Abstract
The American Academy of Pediatrics (AAP) has endorsed Critical Congenital Heart Disease (CCHD) screening using pulse oximetry nationwide, but, however, acknowledges that altitude may impact failure rates and alternative algorithms may be required at high altitudes. We therefore evaluated a modified screening protocol at an altitude of 6200 feet with the hypothesis that modifications could decrease failure rates. We evaluated 2001 well, newborn infants ≥35 weeks gestation using a modified protocol, which included a lower saturation cutoff for the first screen (85% instead of the AAP recommended 90%) and an oxygen hood intervention between the first two screens. Using our modified screening algorithm, we found a 0.3% failure rate, which was similar to the 0.2% sea-level rate and statistically different from the 1.1% rate identified in a recent study at similar altitude. Had the AAP protocol been used, the failure rate would have increased to 0.8%, which is similar to prior reports near this altitude. Echocardiograms were performed on failing newborns with no CCHD identified. A Birth Defects Registry Database review demonstrated one newborn with CCHD was missed after meeting AAP passing criteria. Overall, this study demonstrates that an alternative algorithm can be implemented at moderate altitude with decreased failure rate and comparable false negative rate.
1. Introduction
Studies in both the United States and Europe have convincingly demonstrated that pulse oximetry performed in late preterm to term newborns after the transition period can substantially reduce the number of newborns discharged from hospitals with undetected critical congenital heart disease (CCHD) [1,2]. Historically, CCHD diagnosis has relied on prenatal imaging and clinical assessment. While there has been an overall increase in the rate of prenatal CCHD diagnosis and a decrease in the rate of delayed diagnosis, recent studies have demonstrated that the prenatal diagnosis rate remains low at <30%, and the rate of delayed diagnosis continues to remain unacceptably high [3,4,5,6]. Pulse oximetry can help to decrease the rate of delayed diagnosis by identifying 12 different CCHD lesions, seven of which are primary targets and five of which are secondary targets of pulse oximetry screening. Primary targets, or those defects which always or almost always present with hypoxemia, include d-transposition of the great arteries, truncus arteriosus, total anomalous pulmonary venous return, tricuspid atresia, pulmonary atresia (with intact ventricular septum), hypoplastic left heart syndrome, and Tetralogy of Fallot. Secondary targets, or those defects which sometimes present with hypoxemia, include double outlet right ventricle, Ebstein anomaly, coarctation/hypoplasia of the aortic arch, aortic arch interruption, and single ventricle physiology [1,7,8]. The addition of CCHD screening to clinical assessment has been shown to significantly improve the sensitivity for CCHD detection (from 77.4% to 93.2%) [9]. Therefore, in 2011, the Secretary of Health and Human Services added CCHD screening using pulse oximetry to the Recommended Uniform Screening Panel [10]. The American Academy of Pediatrics (AAP) endorsed screening in January 2012 [11]. In their policy statement, the AAP recommended the use of a specific algorithm presented in a Pediatrics Special Article by Kemper et al., which was based on a three-stage model initially proposed by de-Wahl Granelli [8,12]. Since that time, CCHD screening with pulse oximetry has been adopted in many states (Available online: https://www.newsteps.org/quality-practice-resources/disease-specific-activitie/cchd) [13].
However, both the AAP policy statement and the article by Kemper et al. include the caveat that “algorithm cutoffs may need to be adjusted in high-altitude nurseries” [1,8]. This is due to the fact that many studies have shown that average oxygen saturations in newborns are lower and show more variability at even moderate elevation, as demonstrated in Figure 1 [1,14,15,16,17,18,19,20,21,22]. However, there are few studies that have evaluated CCHD screening algorithms at moderate or higher altitude.
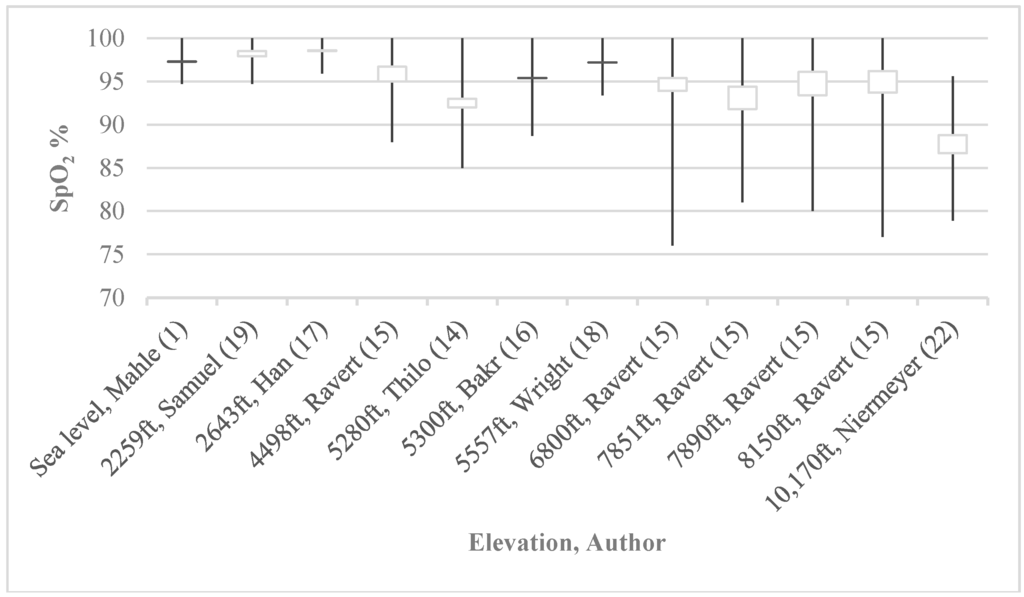
Figure 1.
Historical SpO2 at various altitudes. Average oxygen saturations in newborns are lower and more variable at higher altitudes (closed boxes indicate saturation ranges while whiskers indicate ±2 SD).
Because of the significant opportunity to decrease morbidity and mortality by improving CCHD detection in the neonatal period and the known challenges with pulse oximetry at higher altitude, multiple stakeholders including the Colorado Newborn Screening Advisory Committee, the AAP, and the Secretary of Health and Human Services have requested additional studies be performed and alternative algorithms be trialed in these populations [1,8].
2. Materials and Methods
2.1. The Modified Protocol
Our alternative protocol, shown in Figure 2, was based on the AAP protocol with two major modifications: a lower first screen oxygen saturation cutoff and an oxygen hood intervention.
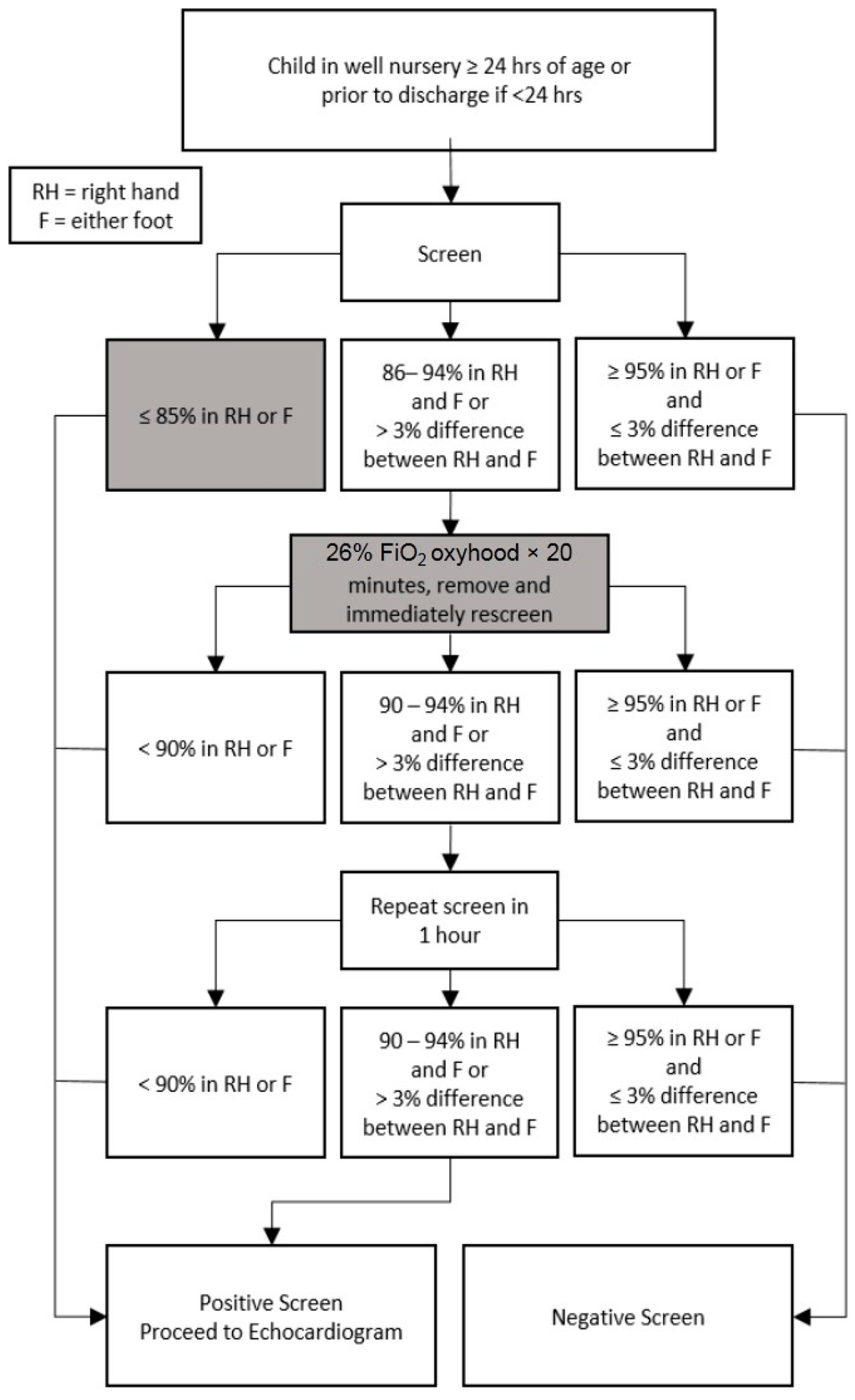
Figure 2.
Modified critical congenital heart disease (CCHD) screening protocol. Our modified CCHD screening protocol with deviations from the American Academy of Pediatrics (AAP) protocol highlighted. Modifications, indicated by gray boxes, include a decreased first screen saturation cutoff from 90% to 85% as well as the oxygen hood intervention between the first and second screens.
The main goal of manipulating the first screen saturation criteria was to allow a subset of otherwise failing newborns (those with saturations 85%–89%) more time to transition and the opportunity to be re-screened. These re-screens were performed in accordance with AAP protocol cut-offs. The 85% cutoff used in the first screen was based on a study by Thilo et al. performed in Denver, CO, USA, which showed a range of post-ductal saturations between 80% and 98% where 85% was two standard deviations below the post-ductal mean saturation [14]. Despite differences in altitude, it was felt that the majority of healthy newborns should be able to demonstrate saturations >95%, and in an effort to remain as consistent with the AAP protocol as possible and minimize the risk of false negatives, the overall passing criteria were not changed. At the time of study design, no large studies were available that outlined a more specific range of normal newborn saturations at moderate altitude. The oxygen hood intervention was used to establish alveolar oxygen tension equivalent to that at sea level in an attempt to allow for standardization across any altitude.
2.2. Study Design
The study was conducted at 6200 feet (1890 m) in the Memorial Hospital well-baby nursery in Colorado Springs, CO (now part of the University of Colorado Health System), from December 2011 through July 2013. The Memorial Hospital Institutional Review Board gave study approval with a waiver of consent and full waiver of Health Insurance Portability and Accountability Act authorization. Parents were given a fact sheet describing the study and potential risks with 10 families opting out. Screenings were conducted at ≥24 h after birth or as near to hospital discharge as possible for those discharged early.
We prospectively enlisted well newborns at ≥35 0/7 weeks gestation without conditions known to predispose to hypoxemia (including prenatally diagnosed CHD). Trained nursing staff placed Nellcor N600x Pulse Oximeter with OxiMax™ Technology and Nellcor neonatal SpO2 sensors (on loan from Covidien Corporation, Boulder, CO, USA) on the right hand or wrist (pre-ductal) and, simultaneously, on a single foot (post-ductal). When possible, newborns were screened while awake and quiet. Newborn characteristics including weight, gender, method of delivery, gestational age, and the state of the newborn during screening were recorded.
Newborns were initially screened in room air. Those with either pre- or post-ductal saturation ≥95% with ≤3% difference in saturations passed screening and exited the algorithm. Any newborn with a single saturation of ≤85% failed screening and exited the algorithm with subsequent provider notification, additional clinical assessment, and echocardiogram. Newborns with saturations between passing and failing criteria advanced to the next stage of screening.
Prior to the second stage of screening, newborns were placed in an oxygen hood for 20 min with FiO2 designed to replicate sea level atmospheric oxygen tension in an effort to promote pulmonary vasodilation and possibly accelerate neonatal transition. This intervention was performed in the newborn nursery, and did not necessitate admission to the neonatal intensive care unit. The 20 min time period in the oxygen hood was chosen based on the length of time that children undergoing pulmonary hypertension evaluation in the Children’s Hospital of Colorado catheterization lab are given oxygen to evaluate their cardiovascular dynamics compared to the baseline condition. Local barometric pressure was identified by retrospective review as well as analysis of local pressures with a DBX1 barograph barometer (Aquatech Scientific Instruments LLC, Yardley, PA, USA). Appropriate FiO2 was then calculated from a local barometric pressure of 610 using the equation FiO2 = 159/610 based on Dalton’s Law of Partial Pressures where 159 is the partial pressure of oxygen exerted at sea level. This identified 26% FiO2 as the average amount of O2 required to simulate sea level atmospheric oxygen tension.
Following 20 min in the oxygen hood, newborns were placed back in room air and immediately underwent a second screen with pass/fail criteria matching national recommendations (passing criteria were either pre- or post-ductal saturation ≥95% with ≤3% difference in saturations, and failing criteria were any saturation of <90%.). Newborns who again neither passed nor failed the screen were retested after 1 h in room air for one additional screen. Those not achieving passing saturations by the third screen failed and proceeded to echocardiogram.
Protocol completion was defined as a newborn having definitively passed or failed any screen, or having failed after a third indeterminate screen. An indeterminate screen was defined as one in which the newborn did not meet criteria to definitively pass or fail.
Pulse oximetry data obtained in this subset of newborns was analyzed in order to determine the overall failure rate using the modified algorithm. The data was also retrospectively analyzed to calculate the first screen failure rate had the AAP protocol been used in the same newborn cohort. These results were compared to each other and to historical failure rates both at altitude and sea level.
2.3. Statistical Analysis
Characteristics of newborns that passed the screening algorithm were compared to those categorized as failing using Fisher’s exact test, Chi-square, t-test with unequal variances, and standard t test. A p-value < 0.05 was considered statistically significant. Statistical analysis was completed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA). Sample size was a result of the pre-determined 18-month study period, and no power analysis was completed prior to study initiation. Following the 18-month period, Memorial Hospital returned to using the standard AAP protocol for screening newborns.
3. Results
In our study, 2001 newborns were screened using the modified CCHD screening protocol. Newborn characteristics recorded at the time of data acquisition are shown in Table 1. There was no correlation identified between various characteristics and the likelihood of a newborn to pass or fail screening. While the ideal screening state is “calm/quiet”, several newborns in our study were recorded as “crying” during their screen. In order to determine if this impacted results, this group was analyzed separately and was not found to be statistically associated with screening failure.

Table 1.
Newborn characteristics. Various newborn characteristics were recorded at the time of data acquisition. No correlation was identified between characteristics and the likelihood of a newborn to pass or fail screening.
Of the 2001 newborns who underwent screening, 1953 (97.6% with a 95% CI (96.9%, 98.3%)) passed and six (0.3% with a 95% CI (0.01%, 0.5%)) failed screening. This failure rate of 0.3% is statistically different from the failure rate of 1.1% found by Wright et al. (p = 0.0089) [18].
There were 42 patients who did not complete the protocol. Reasons for lack of completion are unclear and may have been due to a variety of reasons including parental refusal, infant discharge, or screenings missed by nursing staff. Forty-one of these newborns were lost to screening after an indeterminate first screen (17 were indeterminate due to saturations 86%–94% while 24 had >3% difference in saturations). Of these, one exited screening due to identification of a murmur, one went on to have a second screen that was only partially documented, and the rest were lost for unknown reasons. There was one newborn who was lost after a second indeterminate screen. To our knowledge, no infants had a change in clinical status or decompensation as a result of screening or oxygen hood exposure. Figure 3 demonstrates how newborns progressed through screening.
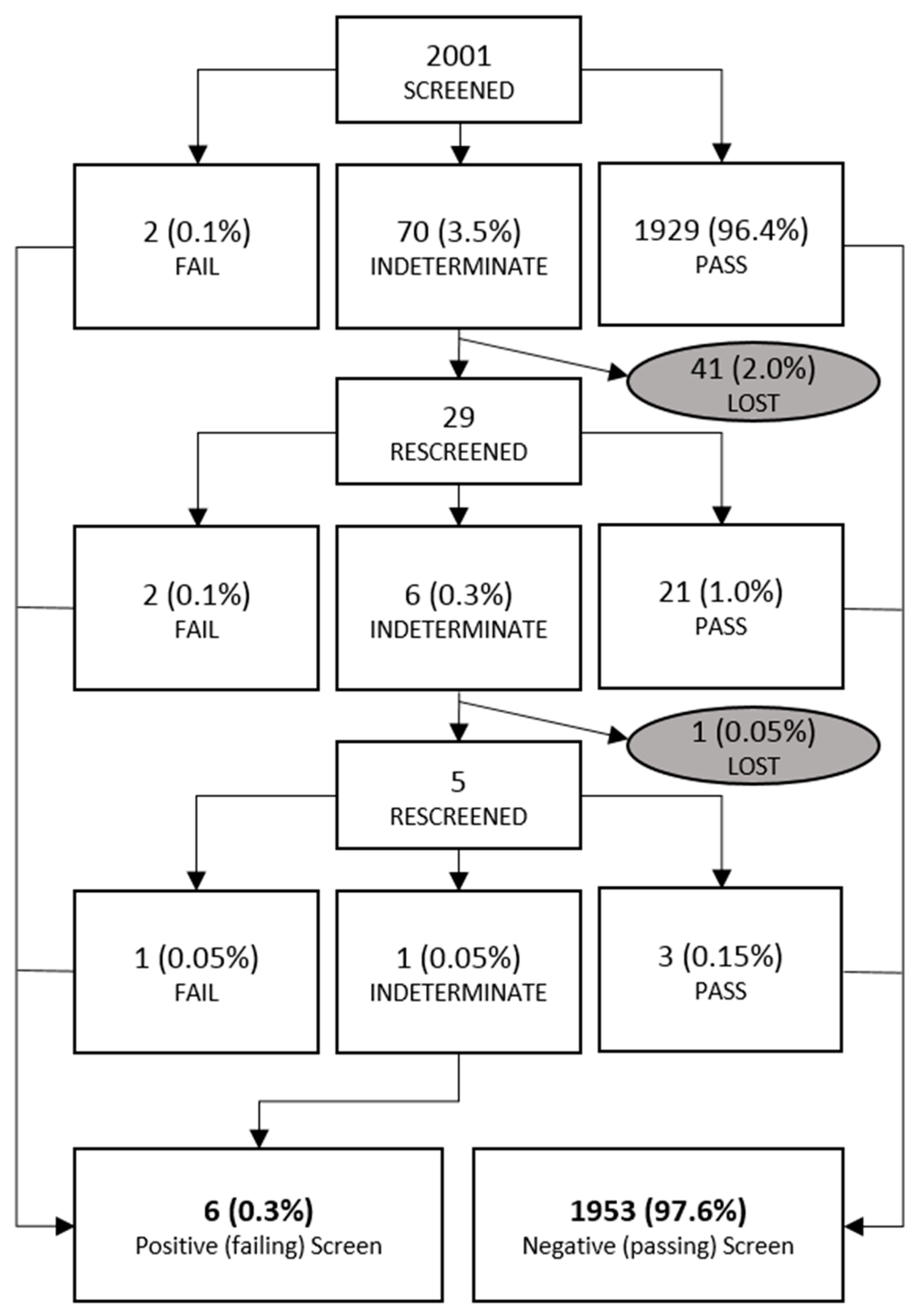
Figure 3.
Newborn flow sheet. Demonstration of where newborns were found to pass, fail, or were lost to screening. Percentages listed are relative to the entire study population.
Among the six newborns who failed screening, two failed their first screen (with pre-ductal/post-ductal saturations of 85%/97% and 81%/82%), two failed their second screen after an indeterminate first screen (with saturations of 89%/90% and 83%/94%), and two failed their third screen (one by meeting failure criteria with saturations of 87%/91%, and one by remaining indeterminate for all three screens). None of the failing newborns had other causes of hypoxemia identified. In some cases, providers continued screening newborns that had failed, and found that two of six newborns would have gone on to pass if allowed one to two additional screens.
Had the AAP protocol been used in the same newborn cohort, the first screen failure rate (using a 90% saturation cutoff) would have increased to 0.70%, and the theoretical overall screening failure rate would have increased to 0.8%.
Echocardiograms were performed on four of six failing newborns (the two newborns who went on to pass with additional screens were considered to have passed and did not undergo an echocardiogram). Likewise, passing newborns did not undergo echocardiography. Of the four failing newborns who were imaged, all had normal echocardiograms—one with no shunt, one with a patent foramen ovale (PFO), one with a patent ductus arteriosus (PDA), and one with both a PFO and a PDA. No CCHD was identified. As above, one newborn who was noted to have a murmur after an indeterminate first screen also underwent an echocardiogram, which revealed a PDA.
In order to address the issue of having a large number of incomplete screens as well as to help determine if our modified pulse oximetry screening algorithm had missed any newborns (false negatives), the Birth Defects Registry Database was queried and was able to provide a list of all children born at Memorial Hospital within the study dates who went on to carry a diagnosis of any form of congenital heart disease. This query revealed 24 children in total. Twenty-three of these newborns were either not included in the study (due to prenatal diagnosis or clinical decompensation prior to 24 h of life) or were found to carry diagnoses of CHD rather than CCHD. The final newborn, however, was found to have a coarctation of the aorta which was identified by physical exam prior to hospital discharge (absent femoral pulses). On chart review, it was found that this newborn had passed their pulse oximetry screen at 24 h of life with a pre-ductal saturation of 97% and a post-ductal saturation of 95%. This resulted in a 0.05% false negative rate for the study.
4. Discussion
The data presented demonstrate that by using a modified algorithm for CCHD screening to account for altitude, the failure rate can be decreased to 0.3%. This is lower than recently published studies of 1.1% at similar altitude utilizing the AAP algorithm [18]. These findings are closer to reported sea-level failure rates of 0.01%–0.15% (comparison shown in Figure 4) [18,23,24,25,26].
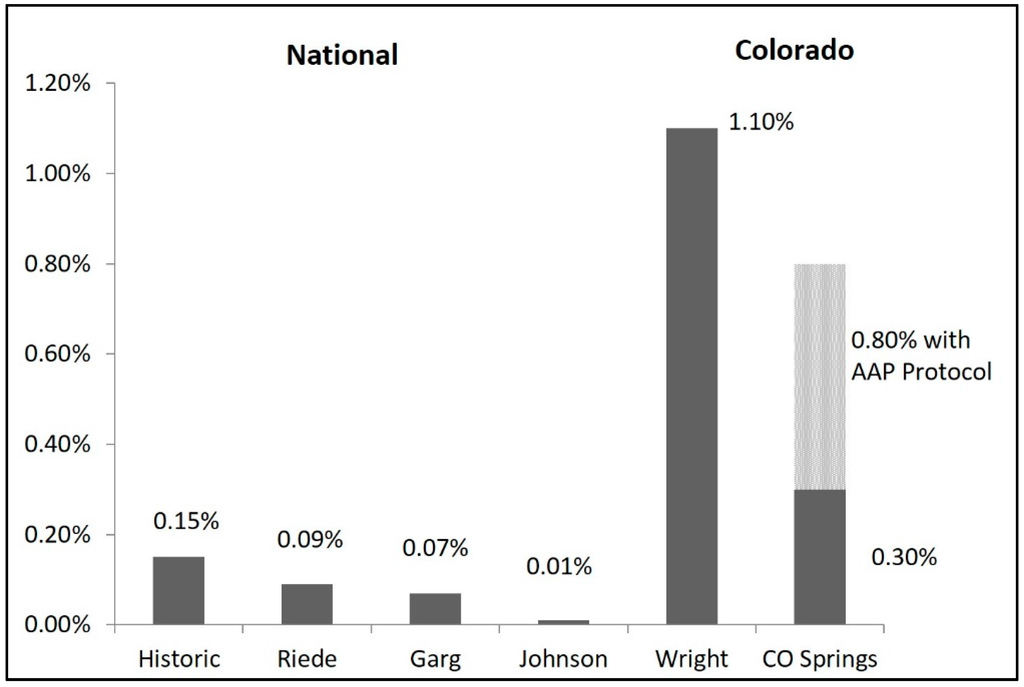
Figure 4.
Colorado screening failure rates as compared to nationwide reports. Compared to national failure rates, failure rates at moderate altitude remain elevated; however, our modified protocol significantly improves on the failure rate found at similar altitude. The hatched bar indicates the theoretical AAP protocol failure rate in our newborn cohort.
The false negative rate for our newborn cohort was one of 2001 (0.05%). This newborn passed screening with a pre-ductal saturation of 97% and post-ductal saturation of 95% at 24 h of life and was subsequently found to have coarctation of the aorta diagnosed after absent femoral pulses were identified on the discharge physical exam. Based on these screening saturation values, this newborn would also have passed the standard AAP protocol and was not missed as a result of our protocol modifications. In reviewing the literature for other studies that report false negative rates, it was found that false negative rates varied between 0.008% and 0.64% [12,23,24,25,26,27,28,29]. Studies that reported which diagnoses had been missed most frequently reported aortic arch obstructions [12,24,26,28]. As previously mentioned, aortic arch anomalies are considered a secondary target of pulse oximetry screening as they may not present with hypoxemia, which was the case with the newborn missed in our cohort. This case further demonstrates the importance of pulse oximetry being used in addition to clinical assessment.
In comparing our modified algorithm with the use of the AAP protocol (90% saturation cutoff used in the first screen) for our newborn cohort, we found that 14 of 2001 newborns would have failed the first screen, increasing the first screen failure rate to 0.7%, and the theoretical overall screening failure rate to 16 of 2001 or 0.8%. Because data is only available for retrospective analysis using the AAP protocol, and no head-to-head comparison was done, it is impossible to determine the true overall screening failure rate given the oxygen hood intervention. However, considering that our 0.8% failure rate using the AAP protocol is not statistically significantly different from the 1.1% failure rate recently identified by Wright et al., our results appear to be consistent with what has previously been found at this altitude [18]. Given that all additional failures using the AAP protocol occurred in the first screen, we suspect the modified oxygen cutoff contributed more to our decreased failure rate than the oxygen hood intervention.
A total of five newborns underwent echocardiography, with no CCHD identified. Based on studies that have demonstrated an incidence of CCHD of 1.4 per 1000 live births, we would have expected to have at least three cases of CCHD in our population [30,31]. We did identify one case of CCHD that was included in the study through our Birth Defects Registry query, and we also identified several more cases that had been diagnosed prenatally or had clinical decompensation prior to the 24 h screening time. This demonstrates that adequate health care access in the urban area tested, improved prenatal screening, and high quality fetal echocardiogram likely impacted the number of patients with CCHD identified through our screening study. This is in alignment with other CCHD screening studies that are not identifying affected newborns [24,25,26]. However, it must be noted that there is no guarantee that all newborns born at Memorial Hospital during our study time period are included in the registry, which is a limitation of the study.
Overall, this study shows that implementation of a modified CCHD screening protocol is possible in a large-volume nursery. While limited by a small sample size, this study suggests that a modified protocol may effectively decrease the screening failure rate when compared to previous studies which have applied the AAP protocol to newborns at moderate altitude. This study also adds to the literature, demonstrating screening saturations that are consistent with published studies at altitude [15,16,17,18,19].
Finally, while CCHD screening using pulse oximetry has been well studied at sea level, and lower oxygen saturations in newborns at altitude have been well documented, exactly how altitude affects newborn oxygen saturation and physiologic transition has not been clearly demonstrated. CCHD screening protocols that account for altitude have been slow to develop and may require larger populations in regions with high and moderate altitude in order to fully understand this phenomena.
5. Conclusions
The widespread use of pulse oximetry in the newborn period has been shown to be a safe and effective screening tool for CCHD, and has become an integral part of standard newborn screening. Questions remain regarding the effectiveness of pulse oximetry for CCHD screening at moderate and high altitudes, and how altitude may impact screening failure rates. Because of the burdens of high false positive rates on patients, families, hospitals, and communities, it is necessary to evaluate alternative screening protocols in order to optimize screening sensitivity while limiting false positive and false negative rates at higher elevations. Novel protocols that account for differences in oxygen tension at various altitudes are indicated to make CCHD screening more applicable worldwide. This study is a first attempt at creating an alternative protocol. Although the algorithm still requires refinement to achieve the best screening outcome, we believe it contributes valuable information on which to build.
We would advocate for further studies that collaborate with birth defects registries and clinical referral centers for cardiac defects in order to more accurately evaluate screening algorithms and the effects of altitude.
Acknowledgments
The authors would like to thank the nurses at Memorial Hospital in Colorado Springs for their invaluable contributions to this study. There was no funding source or grant for this study.
Author Contributions
Mark Duster and Christopher M. Rausch conceptualized and designed the study; Cindy Eller coordinated and supervised data collection; Leilani Russell, Joshua Miller, and Marci Sontag assisted in data analysis; Erin Lueth wrote the paper; Mary Kohn, Marci Sontag, Jason Wright and Christopher M. Rausch critically reviewed and revised the paper; all authors approve the final manuscript as submitted.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CHD | Congenital Heart Disease |
| CCHD | Critical Congenital Heart Disease |
| AAP | American Academy of Pediatrics |
| PVR | Pulmonary Vascular Resistance |
| PDA | Patent Ductus Arteriosus |
| PFO | Patent Foramen Ovale |
References
- Mahle, W.T.; Newburger, J.W.; Matherne, G.P.; Smith, F.C.; Hoke, T.R.; Koppel, R.; Gidding, S.S.; Beekman, R.H., 3rd; Grosse, S.D. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology and Cardiac Surgery, and Committee on Fetus and Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: A scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation 2009, 120, 447–458. [Google Scholar] [PubMed]
- Thangaratinam, S.; Daniels, J.; Ewer, A.K.; Zamora, J.; Khan, K.S. Accuracy of pulse oximetry in screening for congenital heart disease in asymptomatic newborns: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F176–F180. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, M.K.; Silverman, N.H.; Moon-Grady, A.J.; Tong, E.; Nourse, J.; Sorenson, B.; Lee, J.; Hornberger, L.K. Prenatal detection of congenital heart disease. J. Pediatr. 2009, 155, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Liberman, R.F.; Getz, K.D.; Lin, A.E.; Higgins, C.A.; Sekhavat, S.; Markenson, G.R.; Anderka, M. Delayed diagnosis of critical congenital heart defects: Trends and associated factors. Pediatrics 2014, 134, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.H.; Bradshaw, E.; Beekman, R.; Mahle, W.T.; Martin, G.R. Critical congenital heart disease screening using pulse oximetry. J. Pediatr. 2013, 162, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Wren, C.; Richmond, S.; Donaldson, L. Presentation of congenital heart disease in infancy: Implications for routine examination. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 80, F49–F53. [Google Scholar] [CrossRef] [PubMed]
- Congenital Heart Defects (CHDs) CDC Fact Sheet. Centers for Disease Control and Prevention. Available online: http://www.cdc.gov/ncbddd/heartdefects/cchd-facts.html (accessed on 13 April 2016).
- Kemper, A.R.; Mahle, W.T.; Martin, G.R.; Cooley, W.C.; Kumar, P.; Morrow, W.R.; Kelm, K.; Pearson, G.D.; Glidewell, J.; Grosse, S.D.; et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics 2011, 128, e1259–e1267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.M.; Ma, X.J.; Ge, X.L.; Liu, F.; Yan, W.L.; Wu, L.; Ye, M.; Liang, X.; Zhang, J.; Gao, Y.; et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: A prospective study. Lancet 2014, 384, 747–754. [Google Scholar] [CrossRef]
- Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. HHS Secretary Adopts Recommendation to Add Critical Congenital Heart Disease to the Recommended Uniform Screening Panel; Department of Health and Human Services: Washington, DC, USA, 2011. Available online: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf (accessed on 13 April 2016).
- Mahle, W.T.; Martin, G.R.; Beekman, R.H.; Morrow, W.R.; Rosenthal, G.L.; Snyder, C.S.; Minich, L.L.; Mital, S.; Towbin, J.A.; Tweddell, J.S. Endorsement of health and human services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics 2012, 129, 190–192. [Google Scholar] [PubMed]
- De-Wahl Granelli, A.; Wennergren, M.; Sandberg, K.; Mellander, M.; Bejlum, C.; Inganas, L.; Eriksson, M.; Seqerdahl, N.; Aqren, A.; Ekman-Joelsson, B.M.; et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: A Swedish prospective screening study in 39,821 newborns. BMJ 2009, 338. [Google Scholar] [CrossRef] [PubMed]
- NewSTEPs CCHD Newborn Screening Status. The Association of Public Health Laboratories. Available online: https://www.newsteps.org/cchd (accessed on 1 March 2015).
- Thilo, E.H.; Park-Moore, B.; Berman, E.R.; Carson, B.S. Oxygen saturation by pulse oximetry in healthy newborns at an altitude of 1610 m (5280 ft). What is normal? Am. J. Dis. Child. 1991, 145, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Ravert, P.; Detwiler, T.L.; Dickinson, J.K. Mean oxygen saturation in well neonates at altitudes between 4498 and 8150 feet. Adv. Neonatal Care. 2011, 11, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.F.; Habib, H.S. Normal values of pulse oximetry in newborns at high altitude. J. Trop. Pediatr. 2005, 51, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Han, L.M.; Klewer, S.E.; Blank, K.M.; Seckeler, M.D.; Barber, B.J. Feasibility of pulse oximetry screening for critical congenital heart disease at 2643-foot elevation. Pediatr. Cardiol. 2013, 34, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Kohn, M.; Niermeyer, S.; Rausch, C.M. Feasibility of critical congenital heart disease newborn screening at moderate altitude. Pediatrics 2014, 133, e561–e569. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.Y.; Bromiker, R.; Mimouni, F.B.; Picard, E.; Lahav, S.; Mandel, D.; Goldberg, S. Newborn oxygen saturation at mild altitude versus sea level: Implications for neonatal screening for critical congenital heart disease. Acta Paediatr. 2013, 102, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.Y.; Ou, C.N. Validation of oxygen saturation monitoring in neonates. Am. J. Crit. Care 2007, 16, 168–178. [Google Scholar] [PubMed]
- Shiao, S.U. Functional versus fractional oxygen saturation readings: Bias and agreement using simulated solutions and adult blood. Biol. Res. Nurs. 2002, 3, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Niermeyer, S.; Shaffer, E.M.; Thilo, E.; Corbin, C.; Moore, L.G. Arterial oxygenation ad pulmonary arterial pressure in healthy neonates and infants at high altitude. J. Pediatr. 1993, 123, 767–772. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Brown, K.; Zamora, J.; Khan, K.S.; Ewer, A.K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: A systematic review and meta-analysis. Lancet 2012, 379, 2459–2464. [Google Scholar] [CrossRef]
- Riede, F.T.; Worner, C.; Dahnert, I.; Mockel, A.; Kostelka, M.; Schneider, P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine—Results from a prospective multicenter study. Eur. J. Pediatr. 2010, 169, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Garg, L.F.; van Naarden Braun, K.; Knapp, M.M.; Anderson, T.M.; Koppel, R.I.; Hirsch, D.; Beres, L.M.; Sweatlock, J.; Olney, R.S.; Glidewell, J.; et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics 2013, 132, e314–e323. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.C.; Lieberman, E.; O’Leary, E.; Geggel, R.L. Prenatal and newborn screening for critical congenital heart disease: Findings from a nursery. Pediatrics 2014, 134, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Bhola, K.; Kluckow, M.; Evans, N. Post-implementation review of pulse oximetry screening of well newborns in an Australian tertiary maternity hospital. J. Paediatr. Child Health 2014, 50, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Turska, K.A.; Borszewska Kornacka, M.K.; Blaz, W.; Kawalec, W.; Zuk, M. Early screening for critical congenital heart defects in asymptomatic newborns in Mazovia province: Experience of the POLKARD pulse oximetry programme 2006–2008 in Poland. Kardiol. Polska 2012, 70, 370–376. [Google Scholar]
- Ewer, A.K.; Furmston, A.T.; Middleton, L.J.; Deeks, J.J.; Daniels, J.P.; Pattison, H.M.; Powell, R.; Roberts, T.E.; Barton, P.; Auquste, P.; et al. Pulse oximetry as a screening test for congenital heart defects in newborn infants: A test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol. Assess. 2012, 16, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Olney, R.S.; Ailes, E.C.; Sontag, M.K. Detection of critical congenital heart defects: Review of contributions from prenatal and newborn screening. Semin. Perinatol. 2015, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
