Wang, X.; Wang, Y.; Yao, J.; Cui, L.; Pan, L.; Wang, Z.; et al. Benzene Derivatives from Ink Lead to False Positive Results in Neonatal Hyperphenylalaninemia Screening with Ninhydrin Fluorometric Method. Int. J. Neonatal Screen. 2020, 6, 14. https://doi.org/10.3390/ijns6010014
Feng S, Mei J, Yang L, Luo P, Wang X, Wang Y, Yao J, Cui L, Pan L, Wang Z, et al. Benzene Derivatives from Ink Lead to False Positive Results in Neonatal Hyperphenylalaninemia Screening with Ninhydrin Fluorometric Method. International Journal of Neonatal Screening. 2020; 6(1):14. https://doi.org/10.3390/ijns6010014
Chicago/Turabian StyleFeng, Shuren, Joanne Mei, Lu Yang, Ping Luo, Xiaonan Wang, Yuan Wang, Jingyi Yao, Lan Cui, Lei Pan, Zefang Wang, and et al. 2020. "Benzene Derivatives from Ink Lead to False Positive Results in Neonatal Hyperphenylalaninemia Screening with Ninhydrin Fluorometric Method" International Journal of Neonatal Screening 6, no. 1: 14. https://doi.org/10.3390/ijns6010014
APA StyleFeng, S., Mei, J., Yang, L., Luo, P., Wang, X., Wang, Y., Yao, J., Cui, L., Pan, L., Wang, Z., & Xin, L. (2020). Benzene Derivatives from Ink Lead to False Positive Results in Neonatal Hyperphenylalaninemia Screening with Ninhydrin Fluorometric Method. International Journal of Neonatal Screening, 6(1), 14. https://doi.org/10.3390/ijns6010014






