Cystic Fibrosis Newborn Screening in Portugal: PAP Value in Populations with Stringent Rules for Genetic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Population in the Study
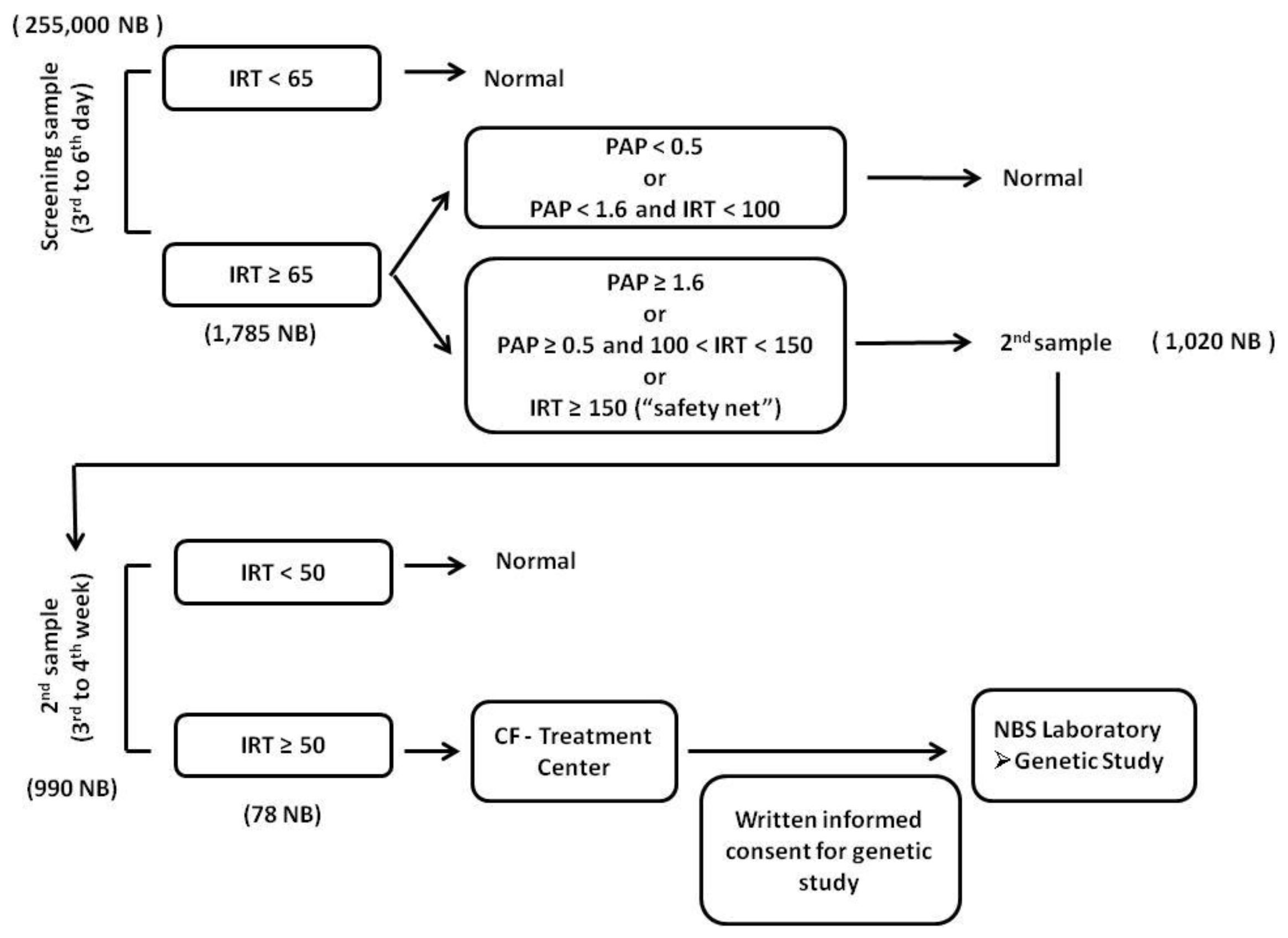
2.2. CF-NBS Algorithm
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARMS | Amplification-Refractory Mutation System |
| CF | Cystic Fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CFSPID | Cystic fibrosis screen positive with an inconclusive diagnosis |
| DBS | dry blood spots |
| FE | fecal elastase |
| IRT | Immunoreactive trypsinogen |
| NB | newborn |
| NBS | Newborn screening |
| PAP | Pancreatitis associated protein |
| PI | pancreatic insufficient |
| PS | pancreatic sufficient |
| SCT | Sweat chloride (Cl−) test |
| PPV | Positive predictive value |
References
- Riordan, J.R. The cystic fibrosis transmembrane conductance regulator. Annu. Rev. Physiol. 1993, 55, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Zielenski, J.; Tsui, L.C. Cystic fibrosis: Genotypic and phenotypic variations. Annu. Rev. Genet. 1995, 29, 777–807. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.P.; Gregory, R.J.; Thompson, S.; Souza, D.W.; Paul, S.; Mulligan, R.C.; Smith, A.E.; Welsh, M.J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 1991, 253, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Bear, C.E.; Li, C.H.; Kartner, N.; Bridges, R.J.; Jensen, T.J.; Ramjeesingh, M.; Riordan, J.R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 1992, 68, 809–818. [Google Scholar] [CrossRef]
- Welsh, M.J.; Ramsey, B.W.; Accurso, F.; Cutting, G.R. Cystic fibrosis. In Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2013; pp. 5121–5188. [Google Scholar]
- Brennan, M.-L.; Schrijver, I. Cystic Fibrosis A Review of Associated Phenotypes, Use of Molecular Diagnostic Approaches, Genetic Characteristics, Progress, and Dilemmas. J. Mol. Diagn. 2016, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Modifier genes in Mendelian disorders: The example of cystic fibrosis. Ann. N. Y. Acad. Sci. 2010, 1214, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Sosnay, P.R.; Siklosi, K.R.; Van Goor, F.; Kaniecki, K.; Yu, H.; Sharma, N.; Ramalho, A.S.; Amaral, M.D.; Dorfman, R.; Zielenski, J.; et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat. Genet. 2013, 45, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Cuppens, H.; Macek, M., Jr.; Cassiman, J.J.; Kerem, E.; Durie, P.; Tullis, E.; Assael, B.M.; Bombieri, C.; Brown, A.; et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J. Cyst. Fibros. 2008, 7, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, J.L.; Macek, M., Jr.; Fine, J.P.; Farrell, P.M. Cystic fibrosis: A worldwide analysis of CFTR mutations—Correlation with incidence data and application to screening. Hum. Mutat. 2002, 19, 575–606. [Google Scholar] [CrossRef] [PubMed]
- Lucotte, G.; Hazout, S.; De Braekeleer, M. Complete map of cystic fibrosis mutation DF508 frequencies in Western Europe and correlation between mutation frequencies and incidence of disease. Hum. Biol. 1995, 67, 797–803. [Google Scholar] [PubMed]
- Jennings, M.T.; Dezube, R.; Paranjape, S.; West, N.E.; Hong, G.; Braun, A.; Grant, J.; Merlo, C.A.; Lechtzin, N. An Observational Study of Outcomes and Tolerances in Patients with Cystic Fibrosis Initiated on Lumacaftor/Ivacaftor. Ann. Am. Thorac. Soc. 2017, 141, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Amaral, M.D. Progress in therapies for cystic fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Farrell, P.M.; Kosorok, M.R.; Rock, M.J.; Laxova, A.; Zeng, L.; Lai, H.C.; Hoffman, G.; Laessig, R.H.; Splaingard, M.L.; et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics 2001, 107, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Lai, H.J.; Li, Z.; Kosorok, M.R.; Laxova, A.; Green, C.G.; Collins, J.; Hoffman, G.; Laessig, R.; Rock, M.J.; Splaingard, M.L. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: Enough is enough! J. Pediatr. 2005, 147 (Suppl. 3), S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Massie, J.; Sontag, M.; Southern, K.W. Newborn screening for cystic fibrosis. Lancet Respir. Med 2016, 4, 653–661. [Google Scholar] [CrossRef]
- Farrell, P.M.; Sommerburg, O. Toward quality improvement in cystic fibrosis newborn screening: Progress and continuing challenges. J. Cyst. Fibros. 2016, 15, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Döring, G. Cystic fibrosis. Lancet, 2003; 361, 681–689. [Google Scholar]
- Farrell, P.M.; Rosenstein, B.J.; White, T.B.; Accurso, F.J.; Castellani, C.; Cutting, G.R.; Durie, P.R.; Legrys, V.A.; Massie, J.; Parad, R.B.; et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J. Pediatr. 2008, 153, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Servidoni, M.F.; Vinagre, A.M.; Ramalho, A.S.; Bonadia, L.C.; Felício, V.; Ribeiro, M.A.; Uliyakina, I.; Marson, F.A.; Kmit, A.; et al. Measurements of CFTR-mediated Cl- secretion in human rectal biopsies constitute a robust biomarker for Cystic Fibrosis diagnosis and prognosis. PLoS ONE 2012, 7, e47708. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.; Farrell, P.M. New challenges in the diagnosis and management of cystic fibrosis. J. Pediatr. 2015, 166, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, L.; Rocha, H.; Sousa, C.; Marcão, A.; Fonseca, H.; Bogas, M.; Osório, R.V. Four years of expanded newborn screening in Portugal with tandem mass spectrometry. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 3), S133–S138. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Krulisova, V.; Hammermann, J.; Lindner, M.; Stahl, M.; Muckenthaler, M.; Kohlmueller, D.; Happich, M.; Kulozik, A.E.; Votava, F.; et al. Comparison of different IRT-PAP protocols to screen newborns for cystic fibrosis in three central European populations. J. Cyst. Fibros. 2014, 13, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ferrie, R.M.; Schwarz, M.J.; Robertson, N.H.; Vaudin, S.; Super, M.; Malone, G.; Little, S. Development, multiplexing, and application of ARMS tests for common mutations in the CFTR gene. Am. J. Hum. Genet. 1992, 51, 251–262. [Google Scholar] [PubMed]
- Sarles, J.; Berthézène, P.; Le Louarn, C.; Somma, C.; Perini, J.M.; Catheline, M.; Mirallié, S.; Luzet, K.; Roussey, M.; Farriaux, J.P.; et al. Combining immunoreactive trypsinogen and pancreatitis-associated protein assays, a method of newborn screening for cystic fibrosis that avoids DNA analysis. J. Pediatr. 2005, 147, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Wiley, V. Newborn screening methods for cystic fibrosis. Paediatr. Respir. Rev. 2003, 4, 272–277. [Google Scholar] [CrossRef]
- Vernooij-van Langen, A.M.; Loeber, J.G.; Elvers, B.; Triepels, R.H.; Roefs, J.; Gille, J.J.; Reijntjens, S.; Dompeling, E.; Dankert-Roelse, J.E. The influence of sex, gestational age, birth weight, blood transfusion, and timing of the heel prick on the pancreatitis-associated protein concentration in newborn screening for cystic fibrosis. J. Inherit. Metab. Dis. 2013, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Krulišová, V.; Balaščaková, M.; Skalická, V.; Piskáčková, T.; Holubová, A.; Paděrová, J.; Křenková, P.; Dvořáková, L.; Zemková, D.; Kračmar, P.; et al. Prospective and parallel assessments of cystic fibrosis newborn screening protocols in the Czech Republic: IRT/DNA/IRT versus IRT/PAP and IRT/PAP/DNA. Eur. J. Pediatr. 2012, 171, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Lindner, M.; Muckenthaler, M.; Kohlmueller, D.; Leible, S.; Feneberg, R.; Kulozik, A.E.; Mall, M.A.; Hoffmann, G.F. Initial evaluation of a biochemical cystic fibrosis newborn screening by sequential analysis of immunoreactive trypsinogen and pancreatitis-associated protein (IRT/PAP) as a strategy that does not involve DNA testing in a Northern European population. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 2), S263–S271. [Google Scholar] [CrossRef] [PubMed]
- Weidler, S.; Stopsack, K.H.; Hammermann, J.; Sommerburg, O.; Mall, M.A.; Hoffmann, G.F.; Kohlmüller, D.; Okun, J.G.; Macek, M., Jr.; Votava, F.; et al. A product of immunoreactive trypsinogen and pancreatitis-associated protein as second-tier strategy in cystic fibrosis newborn screening. J. Cyst. Fibros. 2016, 15, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Amaral, M.; Barreto, C.; Pacheco, P.; Lavinha, J. Complex cystic fibrosis allele R334W-R1158X results in reduced levels of correctly processed mRNA in a pancreatic sufficient patient. Hum. Mutat. 1996, 8, 134–139. [Google Scholar] [CrossRef]
- Report of a Joint Meeting of WHO/ECFTN/ICF(M)A/ECFS. The Molecular Genetics Epidemiology of Cystic Fibrosis. Available online: http://www.who.int/genomics/public/geneticdiseases/en/index2.html#CF (accessed on 27 November 2017).
- Pereira, L.; Azevedo, P.; Cavaco, J.; Felix, M.; Gamboa, F.; Amorim, A.; Vaz, L.; Rocha, H.; Goncalves, J.; Freitas, C.; et al. Genetic characterization of cystic fibrosis patients in Portugal. In Proceedings of the 23rd Annual Congress of European Respiratory Society, Barcelona, Spain, 7–11 September 2013. [Google Scholar]
- Lundman, E.; Gaup, H.J.; Bakkeheim, E.; Olafsdottir, E.J.; Rootwelt, T.; Storrøsten, O.T.; Pettersen, R.D. Implementation of newborn screening for cystic fibrosis in Norway. Results from the first three years. J. Cyst. Fibros. 2016, 15, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease. 1968. Available online: http://apps.who.int/iris/handle/10665/37650/17/WHO_PHP_34.pdf (accessed on 27 November 2017).
- Silva, A.; Amorim, A.; Azevedo, P.; Lopes, C.; Gamboa, F. Cystic fibrosis—characterization of the adult population in Portugal. Rev. Port. Pneumol. 2006, 22, 141–145. [Google Scholar] [CrossRef] [PubMed]
| Sampling Day | Birth Weight (g) | Newborn Screening Results | Sweat Test (nmol/L) | Fecal Elastase (µg/g) | CFTR Gene | |||
|---|---|---|---|---|---|---|---|---|
| 1st IRT (ng/mL) | PAP (ng/mL) | 2nd IRT (ng/mL) | Allele 1 | Allele 2 | ||||
| P1 (5, 15) | 3040 | 94 | 5.4 | 158 | 116 | 1 | p.F508del | p.F508del |
| P2 (21, --) | 3375 | 130 | 3.9 | n.d. | 112 | <30 | p.F508del | p.F508del |
| P3 (6, 17) | 2700 | 210 | >8.8 | 287 | 110 | <15 | p.F508del | ? |
| P4 (23, 28) | 3170 | 190 | >8.8 | 195 | 109 | 1 | p.F508del | p.F508del |
| P5 (3, 13) | 2555 | 260 | 4.3 | 344 | 128 | <15 | p.F508del | p.F508del |
| P6 (4, 27) | 2300 | 266 | >8.8 | 360 | 115 | n.a. | p.F508del | p.F508del |
| P7 (5, 15) | 2695 | 443 | >8.8 | 531 | 120 | <15 | p.F508del | p.F508del |
| P8 (2, 5) | 2710 | 101 | >8.8 | 101 | 121 | <30 | p.F508del | p.F508del |
| P9 (4, 30) | 2870 | 110 | 4.6 | 123 | 108 | <15 | p.F508del | p.F508del |
| P10 (3, 16) | 3825 | 414 | >8.8 | 265 | 84 | <15 | p.F508del | p.F508del |
| P11 (5, 19) | 3855 | 238 | 5.2 | 281 | 93 | 107 | p.F508del | p.R792X |
| P12 (4, 21) | 3750 | 241 | 7.8 | 333 | 110 | <15 | p.F508del | p.F508del |
| P13 (5, 21) | 2860 | 305 | 5.1 | 135 | 87 | 28 | p.F508del | p.F508del |
| P14 (5, 18) | 3380 | 156 | 4.7 | 219 | 95 | <30 | p.F508del | Ex 3 total deletion |
| P15 (6, 23) | 3850 | 133 | 7.6 | 157 | 78 | <5 | p.F508del | p.F508del |
| P16 (3, --) | 3438 | 91 | >8.8 | n.d. | 90 | <30 | p.F508del | p.F508del |
| P17 (5, 27) | 3320 | 113 | 5.8 | 133 | 104 | <15 | p.F508del | p.F508del |
| P18 (5, 12) | 2860 | 463 | >8.8 | 624 | 76 | <5 | p.F508del | p.F508del |
| P19 (4, 14) | 1950 | 215 | >8.8 | 155 | 100 | 20 | p.G85E | p.R1066C |
| P20 (4, 12) | n.a. | 255 | >8.8 | 282 | 89 | <10 | p.F508del | p.F508del |
| P21 (3, 17) | 3120 | 259 | >8.8 | 185 | n.a. | n.a. | p.N1303K | p.R1066C |
| P22 (3, 20) | 3325 | 70 | 2.3 | 55 | 70 | 312 | p.F508del | p.V232D |
| P23 (4, 23) | 2560 | 107 | 7.3 | 191 | 78 | 25 | p.F508del | p.F508del |
| P24 (3, 17) | 2500 | 258 | 5.6 | 269 | n.a. | <15 | p.F508del | p.F508del |
| P25 (5, 18) | 2950 | 240 | 9.3 | 244 | 88 | 13 | p.F508del | p.F508del |
| P265, 18) | 3265 | 113 | ≥8.8 | 136 | 83 | <15 | p.F508del | p.F508del |
| P27 (3, 15) | 2760 | 160 | 9.0 | 172 | 122 | 72 | p.F508del | p.N1303K |
| P28 (13, 28) | 2235 | 67 | ≥8.8 | 51 | n.a. | <5 | p.F508del | p.F508del |
| P29 (5, 21) | 3640 | 75 | 1.6 | 123 | 134 | <15 | p.F508del | p.R1162X |
| P30 (4, 15) | 3100 | 397 | ≥8.8 | 306 | 69 | 40 | p.F508del | p.G85E |
| P31 (3, 28) | 3070 | 103 | ≥8.8 | 83 | 88 | <30 | p.F508del | p.F508del |
| P32 (4, --) | 2860 | 202 | 2.7 | n.d. | n.a. | n.a. | p.F508del | p.N1303K |
| P33 (10, 28) | 3840 | 54 | 7.5 | 49 | n.a. | <10 | p.F508del | p.F508del |
| P34 (5, 49) | 3555 | 108 | ≥8.8 | 11 | 110 | <100 | p.G542X | p.G542X |
| Screened newborns (n) | 255,000 |
| Confirmed CF patients (n) | 34 |
| False positives referred for SCT (n) | 46 |
| False negatives, including cases with meconium ileus (n) | 2 |
| Sensitivity, % [IC 95%] | 94.44% [81.85–98.47%] |
| Specificity, % [IC 95%] | 99.98% [99.98–99.99%] |
| PPV, % [IC 95%] | 41.03% [30.78–52.11%] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcão, A.; Barreto, C.; Pereira, L.; Vaz, L.G.; Cavaco, J.; Casimiro, A.; Félix, M.; Silva, T.R.; Barbosa, T.; Freitas, C.; et al. Cystic Fibrosis Newborn Screening in Portugal: PAP Value in Populations with Stringent Rules for Genetic Studies. Int. J. Neonatal Screen. 2018, 4, 22. https://doi.org/10.3390/ijns4030022
Marcão A, Barreto C, Pereira L, Vaz LG, Cavaco J, Casimiro A, Félix M, Silva TR, Barbosa T, Freitas C, et al. Cystic Fibrosis Newborn Screening in Portugal: PAP Value in Populations with Stringent Rules for Genetic Studies. International Journal of Neonatal Screening. 2018; 4(3):22. https://doi.org/10.3390/ijns4030022
Chicago/Turabian StyleMarcão, Ana, Celeste Barreto, Luísa Pereira, Luísa Guedes Vaz, José Cavaco, Ana Casimiro, Miguel Félix, Teresa Reis Silva, Telma Barbosa, Cristina Freitas, and et al. 2018. "Cystic Fibrosis Newborn Screening in Portugal: PAP Value in Populations with Stringent Rules for Genetic Studies" International Journal of Neonatal Screening 4, no. 3: 22. https://doi.org/10.3390/ijns4030022
APA StyleMarcão, A., Barreto, C., Pereira, L., Vaz, L. G., Cavaco, J., Casimiro, A., Félix, M., Silva, T. R., Barbosa, T., Freitas, C., Nunes, S., Felício, V., Lopes, L., Amaral, M., & Vilarinho, L. (2018). Cystic Fibrosis Newborn Screening in Portugal: PAP Value in Populations with Stringent Rules for Genetic Studies. International Journal of Neonatal Screening, 4(3), 22. https://doi.org/10.3390/ijns4030022





