Methodological and Procedural Considerations for Developing Decision Analytic Models to Assess the Health Economic Impacts of Newborn Bloodspot Screening: A Systematic Methodological Review
Abstract
1. Introduction
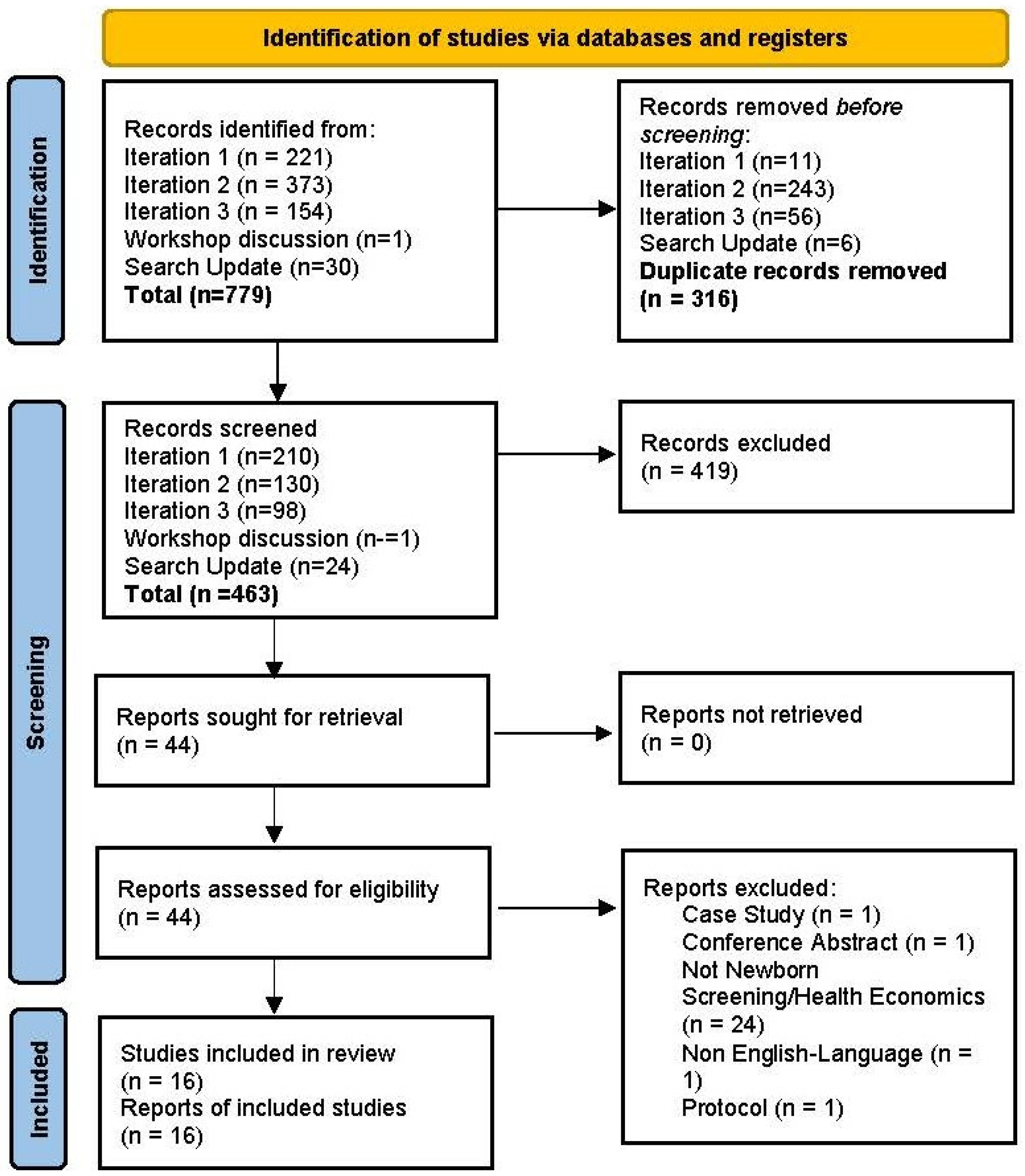
2. Materials and Methods
3. Results
3.1. The Screening Decision Node
3.1.1. Decision Criteria
3.1.2. Decision Variables
3.2. Defining the Decision Problem Scope and Model Structure
3.2.1. Decision Problem Scope
3.2.2. Defining the Model Structure
3.2.3. Selecting the Modelling Method
3.3. The Target Condition
3.4. The Screening Test/Protocol
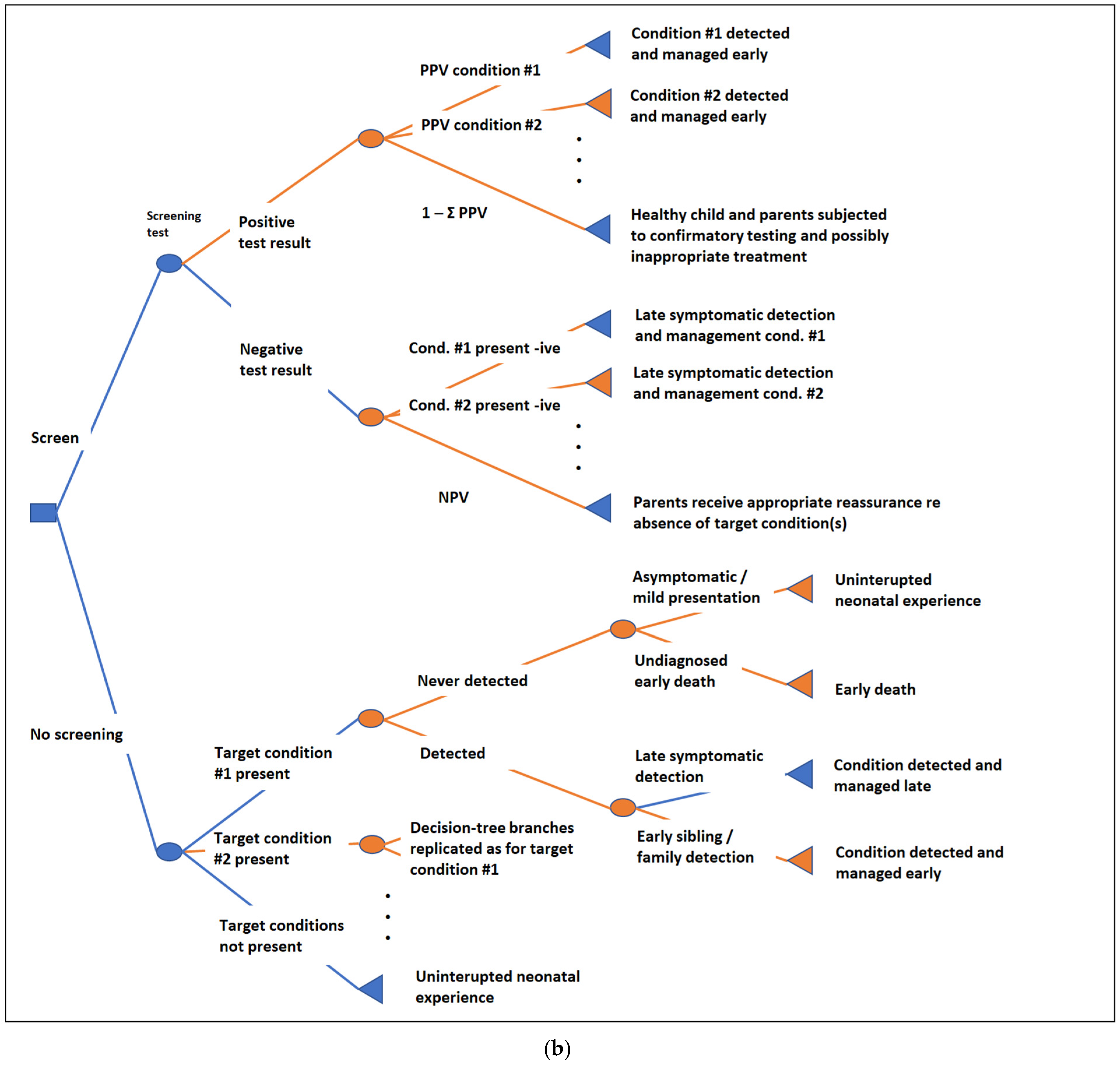
3.5. Outcome Nodes
3.6. Other Considerations
3.7. Recommendations Regarding Decision Processes and Methods Research
3.7.1. Supporting NBS Decision Making
3.7.2. NBS Model Structure and Methods
3.7.3. Data and Estimation of Model Parameters
3.7.4. Overarching Recommendations
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSTG | Blood Spot Task Group of the UKNSC |
| DBS | Dried Bloodspot |
| DHSC | Department of Health and Social Care |
| HIQA | Health Information and Quality Authority |
| NBS | Newborn Bloodspot Screening |
| OHID | Office of Health Improvement and Disparities |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| SCHARR | Sheffield Centre for Health and Related Research |
| SCID | Severe Combined Immunodeficiency |
| UKNSC | UK National Screening Committee |
References
- Therrell, B.L.; Padilla, C.D.; Borrajo, G.J.C.; Khneisser, I.; Schielen, P.C.J.I.; Knight-Madden, J.; Malherbe, H.L.; Kase, M. Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020–2023). Int. J. Neonatal Screen. 2024, 10, 38. [Google Scholar] [CrossRef]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A New Framework for Developing and Evaluating Complex Interventions: Update of Medical Research Council Guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef]
- Lombardo, S.; Seedat, F.; Elliman, D.; Marshall, J. Policy-Making and Implementation for Newborn Bloodspot Screening in Europe: A Comparison between EURORDIS Principles and UK Practice. Lancet Reg. Health-Eur. 2023, 33, 100714. [Google Scholar] [CrossRef]
- UK NSC Blood Spot Task Group Terms of Reference. GOV.UK. Available online: https://www.gov.uk/government/publications/uk-nsc-blood-spot-task-group-terms-of-reference/uk-nsc-blood-spot-task-group-terms-of-reference (accessed on 25 April 2025).
- Karnon, J.; Goyder, E.; Tappenden, P.; McPhie, S.; Towers, I.; Brazier, J.; Madan, J. A Review and Critique of Modelling in Prioritising and Designing Screening Programmes. Health Technol. Assess. 2007, 11. [Google Scholar] [CrossRef] [PubMed]
- Langer, A.; Holle, R.; John, J. Specific Guidelines for Assessing and Improving the Methodological Quality of Economic Evaluations of Newborn Screening. BMC Health Serv. Res. 2012, 12, 300. [Google Scholar] [CrossRef]
- Chilcott, J.B.; Sutton, A.; Bessey, A.; Lombardo, S.; Visintin, C.; Marshall, J.; Rivero-Arias, O.; Taylor-Phillips, S. Methodological and Procedural Considerations for Developing Decision Analytic Clinical and Cost-Effectiveness Models to Assess Newborn Bloodspot Screening: Study Protocol. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023461551 (accessed on 25 April 2025).
- Png, M.E.; Yang, M.; Taylor-Phillips, S.; Ratushnyak, S.; Roberts, N.; White, A.; Hinton, L.; Boardman, F.; McNiven, A.; Fisher, J.; et al. Benefits and Harms Adopted by Health Economic Assessments Evaluating Antenatal and Newborn Screening Programmes in OECD Countries: A Systematic Review of 336 Articles and Reports. Soc. Sci. Med. 2022, 314, 115428. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, P.; Visser, L.A.; Buyukkaramikli, N.; van der Ploeg, C.P.B.; van den Akker-van Marle, M.E. The Methodological Quality and Challenges in Conducting Economic Evaluations of Newborn Screening: A Scoping Review. Int. J. Neonatal Screen. 2020, 6, 23. [Google Scholar] [CrossRef]
- Phillips, K.A.; Deverka, P.A.; Marshall, D.A.; Wordsworth, S.; Regier, D.A.; Christensen, K.D.; Buchanan, J. Methodological Issues in Assessing the Economic Value of Next-Generation Sequencing Tests: Many Challenges and Not Enough Solutions. Value Health 2018, 21, 1033–1042. [Google Scholar] [CrossRef]
- Ho, V.H.; Giguere, Y.; Reinharz, D. Expected Benefits and Challenges of Using Economic Evaluations to Make Decisions About the Content of Newborn Screening Programs in Vietnam: A Scoping Review of the Literature. J. Inborn Errors Metab. Screen. 2023, 11, e20220011. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Lawson, D.O.; Puljak, L.; Allison, D.B.; Thabane, L. A Tutorial on Methodological Studies: The What, When, How and Why. BMC Med. Res. Methodol. 2020, 20, 226. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Grosse, S.D.; Teutsch, S.M.; Haddix, A.C. Lessons from Cost-Effectiveness Research for United States Public Health Policy. Annu. Rev. Public Health 2007, 28, 365–391. [Google Scholar] [CrossRef]
- Grosse, S.D.; Prosser, L.A.; Asakawa, K.; Feeny, D. QALY Weights for Neurosensory Impairments in Pediatric Economic Evaluations: Case Studies and a Critique. Expert. Rev. Pharmacoecon Outcomes Res. 2010, 10, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D. Showing Value in Newborn Screening: Challenges in Quantifying the Effectiveness and Cost-Effectiveness of Early Detection of Phenylketonuria and Cystic Fibrosis. Healthcare 2015, 3, 1133–1157. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Thompson, J.D.; Ding, Y.; Glass, M. The Use of Economic Evaluation to Inform Newborn Screening Policy Decisions: The Washington State Experience: Economic Evaluation to Inform NBS Policy Decisions. Milbank Q. 2016, 94, 366–391. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Van Vliet, G. Challenges in Assessing the Cost-Effectiveness of Newborn Screening: The Example of Congenital Adrenal Hyperplasia. Int. J. Neonatal Screen. 2020, 6, 25. [Google Scholar] [CrossRef]
- Wright, S.J.; Jones, C.; Payne, K.; Dharni, N.; Ulph, F. The Role of Information Provision in Economic Evaluations of Newborn Bloodspot Screening: A Systematic Review. Appl. Health Econ. Health Policy 2015, 13, 615–626. [Google Scholar] [CrossRef]
- Castilla-Rodríguez, I.; Vallejo-Torres, L.; Couce, M.L.; Valcárcel-Nazco, C.; Mar, J.; Serrano-Aguilar, P. Cost-Effectiveness Methods and Newborn Screening Assessment. In Rare Diseases Epidemiology: Update and Overview; Posada De La Paz, M., Taruscio, D., Groft, S.C., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 1031, pp. 267–281. [Google Scholar] [CrossRef]
- Grosse, S.D.; Rogowski, W.H.; Ross, L.F.; Cornel, M.C.; Dondorp, W.J.; Khoury, M.J. Population Screening for Genetic Disorders in the 21st Century: Evidence, Economics, and Ethics. Public Health Genom. 2009, 13, 106–115. [Google Scholar] [CrossRef]
- Prosser, L.A.; Grosse, S.D.; Kemper, A.R.; Tarini, B.A.; Perrin, J.M. Decision Analysis, Economic Evaluation, and Newborn Screening: Challenges and Opportunities. Genet. Med. 2012, 14, 703–712. [Google Scholar] [CrossRef]
- Prieto-Gonzalez, D.; Castilla-Rodriguez, I.; Gonzalez, E.; Couce, M.L. Towards the Automated Economic Assessment of Newborn Screening for Rare Diseases. J. Biomed. Inform. 2019, 95, 103216. [Google Scholar] [CrossRef]
- Ulph, F.; Wright, S.; Dharni, N.; Payne, K.; Bennett, R.; Roberts, S.; Walshe, K.; Lavender, T. Provision of Information about Newborn Screening Antenatally: A Sequential Exploratory Mixed-Methods Project. Health Technol. Assess. 2017, 21, 1–240. [Google Scholar] [CrossRef]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease. World Health Organization, 1968. Available online: https://iris.who.int/handle/10665/37650 (accessed on 25 April 2025).
- Key Principles for Newborn Screening: A EURORDIS Position Paper. EURORDIS Rare Diseases Europe, 2021. Available online: https://www.eurordis.org/publications/key-principles-for-newborn-screening/ (accessed on 25 April 2025).
- UK NSC Ethical Framework for Screening. GOV.UK. Available online: https://www.gov.uk/government/publications/uk-nsc-ethical-framework-for-screening (accessed on 25 April 2025).
- Fischer, K.E.; Rogowski, W.H. Funding Decisions for Newborn Screening: A Comparative Review of 22 Decision Processes in Europe. Int. J. Environ. Res. Public Health 2014, 11, 5403–5430. [Google Scholar] [CrossRef]
- Ding, Y.; Thompson, J.D.; Kobrynski, L.; Ojodu, J.; Zarbalian, G.; Grosse, S.D. Cost-Effectiveness/Cost-Benefit Analysis of Newborn Screening for Severe Combined Immune Deficiency in Washington State. J. Pediatr. 2016, 172, 127–135. [Google Scholar] [CrossRef]
- Sontag, M.K.; Sarkar, D.; Comeau, A.M.; Hassell, K.; Botto, L.D.; Parad, R.; Rose, S.R.; Wintergerst, K.A.; Smith-Whitley, K.; Singh, S.; et al. Case Definitions for Conditions Identified by Newborn Screening Public Health Surveillance. Int. J. Neonatal Screen. 2018, 4, 16. [Google Scholar] [CrossRef]
- Blom, M.; Zetterström, R.H.; Stray-Pedersen, A.; Gilmour, K.; Gennery, A.R.; Puck, J.M.; van der Burg, M. Recommendations for Uniform Definitions Used in Newborn Screening for Severe Combined Immunodeficiency. J. Allergy Clin. Immunol. 2022, 149, 1428–1436. [Google Scholar] [CrossRef]
- Mulholland, R.J.; Wu, O.; Noel-Storr, A.; Seyahian, A.; Quinn, T.; Sutton, A.; Hawkins, N. Defining Incidental Findings in Economic Evaluations of Screening Programmes: A Scoping Review [Unpublished report]. Glasgow University on behalf of the NIHR Complex Reviews Synthesis Unit Evidence Synthesis Group, 2024. [Google Scholar]
- Henry, E.; Al-Janabi, H.; Brouwer, W.; Cullinan, J.; Engel, L.; Griffin, S.; Hulme, C.; Kingkaew, P.; Lloyd, A.; Payakachat, N.; et al. Recommendations for Emerging Good Practice and Future Research in Relation to Family and Caregiver Health Spillovers in Health Economic Evaluations: A Report of the SHEER Task Force. PharmacoEconomics 2024, 42, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.; Reitsma, J.B.; Vandenbroucke, J.P.; Glas, A.S.; Bossuyt, P.M. Case–Control and Two-Gate Designs in Diagnostic Accuracy Studies. Clin. Chem. 2005, 51, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Pandor, A.; Eastham, J.; Chilcott, J.; Paisley, S.; Beverley, C. Economics of Tandem Mass Spectrometry Screening of Neonatal Inherited Disorders. Int. J. Technol. Assess. Health Care 2006, 22, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Bessey, A.; Chilcott, J.; Pandor, A.; Paisley, S. The Cost-Effectiveness of Expanding the UK Newborn Bloodspot Screening Programme to Include Five Additional Inborn Errors of Metabolism. Int. J. Neonatal Screen. 2020, 6, 93. [Google Scholar] [CrossRef]
- Simon, N.-J.; Richardson, J.; Ahmad, A.; Rose, A.; Wittenberg, E.; D’Cruz, B.; Prosser, L.A. Health Utilities and Parental Quality of Life Effects for Three Rare Conditions Tested in Newborns. JPRO 2019, 3, 4. [Google Scholar] [CrossRef]
- Dixon, S.; Shackley, P.; Bonham, J.; Ibbotson, R. Putting a Value on the Avoidance of False Positive Results When Screening for Inherited Metabolic Disease in the Newborn. J. Inherit. Metab. Dis. 2012, 35, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ungar, W.J. Challenges in Health State Valuation in Paediatric Economic Evaluation: Are QALYs Contraindicated? PharmacoEconomics 2011, 29, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Matza, L.S.; Patrick, D.L.; Riley, A.W.; Alexander, J.J.; Rajmil, L.; Pleil, A.M.; Bullinger, M. Pediatric Patient-Reported Outcome Instruments for Research to Support Medical Product Labeling: Report of the ISPOR PRO Good Research Practices for the Assessment of Children and Adolescents Task Force. Value Health 2013, 16, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Rowen, D.; Rivero-Arias, O.; Devlin, N.; Ratcliffe, J. Review of Valuation Methods of Preference-Based Measures of Health for Economic Evaluation in Child and Adolescent Populations: Where Are We Now and Where Are We Going? PharmacoEconomics 2020, 38, 325–340. [Google Scholar] [CrossRef]
- Rutter, H.; Savona, N.; Glonti, K.; Bibby, J.; Cummins, S.; Finegood, D.T.; Greaves, F.; Harper, L.; Hawe, P.; Moore, L.; et al. The Need for a Complex Systems Model of Evidence for Public Health. Lancet 2017, 390, 2602–2604. [Google Scholar] [CrossRef]
- Breeze, P.R.; Squires, H.; Ennis, K.; Meier, P.; Hayes, K.; Lomax, N.; Shiell, A.; Kee, F.; De Vocht, F.; O’Flaherty, M.; et al. Guidance on the Use of Complex Systems Models for Economic Evaluations of Public Health Interventions. Health Econ. 2023, 32, 1603–1625. [Google Scholar] [CrossRef]
- Why Evidence Maps are the UK NSC’s First Step in Reviewing Screening Evidence—UK National Screening Committee. Available online: https://nationalscreening.blog.gov.uk/2022/10/28/why-evidence-maps-are-the-uk-nscs-first-step-in-reviewing-screening-evidence/ (accessed on 31 May 2025).
- Briggs, A.H.; Weinstein, M.C.; Fenwick, E.A.L.; Karnon, J.; Sculpher, M.J.; Paltiel, A.D. Model Parameter Estimation and Uncertainty Analysis: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group–6. Med. Decis. Mak. 2012, 32, 722–732. [Google Scholar] [CrossRef]
- Guide to the Methods of Technology Appraisal. National Institute for Health and Care Excellence (NICE), 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK395867/ (accessed on 9 June 2025).
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093. [Google Scholar] [CrossRef]
- iDSI Reference Case for Economic Evaluation. iDSI. Available online: https://idsihealth.org/resource-items/idsi-reference-case-for-economic-evaluation/ (accessed on 25 April 2025).
- Broomfield, J.; Abrams, K.R.; Freeman, S.; Latimer, N.; Rutherford, M.J.; Crowther, M.J.; Project HERCULES, the Cooperative International Neuromuscular Research Group Investigators and Duchenne Regulatory Science Consortium Members. Modeling the Multi-state Natural History of Rare Diseases with Heterogeneous Individual Patient Data: A Simulation Study. Stat. Med. 2024, 43, 184–200. [Google Scholar] [CrossRef]
- Project HERCULES. Duchenne UK. Available online: https://www.duchenneuk.org/project-hercules/ (accessed on 25 April 2025).
- Critical Path Institute—Rare and Orphan Diseases. C-Path. Available online: https://c-path.org/area-of-focus/rare-and-orphan-diseases/ (accessed on 25 April 2025).
- Regier, D.A.; Weymann, D.; Buchanan, J.; Marshall, D.A.; Wordsworth, S. Valuation of Health and Nonhealth Outcomes from Next-Generation Sequencing: Approaches, Challenges, and Solutions. Value Health 2018, 21, 1043–1047. [Google Scholar] [CrossRef]
- Payne, K.; Gavan, S.P.; Wright, S.J.; Thompson, A.J. Cost-Effectiveness Analyses of Genetic and Genomic Diagnostic Tests. Nat. Rev. Genet. 2018, 19, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Newborn Genomes Programme. Genomics England. Available online: https://www.genomicsengland.co.uk/initiatives/newborns (accessed on 25 April 2025).
- Lunke, S.; Bouffler, S.E.; Downie, L.; Caruana, J.; Amor, D.J.; Archibald, A.; Bombard, Y.; Christodoulou, J.; Clausen, M.; Fazio, P.D.; et al. Prospective Cohort Study of Genomic Newborn Screening: BabyScreen+ Pilot Study Protocol. BMJ Open 2024, 14, e081426. [Google Scholar] [CrossRef]
- Brouwer, W.B.F.; Culyer, A.J.; Van Exel, N.J.A.; Rutten, F.F.H. Welfarism vs. Extra-Welfarism. J. Health Econ. 2008, 27, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Rivero-Arias, O.; Wong, R.; Tsuchiya, A.; Bleichrodt, H.; Edwards, R.T.; Norman, R.; Lloyd, A.; Clarke, P. The QALY at 50: One Story Many Voices. Soc. Sci. Med. 2022, 296, 114653. [Google Scholar] [CrossRef] [PubMed]


| Study | SANRA Score | Decision Criteria | Decision Variables | Decision Problem Scope | Defining Model Structure | Selecting Modelling Method | The Target Condition | The Screening Test/Protocol | Outcome Nodes | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Cacciatore et al., 2020 [9] | 12 | √ | √ | √ | √ | √ | √ | |||
| Castilla Rodriguez et al., 2017 [20] | 8 | √ | √ | √ | √ | √ | ||||
| Grosse et al., 2007 [14] | 7 | √ | √ | √ | ||||||
| Grosse et al., 2009 [21] | 8 | √ | √ | |||||||
| Grosse et al., 2010 [15] | 3 | √ | ||||||||
| Grosse et al., 2015 [16] | 9 | √ | √ | |||||||
| Grosse et al., 2016 [17] | 10 | √ | √ | |||||||
| Grosse et al., 2020 [18] | 9 | √ | √ | √ | √ | √ | ||||
| Ho et al., 2023 [11] | 5 | √ | √ | √ | ||||||
| Karnon et al., 2007 [5] | 11 | √ | √ | √ | √ | |||||
| Langer et al., 2012 [6] | 12 | √ | √ | √ | ||||||
| Png, 2022 [8] | 12 | √ | √ | √ | √ | |||||
| Prieto-González et al., 2019 [23] | 12 | √ | ||||||||
| Prosser et al., 2012 [22] | 10 | √ | √ | √ | √ | √ | √ | |||
| Ulph et al., 2017 [24] | 12 | √ | √ | |||||||
| Wright et al., 2015 [19] | 10 | √ |
| Question | Decision Affected |
|---|---|
| Does the heterogeneity of patients’ attributes affect the outcomes in a non-linear fashion? | Modelling approach |
| Does the screening/diagnosis protocol include complex strategies that depend on individual patient characteristics? | Modelling approach |
| Does a positive test result involve conditions other than the target? | Model structure |
| Is the screening strategy a complex intervention, which comprises a series of tests, recalls, etc., that have a remarkable impact on outcomes? | Model structure |
| Is there evidence that ascertainment bias plays a critical role in the affected population? | Model structure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilcott, J.; Bessey, A.; Bonham, J.R.; Castilla-Rodríguez, I.; Davis, S.; Elliman, D.; Hunt, S.; Hyde, C.; Lombardo, S.; Madan, J.; et al. Methodological and Procedural Considerations for Developing Decision Analytic Models to Assess the Health Economic Impacts of Newborn Bloodspot Screening: A Systematic Methodological Review. Int. J. Neonatal Screen. 2025, 11, 96. https://doi.org/10.3390/ijns11040096
Chilcott J, Bessey A, Bonham JR, Castilla-Rodríguez I, Davis S, Elliman D, Hunt S, Hyde C, Lombardo S, Madan J, et al. Methodological and Procedural Considerations for Developing Decision Analytic Models to Assess the Health Economic Impacts of Newborn Bloodspot Screening: A Systematic Methodological Review. International Journal of Neonatal Screening. 2025; 11(4):96. https://doi.org/10.3390/ijns11040096
Chicago/Turabian StyleChilcott, Jim, Alice Bessey, James R. Bonham, Iván Castilla-Rodríguez, Sarah Davis, David Elliman, Sara Hunt, Chris Hyde, Silvia Lombardo, Jason Madan, and et al. 2025. "Methodological and Procedural Considerations for Developing Decision Analytic Models to Assess the Health Economic Impacts of Newborn Bloodspot Screening: A Systematic Methodological Review" International Journal of Neonatal Screening 11, no. 4: 96. https://doi.org/10.3390/ijns11040096
APA StyleChilcott, J., Bessey, A., Bonham, J. R., Castilla-Rodríguez, I., Davis, S., Elliman, D., Hunt, S., Hyde, C., Lombardo, S., Madan, J., Marshall, J., Morris, J., Payne, K., Rivero-Arias, O., Shinkins, B., Shortland, G., Spillane, S., Sutton, A., Taylor-Phillips, S., & Visintin, C. (2025). Methodological and Procedural Considerations for Developing Decision Analytic Models to Assess the Health Economic Impacts of Newborn Bloodspot Screening: A Systematic Methodological Review. International Journal of Neonatal Screening, 11(4), 96. https://doi.org/10.3390/ijns11040096







