Prediction of Congenital Portosystemic Shunt in Neonatal Hypergalactosemia Using Gal-1-P/Gal Ratio, Bile Acid, and Ammonia
Abstract
1. Introduction
2. Materials and Methods
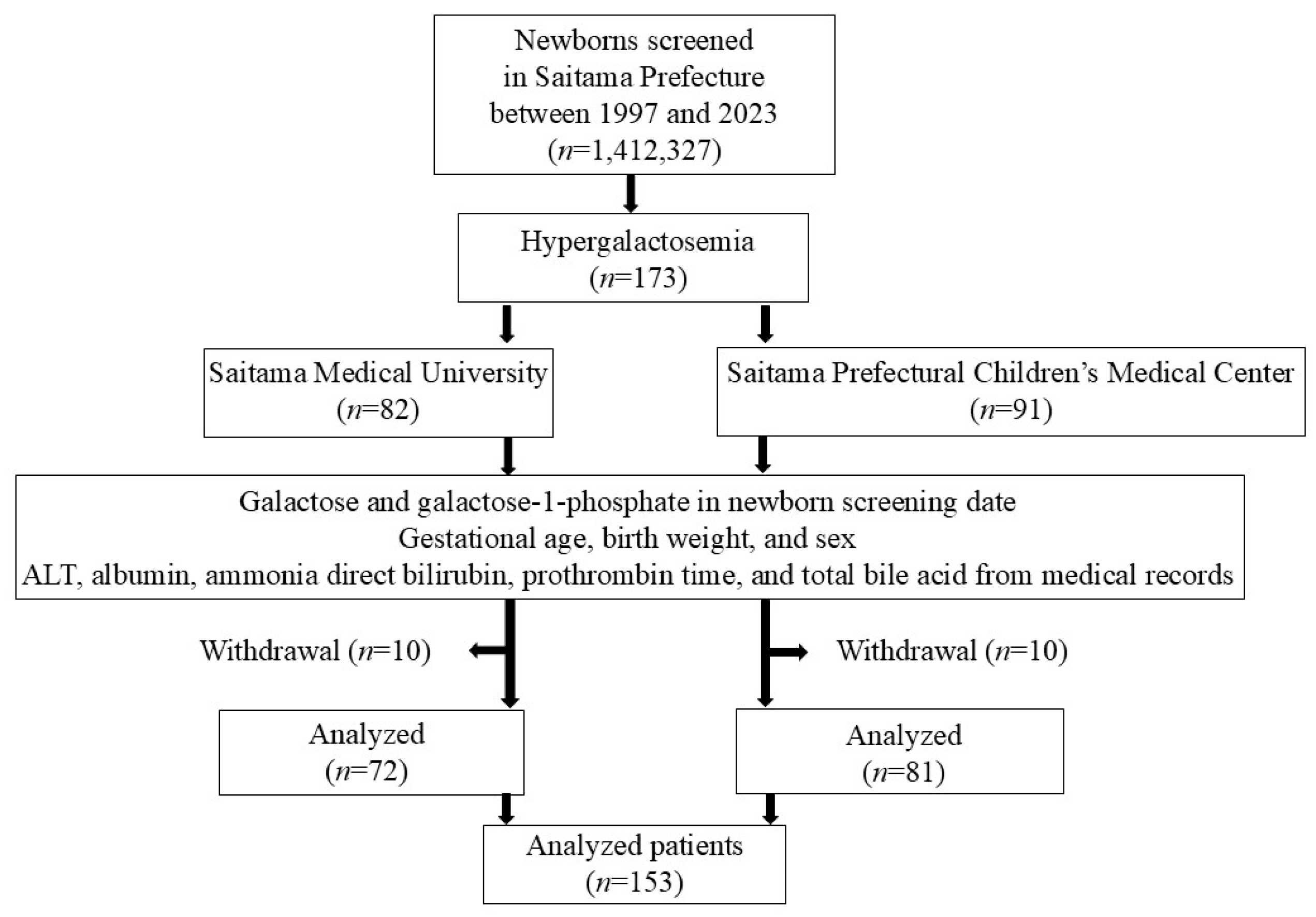
2.1. Newborn Screening
2.2. Cut-Off Values
2.3. Confirmatory Testing
2.4. Handling for Hypergalactosemia
2.5. Definitions of CPSS and Transient Galactosemia
2.6. Types of CPSS
2.7. Statistical Analysis
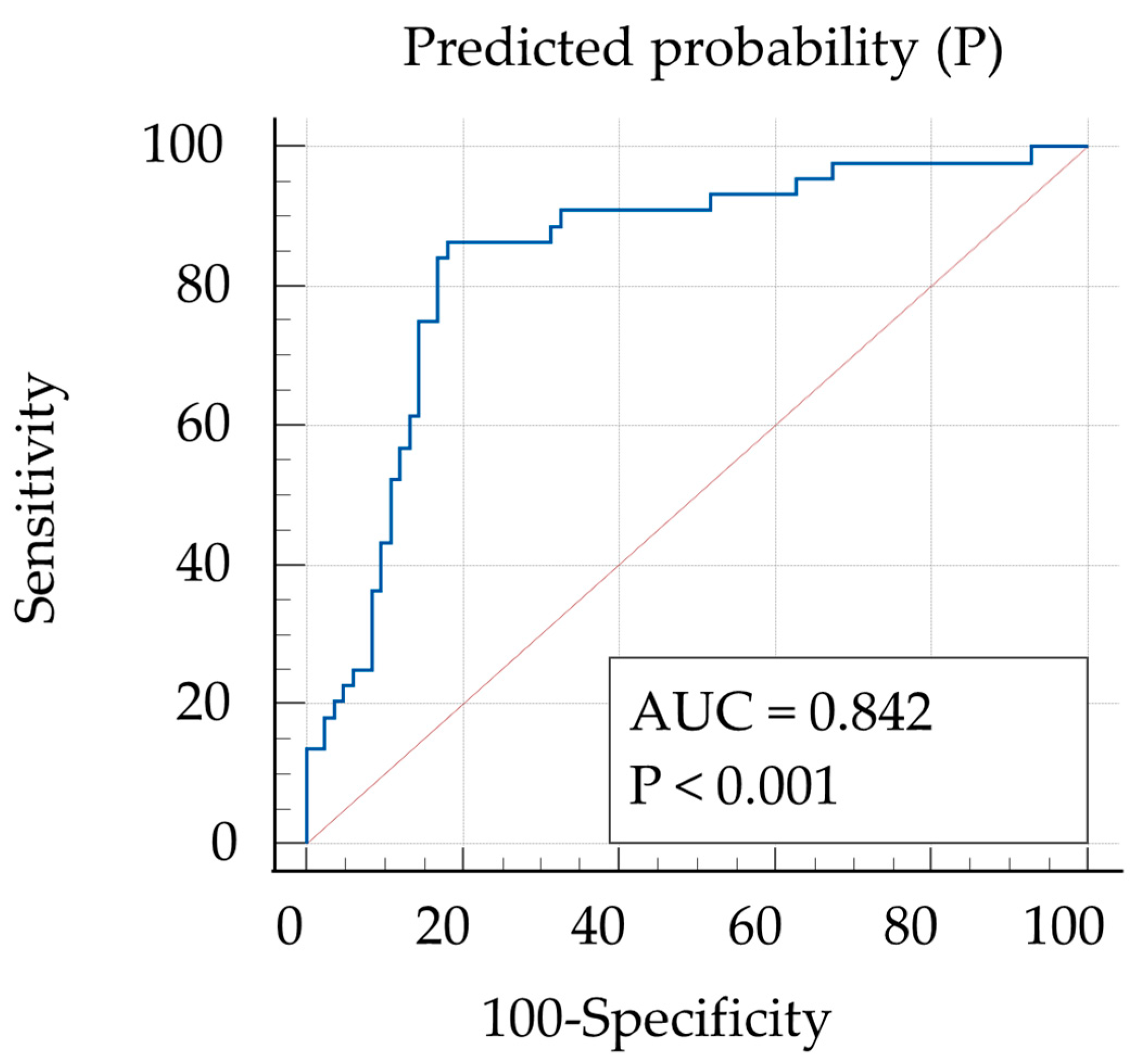
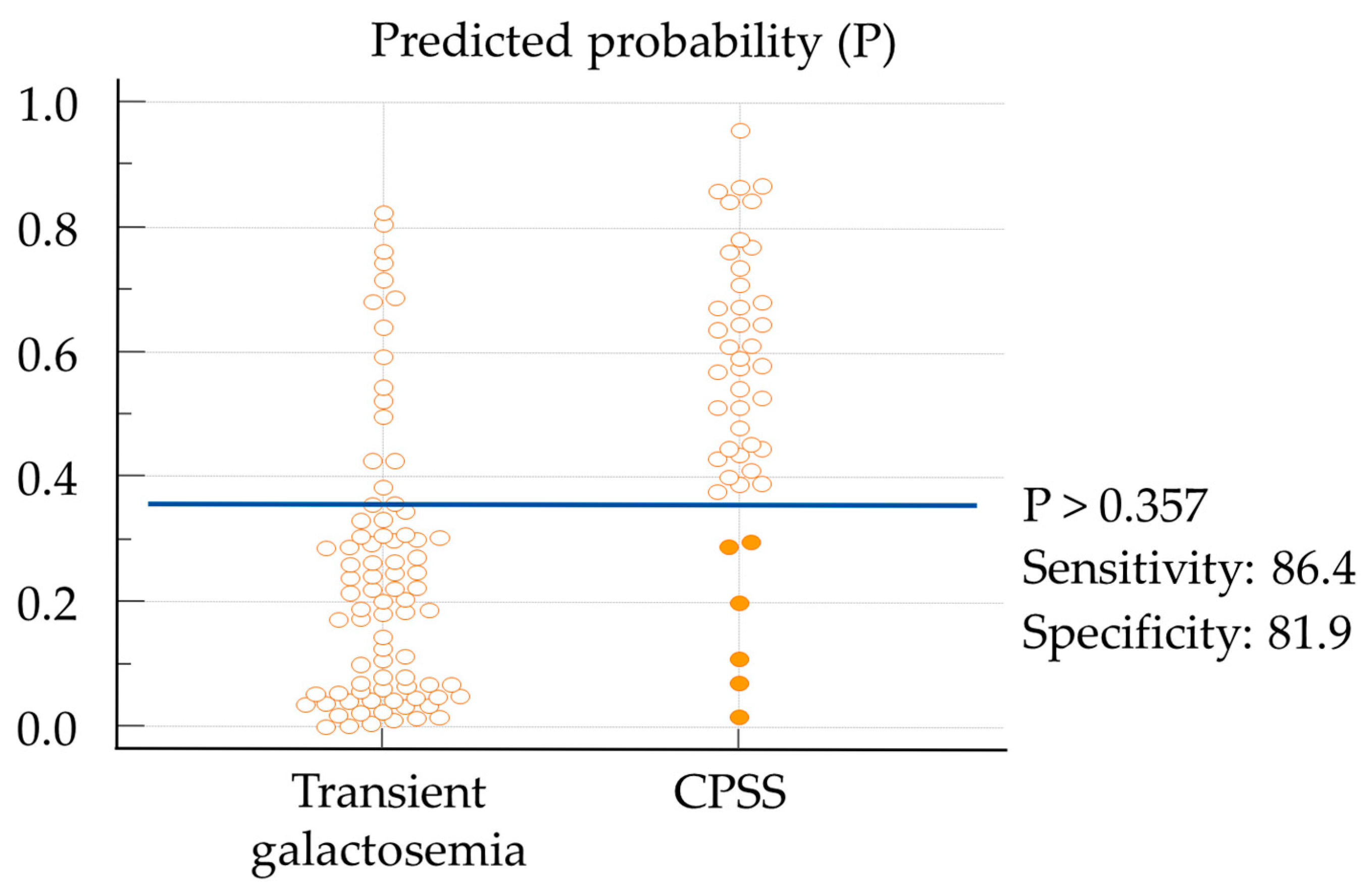
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Nishimura, Y.; Tajima, G.; Bahagia, A.D.; Sakamoto, A.; Ono, H.; Sakura, N.; Naito, K.; Hamakawa, M.; Yoshii, C.; Kubota, M.; et al. Differential Diagnosis of Neonatal Mild Hypergalactosaemia Detected by Mass Screening: Clinical Significance of Portal Vein Imaging. J. Inherit. Metab. Dis. 2004, 27, 11–18. [Google Scholar] [CrossRef]
- Papamichail, M.; Pizanias, M.; Heaton, N. Congenital Portosystemic Venous Shunt. Eur. J. Pediatr. 2018, 177, 285–294. [Google Scholar] [CrossRef]
- Nishigaki, S.; Etani, Y.; Shoji, Y.; Yamada, H.; Matayoshi, K.; Ioi, A.; Inaoka, K.; Takeshima, K.; Ida, S. Clinical Feature of Galactosaemia Identified by Neonatal Mass Screening. J. Mass. Screen. 2014, 24, 267–273. (In Japanese) [Google Scholar]
- Laverdure, N.; Lallier, M.; Dubois, J.; Paganelli, M. Congenital Absence of the Portal Vein: Define the Portosystemic Shunt, Avoid Liver Transplantation. Can. Liver J. 2021, 4, 322–327. [Google Scholar] [CrossRef]
- McLin, V.A.; Franchi-Abella, S.; Brütsch, T.; Bahadori, A.; Casotti, V.; De Ville De Goyet, J.; Dumery, G.; Gonzales, E.; Guérin, F.; Hascoet, S.; et al. Expert Management of Congenital Portosystemic Shunts and Their Complications. JHEP Rep. 2024, 6, 100933. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, P.; Hu, L.; Zhou, W.; Cheng, G. Case Report: Clinical Features of Congenital Portosystemic Shunts in the Neonatal Period. Front. Pediatr. 2021, 9, 778791. [Google Scholar] [CrossRef]
- Konstas, A.A.; Digumarthy, S.R.; Avery, L.L.; Wallace, K.L.; Lisovsky, M.; Misdraji, J.; Hahn, P.F. Congenital Portosystemic Shunts: Imaging Findings and Clinical Presentations in 11 Patients. Eur. J. Radiol. 2011, 80, 175–181. [Google Scholar] [CrossRef]
- Matsuoka, R.; Mochizuki, H.; Kubota, M. Value of the Gal-1-P/Gal Ratio and the Serum Total Bile Acid at the Differential Diagnosis of Hypergalactosemia Detected by Newborn Screening. J. Mass. Screen. 2015, 25, 281–287. (In Japanese) [Google Scholar]
- Fujimura, Y.; Kawamura, M.; Naruse, H. Simultaneous Quantitative Estimation of Galactose-1-Phosphate and Galactose in Blood for the Diagnosis of Galactosemia. Tohoku J. Exp. Med. 1982, 137, 289–295. [Google Scholar] [CrossRef]
- Beutler, E.; Baluda, M.C. Improved Method for Measuring Galactose-1-Phosphate Uridyl Transferase Activity of Erythrocytes. Clin. Chim. Acta 1966, 13, 369–379. [Google Scholar] [CrossRef]
- Fujimura, Y. A New Mass Screening Method of Detecting UDP-Galactose-4-Epimerase Deficiency. Tohoku J. Exp. Med. 1980, 131, 15–22. [Google Scholar] [CrossRef]
- Kikuchi, A.; Arai-Ichinoi, N.; Sakamoto, O.; Matsubara, Y.; Saheki, T.; Kobayashi, K.; Ohura, T.; Kure, S. Simple and Rapid Genetic Testing for Citrin Deficiency by Screening 11 Prevalent Mutations in SLC25A13. Mol. Genet. Metab. 2012, 105, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Kikuchi, A.; Arai-Ichinoi, N.; Sakamoto, O.; Takezawa, Y.; Iwasawa, S.; Niihori, T.; Nyuzuki, H.; Nakajima, Y.; Ogawa, E.; et al. Biallelic GALM Pathogenic Variants Cause a Novel Type of Galactosemia. Genet. Med. 2019, 21, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Superina, R. Congenital Absence of the Portal Vein: Two Cases and a Proposed Classification System for Portasystemic Vascular Anomalies. J. Pediatr. Surg. 1994, 29, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Niwa, T.; Kirikoshi, H.; Fujita, K.; Yoneda, M.; Saito, S.; Nakajima, A. Clinical Classification of Congenital Extrahepatic Portosystemic Shunts. Hepatol. Res. 2010, 40, 585–593. [Google Scholar] [CrossRef]
- Park, J.H.; Cha, S.H.; Han, J.K.; Han, M.C. Intrahepatic Portosystemic Venous Shunt. Am. J. Roentgenol. 1990, 155, 527–528. [Google Scholar] [CrossRef]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Harrel, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-19424-0. [Google Scholar]
- Steyerberg, E.W. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating; Statistics for Biology and Health; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-16398-3. [Google Scholar]
- Badiu Tișa, I.; Achim, A.C.; Cozma-Petruț, A. The Importance of Neonatal Screening for Galactosemia. Nutrients 2022, 15, 10. [Google Scholar] [CrossRef]
- Gatti, R.A.; Manfield, P.; Yi-Yung Hsia, D. Screening Newborn Infantsfor Galactosemia. J. Pediatr. 1966, 69, 1126–1129. [Google Scholar] [CrossRef]
- Aufrance, O.E.; Jones, W.N.; Harris, W.H. Screening for Galactosemia and Phenylketonuria. JAMA 1964, 190, 1126. [Google Scholar] [CrossRef]
- Nishimura, Y.; Tajima, G.; Ono, H.; Naito, K.; Sakura, N.; Yoshii, C.; Hamakawa, M. Overview of 60 Cases of Hypergalactosemia Caused by Portosystemic Shunt Detected by Neonatal Mass Screening Test in Hiroshima Prefecture. Jpn. J. Mass Screen. 2008, 18, 232–237. (In Japanese) [Google Scholar]
- Uchida, H.; Shinkai, M.; Okuyama, H.; Ueno, T.; Inoue, M.; Yasui, T.; Hiyama, E.; Kurihara, S.; Sakuma, Y.; Sanada, Y.; et al. Impact of Portal Flow on the Prognosis of Children with Congenital Portosystemic Shunt: A Multicentric Observation Study in Japan. J. Pediatr. Surg. 2024, 59, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, H.; Miida, T.; Yorifuji, T.; Hirano, K.; Inui, A.; Fujisawa, T.; Tsukahara, H.; Hayashi, H.; Inomata, Y. Metabolic Improvements in Intrahepatic Porto-Systemic Venous Shunt Presenting Various Metabolic Abnormalities by 4-Phenylacetate. Clin. Chim. Acta 2013, 419, 52–56. [Google Scholar] [CrossRef]
- Kim, M.J.; Ko, J.S.; Seo, J.K.; Yang, H.R.; Chang, J.Y.; Kim, G.B.; Cheon, J.-E.; Kim, W.S. Clinical Features of Congenital Portosystemic Shunt in Children. Eur. J. Pediatr. 2012, 171, 395–400. [Google Scholar] [CrossRef]
- Sokollik, C.; Bandsma, R.H.J.; Gana, J.C.; Van Den Heuvel, M.; Ling, S.C. Congenital Portosystemic Shunt: Characterization of a Multisystem Disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Nii, A.; Takehara, H.; Kuyama, H.; Shimada, M. Successful Preemptive Surgical Division of Type 2-Congenital Extrahepatic Portosystemic Shunt in Children. J. Med. Invest. 2009, 56, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.B.; Jordan, G.; Katz, D.; Sundaram, S.S.; Boster, J.; Brigham, D.; Ladd, P.; Chan, C.M.; Shay, R.L.; Ochmanek, E.; et al. Congenital Portosystemic Shunts: Variable Clinical Presentations Requiring a Tailored Endovascular or Surgical Approach. JPGN Rep. 2023, 4, e279. [Google Scholar] [CrossRef]
- Murayama, K. Significant Correlations between the Flow Volume of Patent Ductus Venosus and Early Neonatal Liver Function: Possible Involvement of Patent Ductus Venosus in Postnatal Liver Function. Arch. Dis. Child. Fetal Neonatal 2005, 91, F175–F179. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-0-470-58247-3. [Google Scholar]
- Ifuku, T.; Suzuki, S.; Nagatomo, Y.; Yokoyama, R.; Yamamura, Y.; Nakatani, K. Congenital Portosystemic Venous Shunt Associated with 22q11.2 Deletion Syndrome: A Case Report. BMC Pediatr. 2022, 22, 379. [Google Scholar] [CrossRef]
- Takama, Y.; Nakamura, T.; Santo, K.; Yoneda, A. Liver Resection for a Congenital Intrahepatic Portosystemic Shunt in a Child with Hyperammonemia and Hypermanganesemia: A Case Report. Surg. Case Rep. 2020, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Tokuhara, D.; Shimono, T.; Yamamoto, A.; Higashiyama, S.; Kotani, K.; Kawabe, J.; Okano, Y.; Shiomi, S.; Shintaku, H. Role of Per-Rectal Portal Scintigraphy in Long-Term Follow-up of Congenital Portosystemic Shunt. Pediatr. Res. 2014, 75, 658–662. [Google Scholar] [CrossRef] [PubMed]
| Analyzed Patients (n = 153) | |
|---|---|
| Final Diagnosis | n (%) |
| Enzyme deficiency | 18 * (11.8%) |
| Portosystemic shunt | 44 (28.8%) |
| Intrahepatic | 32 |
| Extrahepatic | 12 |
| Citrin deficiency | 7 (4.6%) |
| Transient galactosemia | 83 (54.2%) |
| Other | 1 (0.6%) |
| Case | Type of Shunt | Anatomy | Clinical Features | Treatment | Gal-1-P/Gal | TBA (μmol/L) | NH3 (μg/dL) | P |
|---|---|---|---|---|---|---|---|---|
| 1 | extrahepatic | IMV-LFV | no symptoms | endovascular | 2.73 | 56.7 | 90.00 | 0.51 |
| 2 | extrahepatic | PV-LRV | no symptoms | endovascular | 1.25 | 47.2 | 132.00 | 0.67 |
| 3 | extrahepatic | absence of PV | no symptoms | LT | 0.62 | 20.0 | 90.26 | 0.43 |
| 4 | extrahepatic | hypoplastic PV, SMV-LHV | MR | endovascular | 1.69 | 26.6 | 95.37 | 0.45 |
| 5 | extrahepatic | absence of PV, SMV-azygos vein | unclear | unclear | 0.80 | 80.0 | 115.80 | 0.74 |
| 6 | extrahepatic | SV-LRV | no symptoms | endovascular | 3.97 | 28.3 | 68.12 | 0.30 |
| 7 | extrahepatic | SV-LRV | MR | endovascular | 1.98 | 72.6 | 76.64 | 0.54 |
| 8 | extrahepatic | hypoplastic PV, SV-LRV | Hypermanganesemia, hepatic atrophy | surgical | 0.70 | 77.4 | 109.00 | 0.71 |
| 9 | extrahepatic | PV-RA | no symptoms | surgical | 2.88 | 114.8 | 137.94 | 0.84 |
| 10 | extrahepatic | SV-LRV | no symptoms | surgical | 1.67 | 68.0 | 91.96 | 0.59 |
| 11 | intrahepatic | PDV | heart failure with COA | operation for COA | 0.80 | 137.2 | 68.00 | 0.76 |
| 12 | intrahepatic | PV-LHV | no symptoms | endovascular | 1.69 | 150.6 | 102.00 | 0.86 |
| 13 | intrahepatic | PDV | no symptoms | endovascular | 0.69 | 12.6 | 84.00 | 0.38 |
| CPSS (n = 44) | Transient Galactosemia (n = 83) | p-Value | |
|---|---|---|---|
| Birth weight, g, mean (SD) | 2887.4 (422.0) | 2988.7 (409.1) | 0.19 |
| Sex ratio, male/female | 24:20 | 52:31 | 0.45 |
| Galactose, mg/dL, median (IQR) | 5.65 (4.00–9.58) | 2.80 (1.20–4.60) | <0.001 |
| Total galactose, mg/dL, mean (SD) | 12.95 (5.48) | 17.70 (6.75) | <0.001 |
| Gal-1-P, mg/dL, median (IQR) | 7.20 (3.67–11.40) | 21.30 (8.64–27.80) | <0.001 |
| Gal-1-P/Gal, median (IQR) | 1.05 (0.61–1.95) | 7.69 (1.34–20.90) | <0.001 |
| ALT, U/L, median (IQR) | 18.00 (14.00–26.75) | 18.00 (14.00–22.00) | 0.37 |
| Albumin, g/dL, mean (SD) | 3.63 (0.35) | 3.80 (0.34) | 0.009 |
| Ammonia, μg/dL, median (IQR) | 90.13 (68.03–118.36) | 68.12 (54.50–86.00) | 0.002 |
| Prothrombin time, % median (IQR) | 86.00 (73.55–94.90) | 89.00 (83.00–96.30) | 0.07 |
| Total bile acid, μmol/L, median (IQR) | 53.50 (28.75–79.90) | 17.90 (11.00–41.20) | <0.001 |
| Direct bilirubin, mg/dL, median (IQR) | 0.45 (0.20–0.80) | 0.40 (0.20–0.60) | 0.46 |
| Crude OR | 95%CI | Adjusted OR | 95%CI | Adjusted OR | 95%CI | p-Value | |
|---|---|---|---|---|---|---|---|
| (Enter) | (Selected Variables) | ||||||
| Birth weight, g | 1.00 | 0.99–1.00 | 1.00 | 0.99–1.01 | |||
| Sex, male | 0.72 | 0.34–1.51 | 0.53 | 0.18–1.50 | |||
| Gal-1-P/Gal | 0.87 | 0.80–0.94 | 0.90 | 0.83–0.97 | 0.90 | 0.83–0.97 | <0.001 |
| ALT, IU/L | 1.03 | 0.99–1.07 | 1.03 | 0.97–1.10 | |||
| Albumin, g/dL | 0.23 | 0.07–0.71 | 0.37 | 0.08–1.72 | |||
| Ammonia, μg/dL | 1.02 | 1.00–1.04 | 1.02 | 1.00–1.04 | 1.02 | 1.00–1.04 | 0.03 |
| Prothrombin time, % | 0.98 | 0.95–1.01 | 0.96 | 0.93–1.00 | |||
| Total bile acid, mg/dL | 1.02 | 1.01–1.04 | 1.02 | 1.00–1.04 | 1.02 | 1.00–1.03 | 0.01 |
| Direct bilirubin, mg/dL | 1.84 | 0.73–4.62 | 0.48 | 0.13–1.67 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki-Ajihara, S.; Musha, I.; Arao, M.; Mori, K.; Fujibayashi, S.; Ryo, I.; Kono, T.; Tajima, A.; Mochizuki, H.; Imai-Okazaki, A.; et al. Prediction of Congenital Portosystemic Shunt in Neonatal Hypergalactosemia Using Gal-1-P/Gal Ratio, Bile Acid, and Ammonia. Int. J. Neonatal Screen. 2025, 11, 61. https://doi.org/10.3390/ijns11030061
Suzuki-Ajihara S, Musha I, Arao M, Mori K, Fujibayashi S, Ryo I, Kono T, Tajima A, Mochizuki H, Imai-Okazaki A, et al. Prediction of Congenital Portosystemic Shunt in Neonatal Hypergalactosemia Using Gal-1-P/Gal Ratio, Bile Acid, and Ammonia. International Journal of Neonatal Screening. 2025; 11(3):61. https://doi.org/10.3390/ijns11030061
Chicago/Turabian StyleSuzuki-Ajihara, Sayaka, Ikuma Musha, Masato Arao, Koki Mori, Shunsuke Fujibayashi, Ihiro Ryo, Tomotaka Kono, Asako Tajima, Hiroshi Mochizuki, Atsuko Imai-Okazaki, and et al. 2025. "Prediction of Congenital Portosystemic Shunt in Neonatal Hypergalactosemia Using Gal-1-P/Gal Ratio, Bile Acid, and Ammonia" International Journal of Neonatal Screening 11, no. 3: 61. https://doi.org/10.3390/ijns11030061
APA StyleSuzuki-Ajihara, S., Musha, I., Arao, M., Mori, K., Fujibayashi, S., Ryo, I., Kono, T., Tajima, A., Mochizuki, H., Imai-Okazaki, A., Araki, R., Numakura, C., & Ohtake, A. (2025). Prediction of Congenital Portosystemic Shunt in Neonatal Hypergalactosemia Using Gal-1-P/Gal Ratio, Bile Acid, and Ammonia. International Journal of Neonatal Screening, 11(3), 61. https://doi.org/10.3390/ijns11030061






