The Burden of Congenital Hypothyroidism Without Newborn Screening: Clinical and Cognitive Findings from a Multicenter Study in Algeria
Abstract
1. Introduction
2. Methods
2.1. Objectives
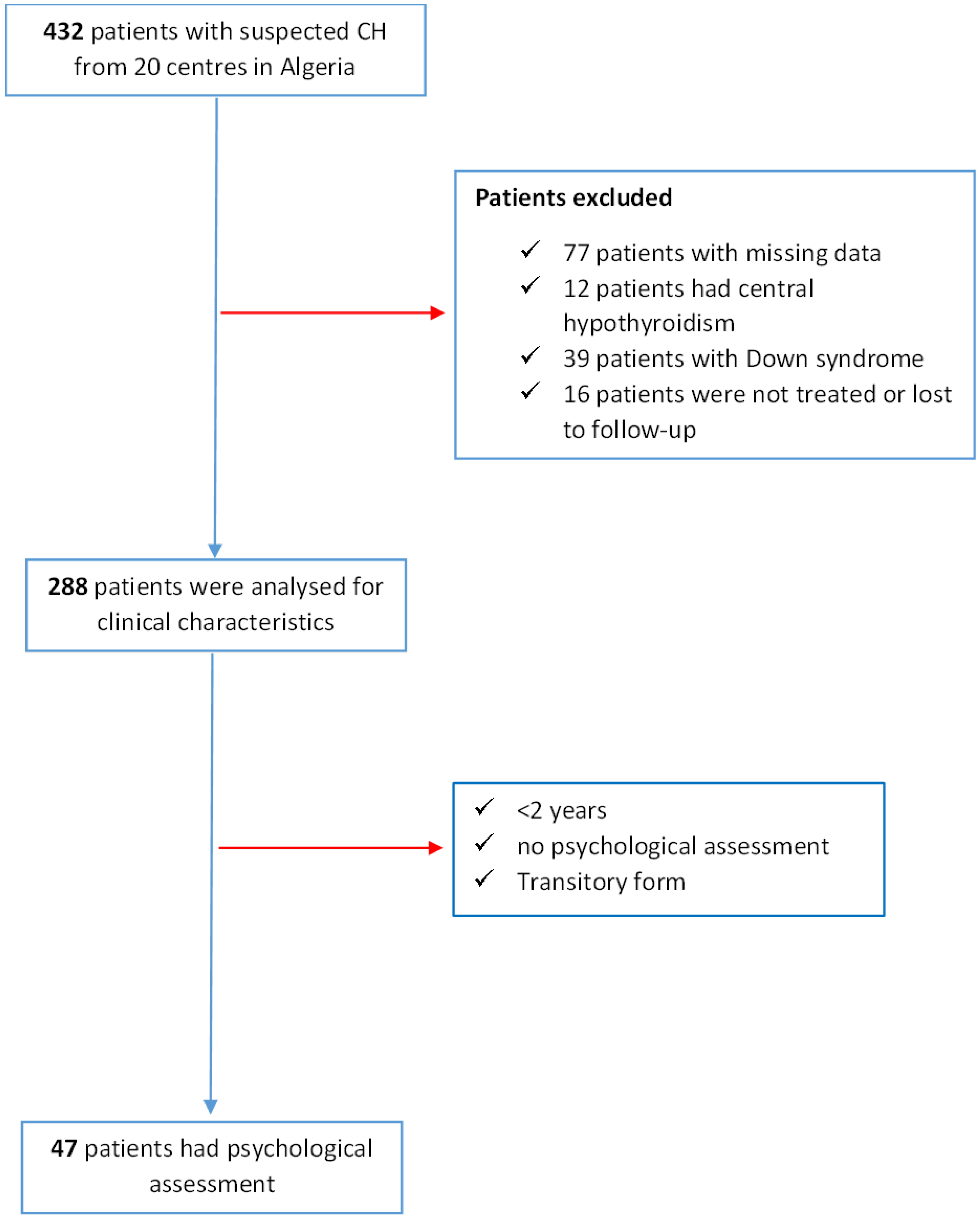
2.2. Study Design and Population
2.3. Data Collection
2.4. Definitions
2.5. Neurodevelopmental Assessment
2.6. Reassessment
2.7. Ethics
2.8. Statistical Analysis
3. Results
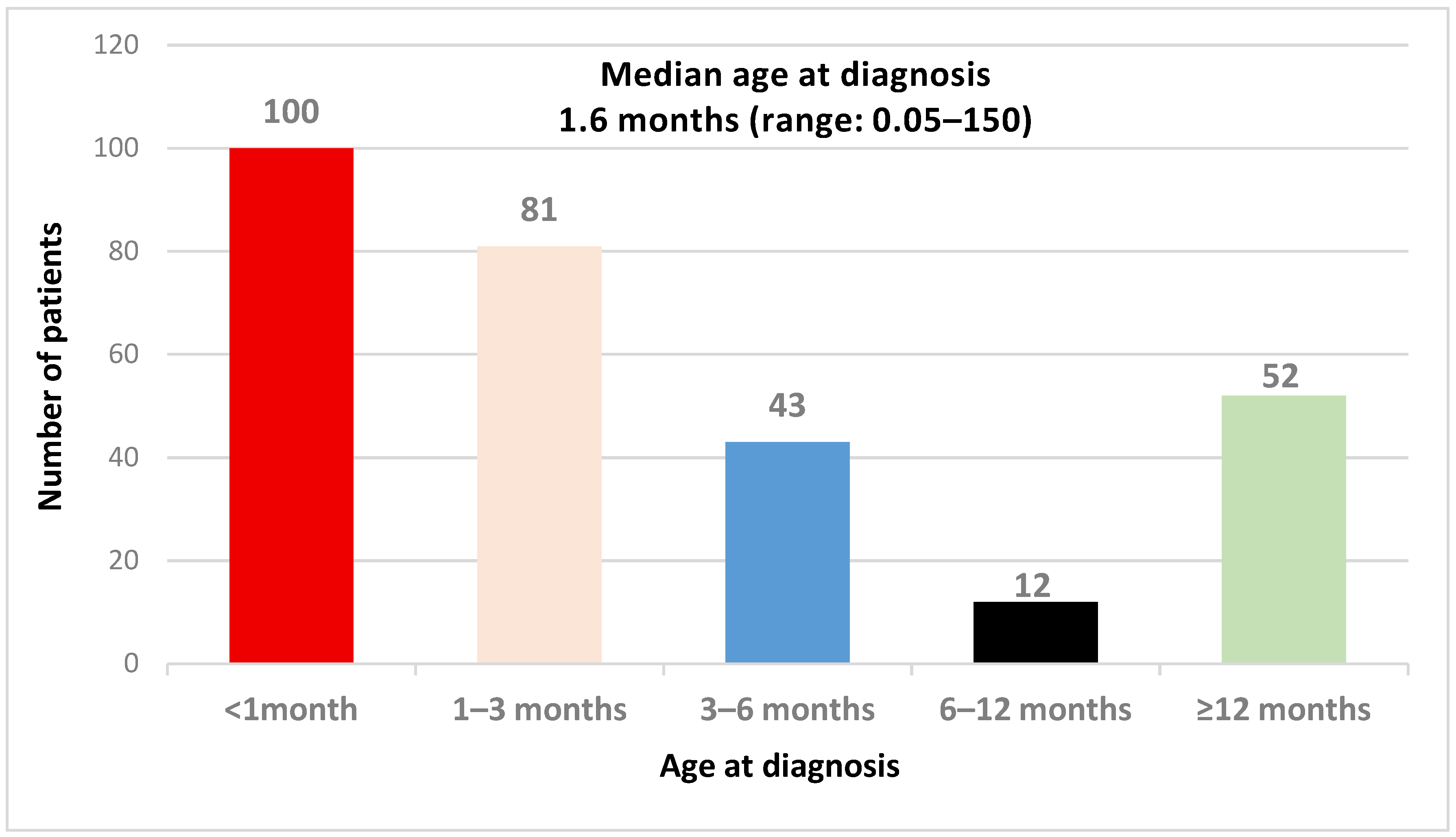
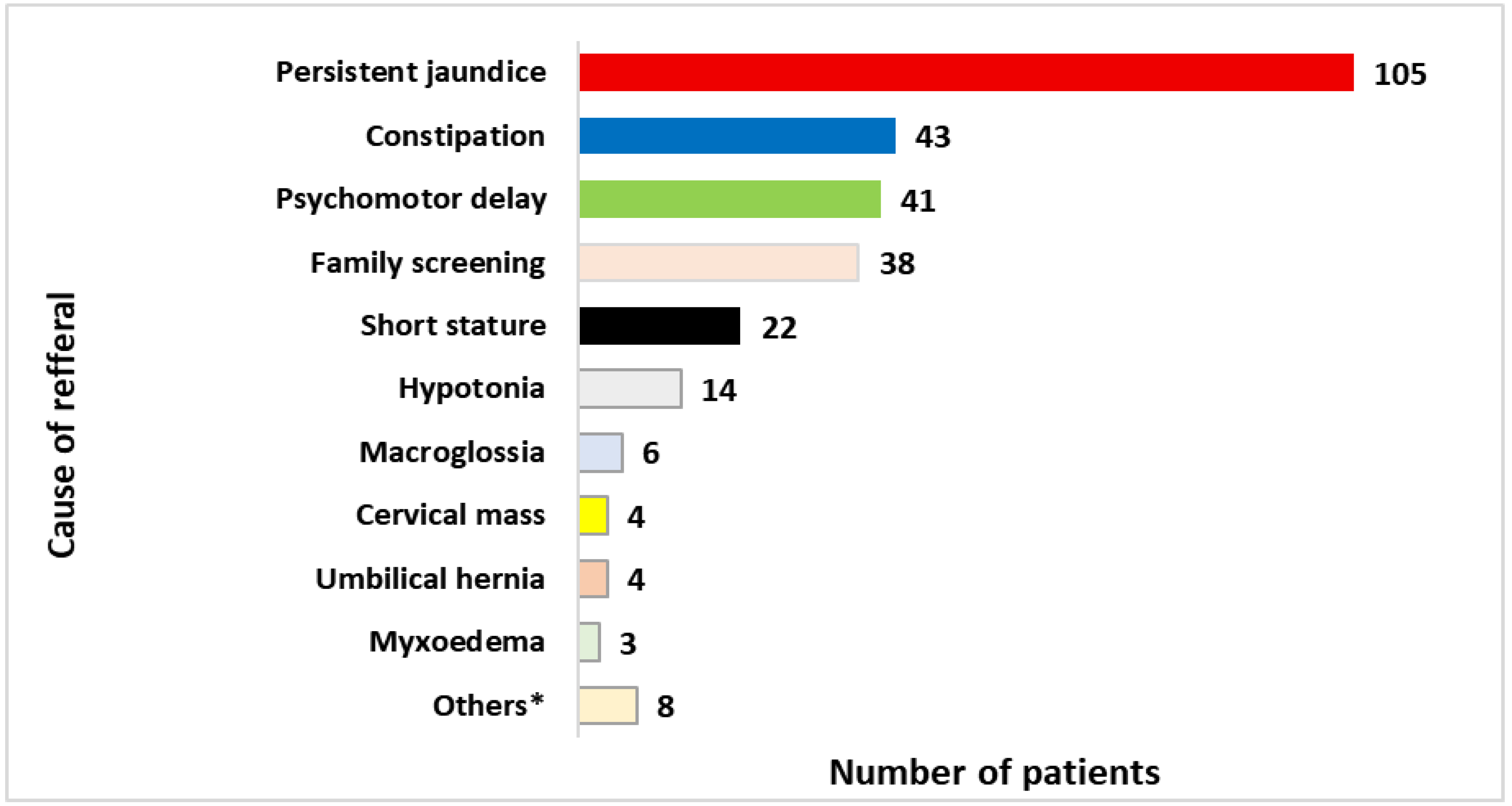
3.1. Characteristics of the CH Population
3.2. Imaging Exams and Aetiological Groups
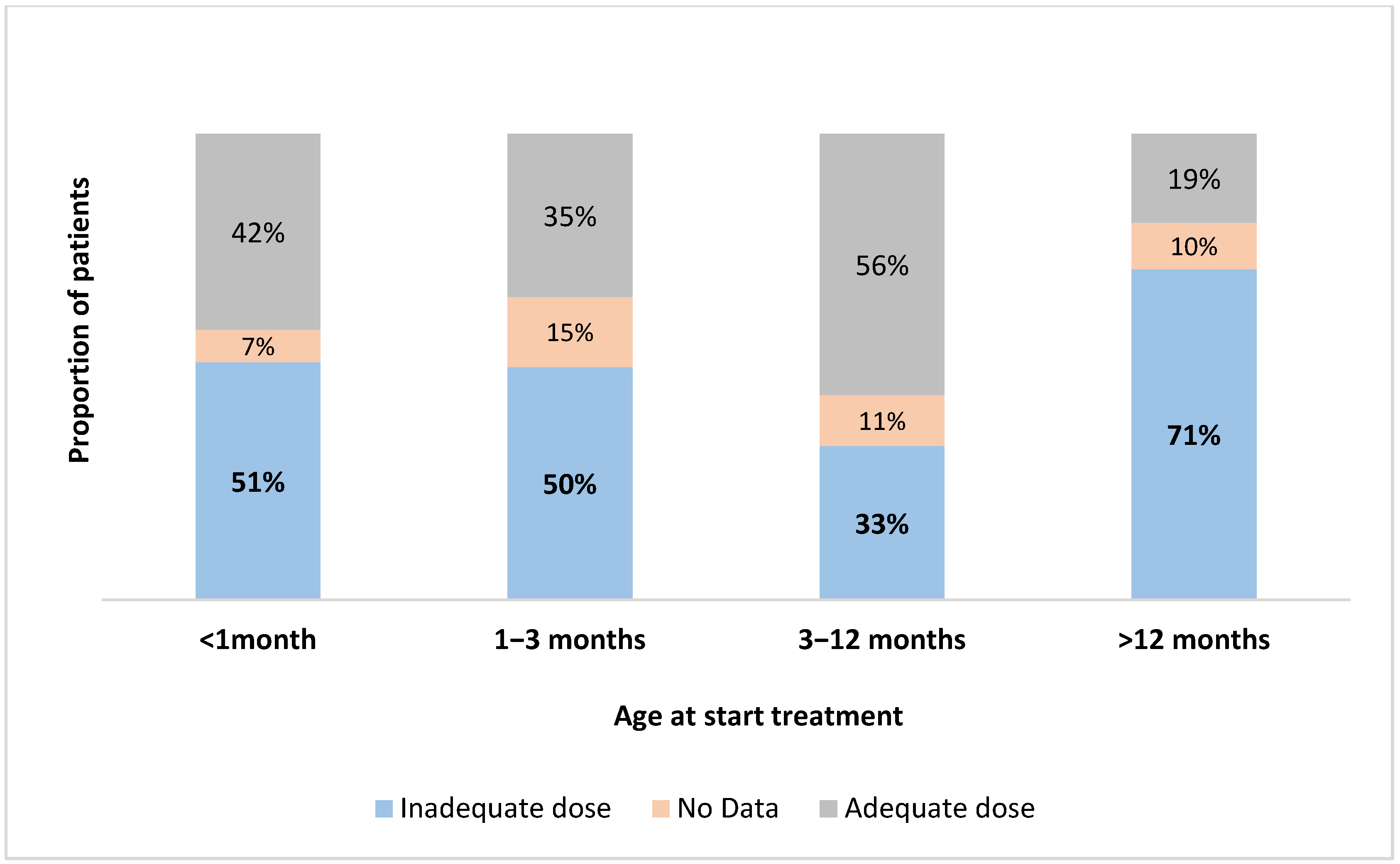
3.3. Treatment
3.4. Outcomes
3.4.1. Transient vs. Permanent CH
3.4.2. Neurodevelopmental Assessment (Table 5)
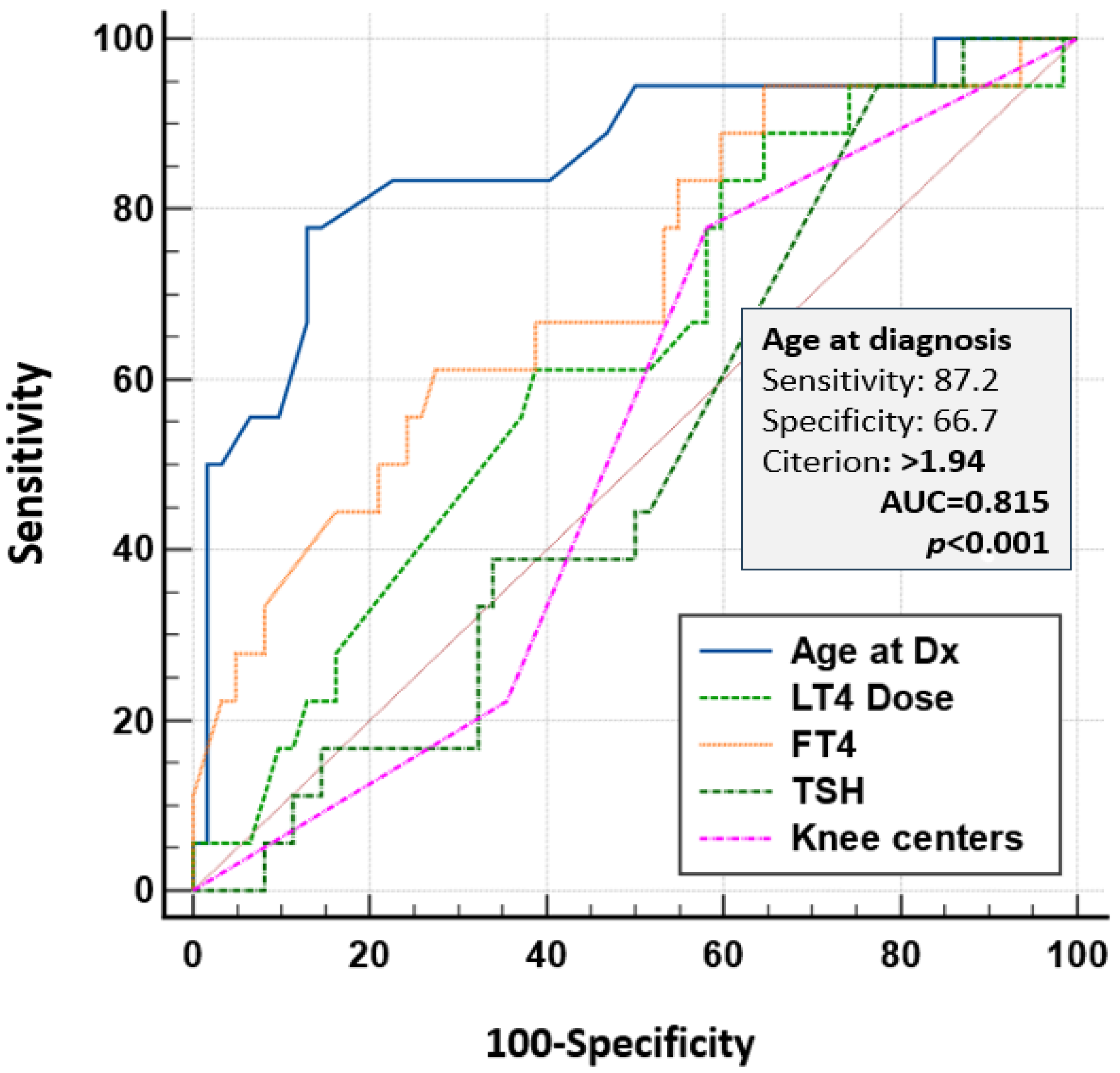
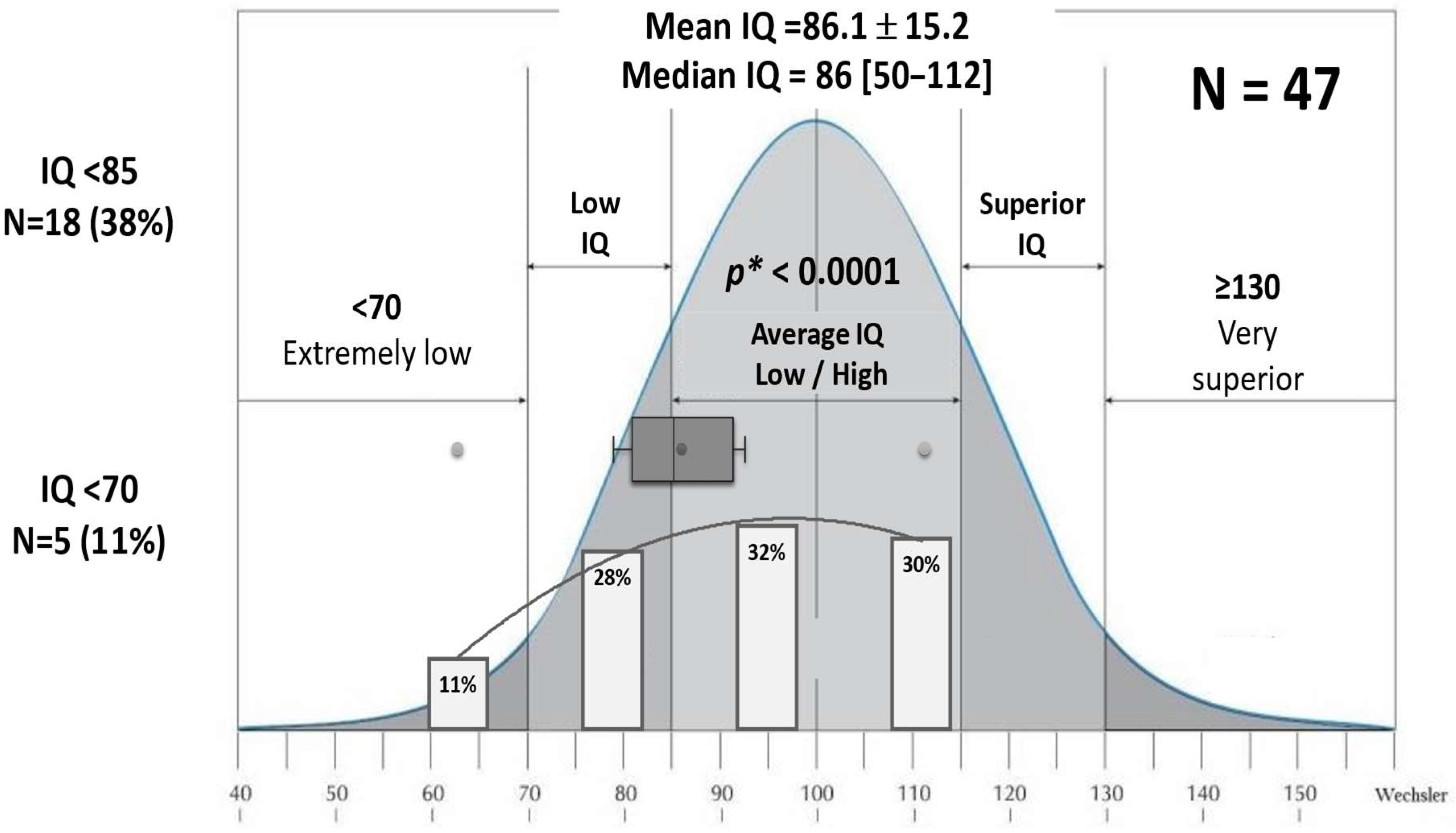
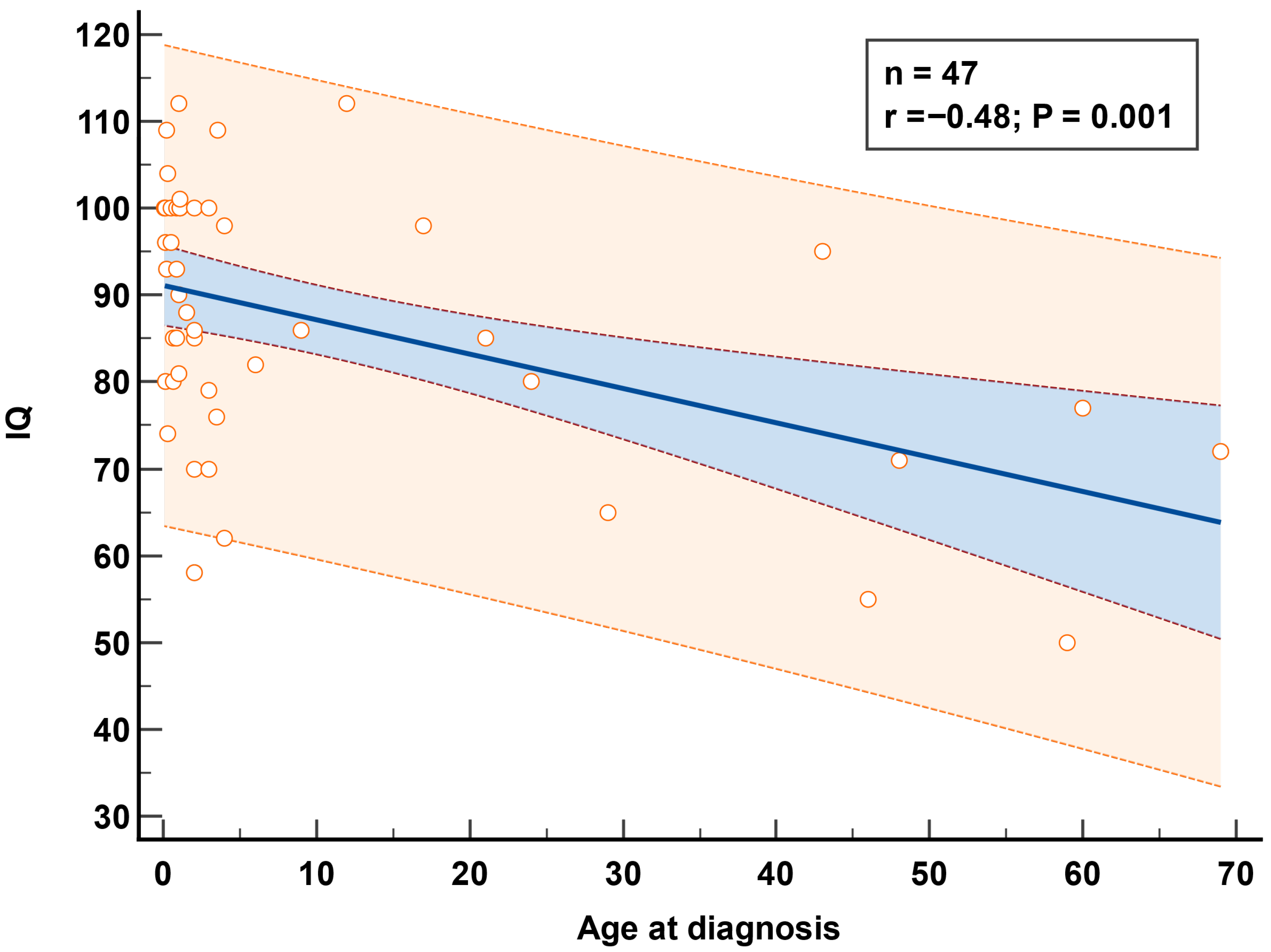
3.4.3. IQ Evaluation
4. Discussion
4.1. The Consequences of Delayed Diagnosis and Treatment
4.2. Etiological Diagnosis and Genetic Factors in Congenital Hypothyroidism
4.3. Permanent and Transient Forms of CH
4.4. Treatment of CH
4.5. Neurodevelopmental Data
4.5.1. Educational Outcomes and Cognitive Impairments
4.5.2. Preventing Delayed Diagnosis and Reducing Cognitive Risks in Congenital Hypothyroidism
4.5.3. Challenges in Timely Screening Implementation
4.5.4. Education and Public Awareness Initiatives
Healthcare Provider Education
Public Awareness Campaigns
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Vliet, G.; Deladoëy, J. Diagnosis, Treatment and Outcome of Congenital Hypothyroidism. Endocr. Dev. 2014, 26, 50–59. [Google Scholar] [CrossRef]
- Grant, D.B.; Smith, I.; Fuggle, P.W.; Tokar, S.; Chapple, J. Congenital Hypothyroidism Detected by Neonatal Screening: Relationship between Biochemical Severity and Early Clinical Features. Arch. Dis. Child. 1992, 67, 87–90. [Google Scholar] [CrossRef][Green Version]
- Alm, J.; Larsson, A.; Zetterstrom, R. Congenital Hypothyroidism in Sweden: Psychomotor Development in Patients Detected by Clinical Signs and Symptoms. Acta Pædiatrica 1981, 70, 907–912. [Google Scholar] [CrossRef]
- He, S.; Ma, X.; Yang, J.; Li, L. Levothyroxine Treatment for Congenital Hypothyroidism Based on Thyroid Function: A 10-Year Clinical Retrospective Study. BMC Endocr. Disord. 2022, 22, 142. [Google Scholar] [CrossRef]
- Donaldson, M.; Jones, J. Optimising Outcome in Congenital Hypothyroidism; Current Opinions on Best Practice in Initial Assessment and Subsequent Management. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 13–22. [Google Scholar] [CrossRef]
- Fasfous, A.F.; Daugherty, J.C. Cultural Considerations in Neuropsychological Assessment of Arab Populations. In Cultural Diversity in Neuropsychological Assessment; Routledge: New York, NY, USA, 2022; pp. 135–150. [Google Scholar]
- Fasfous, A.F.; Al-Joudi, H.F.; Puente, A.E.; Pérez-García, M. Neuropsychological Measures in the Arab World: A Systematic Review. Neuropsychol. Rev. 2017, 27, 158–173. [Google Scholar] [CrossRef]
- Van Trotsenburg, P.; Stoupa, A.; Léger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital Hypothyroidism: A 2020–2021 Consensus Guidelines Update—An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [CrossRef]
- Grosse, S.D.; Van Vliet, G. Prevention of Intellectual Disability through Screening for Congenital Hypothyroidism: How Much and at What Level? Arch. Dis. Child. 2011, 96, 374–379. [Google Scholar] [CrossRef]
- Grob, F.; Lain, S.; Olivieri, A. Newborn Screening for Primary Congenital Hypothyroidism: Past, Present and Future. Eur. Thyroid J. 2025, 14, e240358. [Google Scholar] [CrossRef]
- Kaye, C.I.; Schaefer, G.B.; Bull, M.J.; Enns, G.M.; Gruen, J.R.; Hersh, J.H.; Mendelsohn, N.J.; Saal, H.M. Introduction to the Newborn Screening Fact Sheets. Pediatrics 2006, 118, 1304–1312. [Google Scholar] [CrossRef]
- Fisher, D.A.; Dussault, J.H.; Foley, T.P.; Klein, A.H.; LaFranchi, S.; Larsen, P.R.; Mitchell, M.L.; Murphey, W.H.; Walfish, P.G. Screening for Congenital Hypothyroidism: Results of Screening One Million North American Infants. J. Pediatr. 1979, 94, 700–705. [Google Scholar] [CrossRef]
- Rastogi, M.V.; LaFranchi, S.H. Congenital Hypothyroidism. Orphanet J. Rare Dis. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Pezzuti, I.L.; De Lima, P.P.; Dias, V.M.A. Hipotireoidismo Congĉnito: Perfil Clínico Dos Recém-Nascidos Identificados Pelo Programa de Triagem Neonatal de Minas Gerais. J. Pediatr. 2009, 85, 72–79. [Google Scholar] [CrossRef]
- Raiti, S.; Newns, G.H. Cretinism: Early Diagnosis Its Relation to Mental Prognosis. Arch. Dis. Child. 1971, 46, 692–694. [Google Scholar] [CrossRef][Green Version]
- Alm, J.; Larsson, A.; Zetterström, R. Congenital Hypothyroidism in Sweden Incidence and Age at Diagnosis. Acta Pædiatrica 1978, 67, 1–3. [Google Scholar] [CrossRef]
- LaFranchi, S. Congenital Hypothyroidism: Etiologies, Diagnosis, and Management. Thyroid 1999, 9, 735–740. [Google Scholar] [CrossRef]
- Yunis, K.A.; Nasr, M.R.; Lepejian, G.; Najjar, S.; Daher, R. Short Report: False-Negative Primary Neonatal Thyroid Screening: The Need for Clinical Vigilance and Secondary Screening. J. Med. Screen. 2003, 10, 2–4. [Google Scholar] [CrossRef]
- Nagendra, L.; Bhavani, N.; Pavithran, P.V.; Shenoy, M.; Menon, U.V.; Abraham, N.; Nair, V.; Kumar, H. Etiological Profile, Targeted Levothyroxine Dosing and Impact of Partial Newborn Screening in Congenital Hypothyroidism—A Single Centre Experience. Indian J. Endocrinol. Metab. 2023, 27, 445–449. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, G.A. Congenital Hypothyroidism in the Netherlands. Lancet 1976, 308, 143. [Google Scholar] [CrossRef]
- Wolter, R.; Noël, P.; De Cock, P.; Craen, M.; Ernould, C.; Malvaux, P.; Verstraeten, F.; Simons, J.; Mertens, S.; van Broeck, N.; et al. Neuropsychological Study in Treated Thyroid Dysgenesis. Acta Paediatr. 1979, 68, 41–46. [Google Scholar] [CrossRef]
- Jacobsen, B.B.; Brandt, N.J. Congenital Hypothyroidism in Denmark. Arch. Dis. Child. 1981, 56, 134–136. [Google Scholar] [CrossRef]
- Tarim, O.F.; Yordam, N. Congenital Hypothyroidism in Turkey: A Retrospective Evaluation of 1000 Cases. Turk. J. Pediatr. 1992, 34, 197–202. [Google Scholar] [PubMed]
- Nasheiti NA Childhood Hypothyroidism in Iraq: A Retrospective Study. Int. J. Endocrinol. Metab. 2005, 3, 136–139.
- Chen, C.Y.; Lee, K.T.; Lee, C.T.C.; Ter Lai, W.; Huang, Y. Bin Epidemiology and Clinical Characteristics of Congenital Hypothyroidism in an Asian Population: A Nationwide Population-Based Study. J. Epidemiol. 2013, 23, 85–94. [Google Scholar] [CrossRef]
- Niang, B.; Fall, A.L.; Ba, I.D.; Keita, Y.; Ly, I.D.; Ba, A.; Thiongane, A.; Ndongo, A.A.; Boiro, D.; Thiam, L.; et al. Congenital Hypothyroidism in Dakar: About 28 Cases. Pan Afr. Med. J. 2016, 25, 46. [Google Scholar] [CrossRef]
- Deliana, M.; Batubara, J.R.; Tridjaja, B.; Pulungan, A.B. Hipotiroidisme Kongenital Di Bagian Ilmu Kesehatan Anak RS Ciptomangunkusumo Jakarta, Tahun 1992-2002. Sari Pediatr. 2016, 5, 79. [Google Scholar] [CrossRef][Green Version]
- Saoud, M.; Al-Fahoum, S.; Kabalan, Y. Congenital Hypothyroidism: A Five-Year Retrospective Study at Children’s University Hospital, Damascus, Syria. Qatar Med. J. 2019, 2019, 7. [Google Scholar] [CrossRef]
- Kahssay, M.; Ngwiri, T. High Yield of Congenital Hypothyroidism among Infants Attending Children Hospital, Nairobi, Kenya. Facility Based Study in the Absence of Newborn Screening. J. Pediatr. Endocrinol. Metab. 2025, 38, 51–57. [Google Scholar] [CrossRef]
- Klein, A.H.; Meltzer, S.; Kenny, F.M. Improved Prognosis in Congenital Bypothyroidism Treated before Age Three Months. J. Pediatr. 1972, 81, 912–915. [Google Scholar] [CrossRef]
- Gulshan, A.; Tahmina, B.; Fouzia, M.; Mizanur, R. Neurodevelopmental Outcome of Congenital Hypothyroidism in Children between 1-5 Years of Age. Bangladesh J. Med. Sci. 2011, 10, 245–251. [Google Scholar] [CrossRef]
- John, T.; Anirudhan, D. Physical Growth and Intellectual Function of Children with Congenital Hypothyroidism: An Observational Study. Sri Lanka J. Child Health 2023, 52, 148–154. [Google Scholar] [CrossRef]
- Pulungan, A.B.; Oldenkamp, M.E.; Van Trotsenburg, A.S.P.; Windarti, W.; Gunardi, H. Effect of Delayed Diagnosis and Treatment of Congenital Hypothyroidism on Intelligence and Quality of Life: An Observational Study. Med. J. Indones. 2019, 28, 396–401. [Google Scholar] [CrossRef]
- Léger, J.; Larroque, B.; Norton, J. Influence of Severity of Congenital Hypothyroidism and Adequacy of Treatment on School Achievement in Young Adolescents: A Population-Based Cohort Study. Acta Paediatr. Int. J. Paediatr. 2001, 90, 1249–1256. [Google Scholar] [CrossRef]
- Gunnerbeck, A.; Lundholm, C.; von Döbeln, U.; Zetterström, R.H.; Almqvist, C.; Nordenström, A. Congenital Hypothyroidism and School Achievement in Adolescence: A Population-Based Sibling Control Study. J. Pediatr. 2024, 275, 114240. [Google Scholar] [CrossRef]
- Kreisner, E.; Schermann, L.; Camargo-Neto, E.; Gross, J.L. Predictors of Intellectual Outcome in a Cohort of Brazilian Children with Congenital Hypothyroidism. Clin. Endocrinol. 2004, 60, 250–255. [Google Scholar] [CrossRef]
- Selva, K.A.; Harper, A.; Downs, A.; Blasco, P.A.; LaFranchi, S.H. Neurodevelopmental Outcomes in Congenital Hypothyroidism: Comparison of Initial T4 Dose and Time to Reach Target T4 and TSH. J. Pediatr. 2005, 147, 775–780. [Google Scholar] [CrossRef]
- Léger, J.; Ecosse, E.; Roussey, M.; Lanoë, J.L.; Larroque, B. Subtle Health Impairment and Socioeducational Attainment in Young Adult Patients with Congenital Hypothyroidism Diagnosed by Neonatal Screening: A Longitudinal Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2011, 96, 1771–1782. [Google Scholar] [CrossRef][Green Version]
- Pramono, L.A.; Yuwono, A. Late Diagnosis of Congenital Hypothyroidism in Young Adult. Acta Med. Indones. 2019, 51, 272–274. [Google Scholar][Green Version]
- Makretskaya, N.; Bezlepkina, O.; Kolodkina, A.; Kiyaev, A.; Vasilyev, E.V.; Petrov, V.; Kalinenkova, S.; Malievsky, O.; Dedov, I.I.; Tiulpakov, A. High Frequency of Mutations in’dyshormonogenesis Genes’ in Severe Congenital Hypothyroidism. PLoS ONE 2018, 13, e0204323. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, H.Y.; Kim, Y.M.; Lee, H.; Bae, M.H.; Park, K.H.; Lee, S.M.; Kwak, M.J. Genetic Evaluation of Congenital Hypothyroidism with Gland in Situ Using Targeted Exome Sequencing. Ann. Clin. Lab. Sci. 2021, 51, 73–81. [Google Scholar][Green Version]
- Li, L.; Li, X.; Wang, X.; Han, M.; Zhao, D.; Wang, F.; Liu, S. Mutation Screening of Eight Genes and Comparison of the Clinical Data in a Chinese Cohort with Congenital Hypothyroidism. Endocrine 2023, 79, 125–134. [Google Scholar] [CrossRef]
- Bruellman, R.J.; Watanabe, Y.; Ebrhim, R.S.; Creech, M.K.; Abdullah, M.A.; Dumitrescu, A.M.; Refetoff, S.; Weiss, R.E. Increased Prevalence of TG and TPO Mutations in Sudanese Children with Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2020, 105, 1564–1572. [Google Scholar] [CrossRef]
- Albert, B.B.; Cutfield, W.S.; Webster, D.; Carll, J.; Derraik, J.G.B.; Jefferies, C.; Gunn, A.J.; Hofman, P.L. Etiology of Increasing Incidence of Congenital Hypothyroidism in New Zealand from 1993–2010. J. Clin. Endocrinol. Metab. 2012, 97, 3155–3160. [Google Scholar] [CrossRef]
- Hall, S.; Hutchesson, A.; Kirk, J. Congenital Hypothyroidism, Seasonality and Consanguinity in the West Midlands, England. Acta Paediatr. 1999, 88, 212–215. [Google Scholar] [CrossRef]
- Golbahar, J.; Al-Khayyat, H.; Hassan, B.; Agab, W.; Hassan, E.; Darwish, A. Neonatal Screening for Congenital Hypothyroidism: A Retrospective Hospital Based Study from Bahrain. J. Pediatr. Endocrinol. Metab. 2010, 23, 39–44. [Google Scholar] [CrossRef]
- Bundey, S.; Alam, H. A Five-Year Prospective Study of the Health of Children in Different Ethnic Groups, with Particular Reference to the Effect of Inbreeding. Eur. J. Hum. Genet. 1993, 1, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Gordillo, R.; Kaskel, F.J.; Druschel, C.M.; Woroniecki, R.P. Increased Prevalence of Renal and Urinary Tract Anomalies in Children with Congenital Hypothyroidism. J. Pediatr. 2009, 154, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Danner, E.; Jääskeläinen, J.; Niuro, L.; Huopio, H.; Niinikoski, H.; Viikari, L.; Kero, J.; Sund, R. Comorbidity in Congenital Hypothyroidism—A Nationwide, Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2023, 108, e1695–e1701. [Google Scholar] [CrossRef]
- Stoupa, A.; Carré, A.; Polak, M.; Szinnai, G.; Schoenmakers, N. Genetics of Primary Congenital Hypothyroidism: Three Decades of Discoveries and Persisting Etiological Challenges. Eur. Thyroid J. 2025, 14, e240348. [Google Scholar] [CrossRef] [PubMed]
- Benallègue, A.; Kedji, F. Consanguinité et Santé Publique. Une Étude Algérienne. Arch. Fr. Pediatr. 1984, 41, 435–440. [Google Scholar]
- Nagasaki, K.; Sato, H.; Sasaki, S.; Nyuzuki, H.; Shibata, N.; Sawano, K.; Hiroshima, S.; Asami, T. Re-Evaluation of the Prevalence of Permanent Congenital Hypothyroidism in Niigata, Japan: A Retrospective Study. Int. J. Neonatal Screen. 2021, 7, 27. [Google Scholar] [CrossRef]
- Matejek, N.; Tittel, S.R.; Haberland, H.; Rohrer, T.; Busemann, E.M.; Jorch, N.; Schwab, K.O.; Wölfle, J.; Holl, R.W.; Bettendorf, M. Predictors of Transient Congenital Primary Hypothyroidism: Data from the German Registry for Congenital Hypothyroidism (AQUAPE “HypoDok”). Eur. J. Pediatr. 2021, 180, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Kanike, N.; Davis, A.; Shekhawat, P.S. Transient Hypothyroidism in the Newborn: To Treat or Not to Treat. Transl. Pediatr. 2017, 6, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Schoenmakers, N. MECHANISMS IN ENDOCRINOLOGY: The Pathophysiology of Transient Congenital Hypothyroidism. Eur. J. Endocrinol. 2022, 187, R1–R16. [Google Scholar] [CrossRef]
- Schoen, E.J.; Clapp, W.; To, T.T.; Fireman, B.H. The Key Role of Newborn Thyroid Scintigraphy with Isotopic Iodide ( 123I) in Defining and Managing Congenital Hypothyroidism. Pediatrics 2004, 114, e683–e688. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, T.; Hasegawa, Y.; Higuchi, S.; Satoh, M.; Sawada, H.; Shimura, K.; Takahashi, I.; Takubo, N.; Nagasaki, K. Knowns and Unknowns about Congenital Hypothyroidism: 2022 Update. Clin. Pediatr. Endocrinol. 2023, 32, 11–25. [Google Scholar] [CrossRef]
- Simoneau-Roy, J.; Marti, S.; Deal, C.; Huot, C.; Robaey, P.; Van Vliet, G. Cognition and Behavior at School Entry in Children with Congenital Hypothyroidism Treated Early with High-Dose Levothyroxine. J. Pediatr. 2004, 144, 747–752. [Google Scholar] [CrossRef]
- Rahmani, K.; Yarahmadi, S.; Etemad, K.; Koosha, A.; Mehrabi, Y.; Aghang, N.; Soori, H. Congenital Hypothyroidism: Optimal Initial Dosage and Time of Initiation of Treatment: A Systematic Review. Int. J. Endocrinol. Metab. 2016, 14, e36080. [Google Scholar] [CrossRef]
- Aleksander, P.E.; Brückner-Spieler, M.; Stoehr, A.-M.; Lankes, E.; Kühnen, P.; Schnabel, D.; Ernert, A.; Stäblein, W.; Craig, M.E.; Blankenstein, O.; et al. Mean High-Dose l-Thyroxine Treatment Is Efficient and Safe to Achieve a Normal IQ in Young Adult Patients With Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2018, 103, 1459–1469. [Google Scholar] [CrossRef]
- Rose, S.R.; Wassner, A.J.; Wintergerst, K.A.; Yayah-Jones, N.-H.; Hopkin, R.J.; Chuang, J.; Smith, J.R.; Abell, K.; LaFranchi, S.H.; Wintergerst, K.A.; et al. Congenital Hypothyroidism: Screening and Management. Pediatrics 2023, 151, e2022060419. [Google Scholar] [CrossRef]
- Esposito, A.; Vigone, M.C.; Polizzi, M.; Wasniewska, M.G.; Cassio, A.; Mussa, A.; Gastaldi, R.; Di Mase, R.; Vincenzi, G.; Pozzi, C.; et al. Effect of Initial Levothyroxine Dose on Neurodevelopmental and Growth Outcomes in Children with Congenital Hypothyroidism. Front. Endocrinol. 2022, 13, 923448. [Google Scholar] [CrossRef]
- Vigone, M.C.; Ortolano, R.; Vincenzi, G.; Pozzi, C.; Ratti, M.; Assirelli, V.; Vissani, S.; Cavarzere, P.; Mussa, A.; Gastaldi, R.; et al. Treatment of Congenital Hypothyroidism: Comparison between L-Thyroxine Oral Solution and Tablet Formulations up to 3 Years of Age. Eur. J. Endocrinol. 2022, 186, 45–52. [Google Scholar] [CrossRef]
- Vulsma, T.; Gons, M.H.; de Vijlder, J.J.M. Maternal-Fetal Transfer of Thyroxine in Congenital Hypothyroidism Due to a Total Organification Defect or Thyroid Agenesis. N. Engl. J. Med. 1989, 321, 13–16. [Google Scholar] [CrossRef] [PubMed]
- LaFranchi, S. Congenital Hypothyroidism: A Newborn Screening Success Story? Endocrinologist 1994, 4, 477–486. [Google Scholar] [CrossRef]
- Collaborative, N.E.C.H. Neonatal Hypothyroidism Screening: Status of Patients at 6 Years of Age. J. Pediatr. 1985, 107, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Bargagna, S.; Dinetti, D.; Pinchera, A.; Marcheschi, M.; Montanelli, L.; Presciuttini, S.; Chiovato, L. School Attainments in Children with Congenital Hypothyroidism Detected by Neonatal Screening and Treated Early in Life. Eur. J. Endocrinol. 1999, 140, 407–413. [Google Scholar] [CrossRef][Green Version]
- Hulse, J.A. Outcome for Congenital Hypothyroidism. Arch. Dis. Child. 1984, 59, 23–29. [Google Scholar] [CrossRef]
- Rovet, J.; Ehrlich, R.; Sorbara, D. Intellectual Outcome in Children with Fetal Hypothyroidism. J. Pediatr. 1987, 110, 700–704. [Google Scholar] [CrossRef]
- Murphy, G.H.; Hulse, J.A.; Smith, I.; Grant, D.B. Congenital Hypothyroidism: Physiological and Psychological Factors in Early Development. J. Child Psychol. Psychiatry 1990, 31, 711–725. [Google Scholar] [CrossRef]
- Fuggle, P.W.; Grant, D.B.; Smith, I.; Murphy, G. Intelligence, Motor Skills and Behaviour at 5 Years in Early-Treated Congenital Hypothyroidism. Eur. J. Pediatr. 1991, 150, 570–574. [Google Scholar] [CrossRef]
- Glorieux, J.; Dussault, J.; Van Vliet, G. Intellectual Development at Age 12 Years of Children with Congenital Hypothyroidism Diagnosed by Neonatal Screening. J. Pediatr. 1992, 121, 581–584. [Google Scholar] [CrossRef]
- Bargagna, S.; Chiovato, L.; Dinetti, D.; Montanelli, L.; Giachetti, C.; Romolini, E.; Marcheschi, M.; Pinchera, A. Neuropsychological Development in a Child with Early-Treated Congenital Hypothyroidism as Compared with Her Unaffected Identical Twin. Eur. J. Endocrinol. 1997, 136, 100–104. [Google Scholar] [CrossRef]
- Simons, W.F.; Fuggle, P.W.; Grant, D.B.; Smith, I. Intellectual Development at 10 Years in Early Treated Congenital Hypothyroidism. Arch. Dis. Child. 1994, 71, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Hauri-Hohl, A.; Dusoczky, N.; Dimitropoulos, A.; Leuchter, R.H.-V.; Molinari, L.; Caflisch, J.; Jenni, O.G.; Latal, B. Impaired Neuromotor Outcome in School-Age Children With Congenital Hypothyroidism Receiving Early High-Dose Substitution Treatment. Pediatr. Res. 2011, 70, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Simons, W.F.; Fuggle, P.W.; Grant, D.B.; Smith, I. Educational Progress, Behaviour, and Motor Skills at 10 Years in Early Treated Congenital Hypothyroidism. Arch. Dis. Child. 1997, 77, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Suzuki, M. Congenital Hypothyroidism and Brain Development: Association With Other Psychiatric Disorders. Front. Neurosci. 2021, 15, 772382. [Google Scholar] [CrossRef]
- Romero, M.A.; Goto, M.M.F.; D’Ouro, M.P.C.; Lima, M.C.M.P.; Dutra, V.F.; Mendes-dos-Santos, C.T.; Santos, D.C.C. Analysis of Motor, Cognitive and Language Performance of Infants Undergoing Treatment for Congenital Hypothyroidism. J. Pediatr. 2025, 101, 172–178. [Google Scholar] [CrossRef]
- Habib, M.M.F.; Faddan, H.H.I.A.; Metwalley, K.A.; Ismail, T.A.A.M. Growth and Developmental Milestones in Children with Congenital Hypothyroidism Attending Assiut Health Insurance Clinic. Egypt. J. Community Med. 2022, 40, 233–242. [Google Scholar] [CrossRef]
- Rovet, J.F. Congenital Hypothyroidism: Long-Term Outcome. Thyroid 1999, 9, 741–748. [Google Scholar] [CrossRef]
- Léger, J. Congenital Hypothyroidism: A Clinical Update of Long-Term Outcome in Young Adults. Eur. J. Endocrinol. 2015, 172, R67–R77. [Google Scholar] [CrossRef]
- Smith, D.W.; Klein, A.M.; Henderson, J.R.; Myrianthopoulos, N.C. Congenital Hypothyroidism-Signs and Symptoms in the Newborn Period. J. Pediatr. 1975, 87, 958–962. [Google Scholar] [CrossRef]
- Ahmad, N.; Irfan, A.; Al Saedi, S. Congenital Hypothyroidism: Screening, Diagnosis, Management, and Outcome. J. Clin. Neonatol. 2017, 6, 64. [Google Scholar] [CrossRef]
- Hannon, H.; Therrell, B.; World Health Organization. Hereditary Diseases Programme. In Guidelines on the Prevention and Control of Congenital Hypothyroidism / Prepared by Harry Hannon, Brad Therrell; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]

| Family History | ||
|---|---|---|
| Consanguinity | n, (%) | 75 (26%) |
| Family history of CH in a sibling | n, (%) | 27 (9%) |
| Etiology | ||
| Dysgenesis | n, (%) | 109 (38%) |
| Athyreosis | n, (%) | 52 (18%) |
| Ectopy | n, (%) | 17 (6%) |
| Hypoplasia | n, (%) | 40 (14%) |
| Gland in situ | n, (%) | 150 (52%) |
| Goiter | n, (%) | 25 (9%) |
| Undetermined | n, (%) | 29 (10%) |
| Associated abnormalities | n, (%) | 44 (15%) |
| Heart defects | n, (%) | 23 (8%) |
| Renal defects | n, (%) | 9 (3%) |
| Biological Data | ||
|---|---|---|
| TSH mU/L * | Median (range) | 65.35 (8.12–>100) |
| fT4 pmol/L | Mean ± SDS (range) | 7.6 ± 6.2 (0.01–27) |
| TSH >100 mU/L | n, % | 125 (43.4%) |
| TSH 40–100 mU/L | n, % | 54 (18.7%) |
| TSH 20–40 mU/L | n, % | 32 (11.1%) |
| TSH < 20 mU/L | n, % | 77 (26.7%) |
| fT4 < 5 pmol/L | n, % | 102 (35.4%) |
| Global CH Cohort N = 288 | GIS/Goiter N = 150 | Ectopy/Hypoplasia N = 57 | Athyreosis N = 52 | Undetermined N = 29 | p-Value | |
|---|---|---|---|---|---|---|
| Age at diagnosis (months) | 2 | 1.5 | 4 | 2 | 0.86 | 0.001 * |
| Median (range) | (0.1–375) | (0.05–51) | (0.07–155) | (0.06–150) | (0.13–32) | |
| M/F | 129/159 | 80/69 | 24/33 | 10/42 | 15/15 | 0.0003 |
| Sex ratio | 0.81 | 1.2 | 0.7 | 0.24 | 1 | |
| Consanguinity% (N) | 26% (75) | 31% (47) | 23% (13) | 13.5% (7) | 27% (8) | 0.078 |
| TSH mU/L | 65.3 | 50 | 96.3 | 100 | 47.5 | 0.034 * |
| Median (range) | (8.1–>100) | (8.1–>100) | (10.1–>100) | (17.9–>100) | (10–>100) | |
| TSH > 100 mU/L% (N) | 43% (125) | 35% (53) | 39% (22) | 73% (38) | 41% (12) | <0.0001 |
| fT4 (pmol/L) | 7.6 ± 6.4 | 8.8 ± 6.1 | 7.2 ± 5.5 | 2.6 ± 2.7 | 8.8 ± 2.3 | 0.003 |
| Mean ± SD (range) | (0.01–25.4) | (0.01–25.7) | (0.02–27) | (0.01–10.6) | (0–25.4) | |
| fT4 < 5 pmol/L %, (N) | 35% (102) | 26% (39) | 37% (21) | 60% (31) | 38% (11) | 0.0002 |
| Transient CH N = 24 | Permanent CH N = 23 | p | |
|---|---|---|---|
| Sex ratio M/F | 12/12 | 8/15 | 0.297 |
| Age at diagnosis in months—median (range) | 4.3 (0.16–46) | 2.4 (0.1–12) | 0.440 |
| TSH mU/L—median (range) | 15.98 (4.3–>100) | 100 (7.6–>100) | 0.002 |
| TSH > 100 mU/L, n, (%) | 3 (12.5%) | 11 (48%) | 0.009 |
| TSH < 10 mU/L, n, (%) | 4 (17%) | 0 (0%) | 0.037 |
| fT4 pmol/L—mean (range) | 13.4 (0.1–17.2) | 7.5 (0.1–18.1) | 0.003 |
| fT4 < 10 pmol/L n, (%) | 5 (21%) | 14 (61%) | 0.011 |
| Gland in situ n, (%) | 23 (96%) | 10 (43%) | 0.001 |
| Dysgenesis n, (%) | 1 (5%) | 13 (57%) |
| Neurodevelopmental Data | N, (%) | Age at Evaluation Mean ± SD | Age Treatment Median | Treatment n, % | |
|---|---|---|---|---|---|
| ≤1 Month | ≥3 Months | ||||
| Psychomotor delay | 43 (15%) | 23.3 ± 25.1 months | 13.9 months | 1/43 (2%) | 36/43 (84%) |
| Language delay | 16 (6%) | 18.4 ± 18.1 months | 3.6 months | 2/16 (12.5%) | 9/16 (56%) |
| School-aged children | 88/206 (43%) | 11.6 ± 3.4 years | 3 months | 20/88 (23%) | 47/88 (53%) |
| School failure | 27/88 (31%) | 10.8 ± 3.9 years | 4 months | 6/27 (22%) | 16/27 (59%) |
| IQ assessment N = 47 | |||||
| Normal IQ ≥ 85 | 29 (62%) | 5.1 ± 1.7 years | 1.1 months | 13/29 (45%) | 9/29 (31%) |
| Low IQ < 85 | 13 (28%) | 5.2 ± 1.8 years | 3 months | 2/13 (15%) | 8/13 (61.5%) |
| Very low IQ < 70 | 5 (11%) | 4.8 ± 2.5 years | 29 months | 0/5 (0%) | 4/5 (80%) |
| Cognitive disharmony | 7 (15%) | 5.6 ± 1.3 years | 2 months | 2/7 (28.6%) | 3/7 (43%) |
| Global | IQ < 85 | IQ ≥ 85 | p | ||
|---|---|---|---|---|---|
| N = 47 | N = 18 | N = 29 | |||
| Sex M/F | 15/32 | 5/13 | 10/19 | 0.63 | |
| Age IQ test years—mean (range) | 5.3 ± 1.8 (2.5–9.3) | 5.5 ± 2.1 (3–9.3) | 5.2 ± 1.7 (2.5–8) | 0.68 | |
| Age at treatment in months—median (range) | 2 (0.1–69) | 3.75 (0.2–69) | 1.1(0.1–42.7) | 0.01 | |
| Age start L-T4 | ≤1 month (n,%) | 16 (25.5%) | 3 (17%) | 13 (45%) | 0.05 |
| >1 month (n, %) | 31 (74.5%) | 15 (83%) | 16 (55%) | ||
| Etiology | Dysgenesis | 25 (53%) | 13 (72%) | 12 (41%) | 0.05 |
| Gland in situ | 21 (45%) | 5 (28%) | 16 (55%) | ||
| L-T4 Dose µg/kg/day | <9 µg/kg/day | 27 (54%) | 12 (67%) | 15 (52%) | 0.26 |
| Reference (Original Study) | Country | Period | Diagnosed in Neonatal Period | Late Diagnosis Rates | Neurodevelopmental Outcome |
|---|---|---|---|---|---|
| Raiti 1971 [15] | UK | – | 6% < 1st month 22% < 3 months | 16% < 6 months 55% by 2 years | IQ < 90 (50%) 53% treatment > 6 months |
| De Jonge 1976 [20] | Netherlands | 1972–1974 | 10% <1 month | 50% at 3 months | 34% IQ > 90, 17% IQ < 50 |
| Alm 1978 [3,16] | Sweden | 1969–1975 | 20% | 52% after 3 months | 41% IQ < 85 and/or neurological abnormalities |
| Wolter 1979 [21] | Belgium | – | 7% < 1st month 46% < 3 months | 21% > 1 year | IQ < 80 (23%); Normal IQ if treated <3 months |
| Jacobsen 1981 [22] | Denmark | 1970–1975 | 10% 1st month | 70% by 1 year | 46% intellectual disability |
| Tarim 1992 [23] | Turkey | 1964–1989 | 3.1% | 55.4% after 2 years | 21.4% inability to speak 18.1% inability to walk |
| Nasheiti 2005 [24] | Iraq | 1993–2003 | 25% | 75% beyond neonatal period | 47.5% intellectual disability |
| Chen 2013 [25] | South Asian countries | 1997–2008 | Taiwan 56% <3 months | 22% > 1 years Pakistan, India 70% >1 year | Diagnosis >3 months, higher risk of developmental delay (HR = 1.97) |
| Niang 2016 [26] | Senegal | 2001–2014 | 7% | 78.5% >6 months | 73% intellectual disability 2.5% at school |
| Deliana 2016 [27] | Indonesia | 1992–2002 | Minimal | 53% at 1–5 years 6.7% after 12 years | 62.5% intellectual disability |
| Saoud 2019 [28] | Syria | 2008–2012 | >25% 1st month | 75% beyond neonatal period | 37.1% psychomotor delay 81% diagnosis >6 months |
| Kahssay 2025 [29] | Kenya * | 2015–2020 | 5% < 1st month | 80% 6–11 months 15% > 1–2 years | 60% developmental delay |
| Our study | Algeria | 2005–2023 | 35% < 1st month | 65% ≥1 month | 28% IQ < 85, 11% IQ < 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djermane, A.; Ouarezki, Y.; Boulesnane, K.; Kherra, S.; Bouferoua, F.; Bessahraoui, M.; Selim, N.; Djahlat, L.; Mohammedi, K.; Bouziane Nedjadi, K.; et al. The Burden of Congenital Hypothyroidism Without Newborn Screening: Clinical and Cognitive Findings from a Multicenter Study in Algeria. Int. J. Neonatal Screen. 2025, 11, 78. https://doi.org/10.3390/ijns11030078
Djermane A, Ouarezki Y, Boulesnane K, Kherra S, Bouferoua F, Bessahraoui M, Selim N, Djahlat L, Mohammedi K, Bouziane Nedjadi K, et al. The Burden of Congenital Hypothyroidism Without Newborn Screening: Clinical and Cognitive Findings from a Multicenter Study in Algeria. International Journal of Neonatal Screening. 2025; 11(3):78. https://doi.org/10.3390/ijns11030078
Chicago/Turabian StyleDjermane, Adel, Yasmine Ouarezki, Kamelia Boulesnane, Sakina Kherra, Fadila Bouferoua, Mimouna Bessahraoui, Nihad Selim, Larbi Djahlat, Kahina Mohammedi, Karim Bouziane Nedjadi, and et al. 2025. "The Burden of Congenital Hypothyroidism Without Newborn Screening: Clinical and Cognitive Findings from a Multicenter Study in Algeria" International Journal of Neonatal Screening 11, no. 3: 78. https://doi.org/10.3390/ijns11030078
APA StyleDjermane, A., Ouarezki, Y., Boulesnane, K., Kherra, S., Bouferoua, F., Bessahraoui, M., Selim, N., Djahlat, L., Mohammedi, K., Bouziane Nedjadi, K., Abes, H., Bensalah, M., Lograb, D., Abdelaziz, F., Douiri, D., Djebari, S., Demdoum, M. S., Rouabeh, N., Oussalah, M., ... Ladjouze, A. (2025). The Burden of Congenital Hypothyroidism Without Newborn Screening: Clinical and Cognitive Findings from a Multicenter Study in Algeria. International Journal of Neonatal Screening, 11(3), 78. https://doi.org/10.3390/ijns11030078









