Advancing Neonatal Screening for Pyridoxine-Dependent Epilepsy-ALDH7A1 Through Combined Analysis of 2-OPP, 6-Oxo-Pipecolate and Pipecolate in a Butylated FIA-MS/MS Workflow
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Control Samples
2.2. Sample Preparation, FIA-MS/MS Analysis and Validation
2.3. Statistics
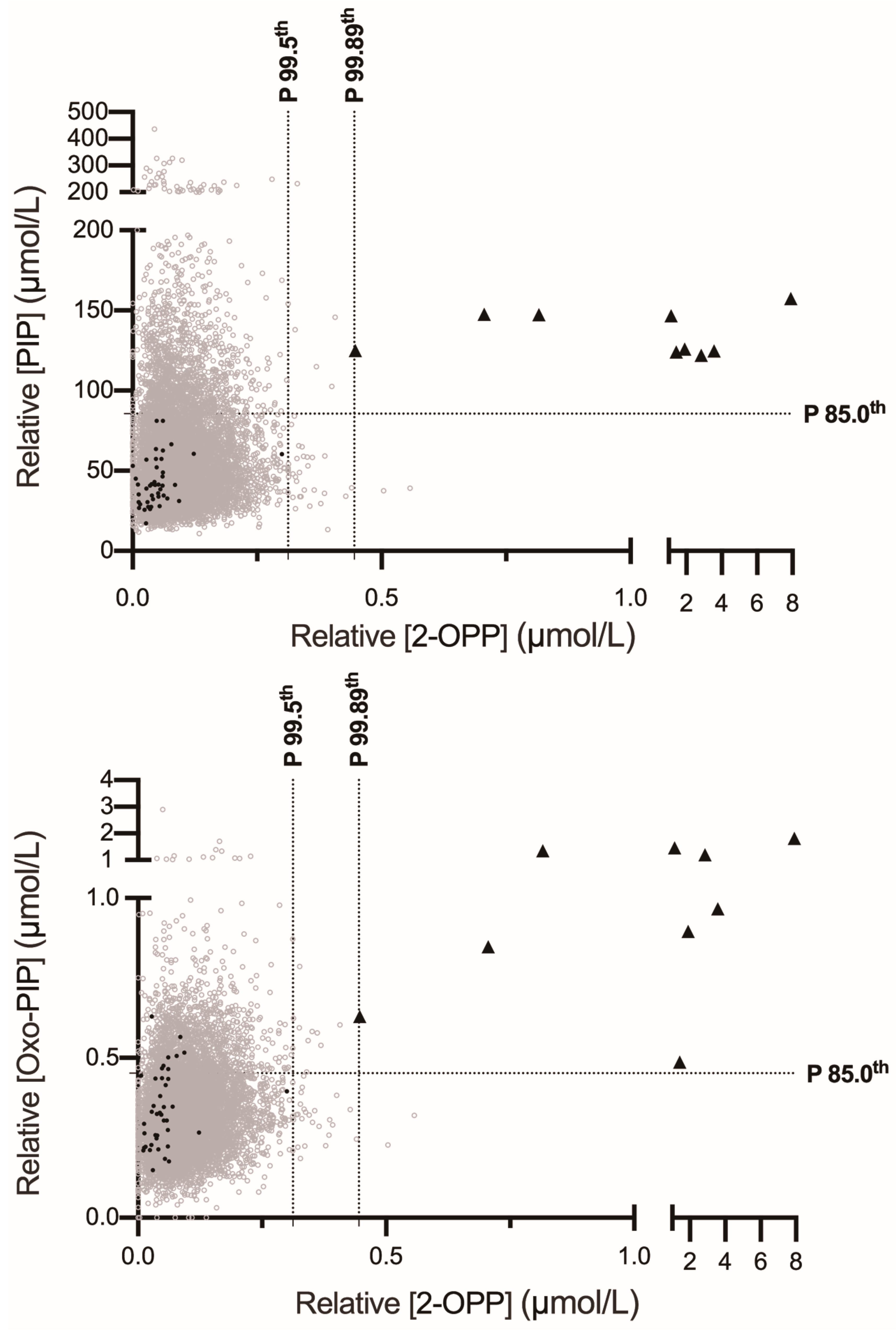
3. Results
3.1. Nine Genetically Confirmed PDE-ALDH7A1 Patients Were Recruited for This Study
3.2. 2-OPP Is a Highly Sensitive and Reasonably Specific Biomarker for PDE-ALDH7A1 Newborn Screening via Butanol-Derivatization FIA-MS/MS
3.3. Combining 2-OPP with Pipecolate or 6-Oxo-Pipecolate Enhances Specificity and Positive Predictive Value in Multiplex FIA-MS/MS Screening for PDE-ALDH7A1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basura, G.J.; Hagland, S.P.; Wiltse, A.M.; Gospe, S.M., Jr. Clinical features and the management of pyridoxine-dependent and pyridoxine-responsive seizures: Review of 63 North American cases submitted to a patient registry. Eur. J. Pediatr. 2009, 168, 697–704. [Google Scholar] [CrossRef]
- Mills, P.B.; Struys, E.; Jakobs, C.; Plecko, B.; Baxter, P.; Baumgartner, M.; Willemsen, M.A.; Omran, H.; Tacke, U.; Uhlenberg, B.; et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat. Med. 2006, 12, 307–309. [Google Scholar] [CrossRef]
- Plecko, B.; Paul, K.; Paschke, E.; Stoeckler-Ipsiroglu, S.; Struys, E.; Jakobs, C.; Hartmann, H.; Luecke, T.; di Capua, M.; Korenke, C.; et al. Biochemical and molecular characterization of 18 patients with pyridoxine-dependent epilepsy and mutations of the antiquitin (ALDH7A1) gene. Hum. Mutat. 2007, 28, 19–26. [Google Scholar] [CrossRef]
- Bok, L.A.; Struys, E.; Willemsen, M.A.; Been, J.V.; Jakobs, C. Pyridoxine-dependent seizures in Dutch patients: Diagnosis by elevated urinary alpha-aminoadipic semialdehyde levels. Arch. Dis. Child. 2007, 92, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Struys, E.A.; Bok, L.A.; Emal, D.; Houterman, S.; Willemsen, M.A.; Jakobs, C. The measurement of urinary Delta(1)-piperideine-6-carboxylate, the alter ego of alpha-aminoadipic semialdehyde, in Antiquitin deficiency. J. Inherit. Metab. Dis. 2012, 35, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Sadilkova, K.; Gospe, S.M., Jr.; Hahn, S.H. Simultaneous determination of alpha-aminoadipic semialdehyde, piperideine-6-carboxylate and pipecolic acid by LC-MS/MS for pyridoxine-dependent seizures and folinic acid-responsive seizures. J. Neurosci. Methods 2009, 184, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Plecko, B.; Hikel, C.; Korenke, G.C.; Schmitt, B.; Baumgartner, M.; Baumeister, F.; Jakobs, C.; Struys, E.; Erwa, W.; Stockler-Ipsiroglu, S. Pipecolic acid as a diagnostic marker of pyridoxine-dependent epilepsy. Neuropediatrics 2005, 36, 200–205. [Google Scholar] [CrossRef]
- Plecko, B.; Stockler-Ipsiroglu, S.; Paschke, E.; Erwa, W.; Struys, E.A.; Jakobs, C. Pipecolic acid elevation in plasma and cerebrospinal fluid of two patients with pyridoxine-dependent epilepsy. Ann. Neurol. 2000, 48, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Hori, T.; Nakajima, M.; Takeshita, K. Plasma levels of pipecolic acid in patients with chronic liver disease. Hepatology 1988, 8, 286–289. [Google Scholar] [CrossRef]
- Peduto, A.; Baumgartner, M.R.; Verhoeven, N.M.; Rabier, D.; Spada, M.; Nassogne, M.C.; Poll-The, B.T.; Bonetti, G.; Jakobs, C.; Saudubray, J.M. Hyperpipecolic acidaemia: A diagnostic tool for peroxisomal disorders. Mol. Genet. Metab. 2004, 82, 224–230. [Google Scholar] [CrossRef]
- Houten, S.M.; Te Brinke, H.; Denis, S.; Ruiter, J.P.; Knegt, A.C.; de Klerk, J.B.; Augoustides-Savvopoulou, P.; Haberle, J.; Baumgartner, M.R.; Coskun, T.; et al. Genetic basis of hyperlysinemia. Orphanet J. Rare Dis. 2013, 8, 57. [Google Scholar] [CrossRef]
- Bok, L.A.; Halbertsma, F.J.; Houterman, S.; Wevers, R.A.; Vreeswijk, C.; Jakobs, C.; Struys, E.; Van Der Hoeven, J.H.; Sival, D.A.; Willemsen, M.A. Long-term outcome in pyridoxine-dependent epilepsy. Dev. Med. Child Neurol. 2012, 54, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, C.R.; Tseng, L.A.; Bok, L.A.; Hartmann, H.; Footitt, E.; Striano, P.; Tabarki, B.M.; Lunsing, R.J.; Stockler-Ipsiroglu, S.; Gordon, S.; et al. Association Between Lysine Reduction Therapies and Cognitive Outcomes in Patients With Pyridoxine-Dependent Epilepsy. Neurology 2022, 99, e2627–e2636. [Google Scholar] [CrossRef]
- Coughlin, C.R., II; van Karnebeek, C.D.; Al-Hertani, W.; Shuen, A.Y.; Jaggumantri, S.; Jack, R.M.; Gaughan, S.; Burns, C.; Mirsky, D.M.; Gallagher, R.C.; et al. Triple therapy with pyridoxine, arginine supplementation and dietary lysine restriction in pyridoxine-dependent epilepsy: Neurodevelopmental outcome. Mol. Genet. Metab. 2015, 116, 35–43. [Google Scholar] [CrossRef]
- Mahajnah, M.; Corderio, D.; Austin, V.; Herd, S.; Mutch, C.; Carter, M.; Struys, E.; Mercimek-Mahmutoglu, S. A Prospective Case Study of the Safety and Efficacy of Lysine-Restricted Diet and Arginine Supplementation Therapy in a Patient With Pyridoxine-Dependent Epilepsy Caused by Mutations in ALDH7A1. Pediatr. Neurol. 2016, 60, 60–65. [Google Scholar] [CrossRef]
- Mercimek-Mahmutoglu, S.; Cordeiro, D.; Cruz, V.; Hyland, K.; Struys, E.A.; Kyriakopoulou, L.; Mamak, E. Novel therapy for pyridoxine dependent epilepsy due to ALDH7A1 genetic defect: L-arginine supplementation alternative to lysine-restricted diet. Eur. J. Paediatr. Neurol. 2014, 18, 741–746. [Google Scholar] [CrossRef]
- van Karnebeek, C.D.; Hartmann, H.; Jaggumantri, S.; Bok, L.A.; Cheng, B.; Connolly, M.; Coughlin, C.R., 2nd; Das, A.M.; Gospe, S.M., Jr.; Jakobs, C.; et al. Lysine restricted diet for pyridoxine-dependent epilepsy: First evidence and future trials. Mol. Genet. Metab. 2012, 107, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Yuzyuk, T.; Thomas, A.; Viau, K.; Liu, A.; De Biase, I.; Botto, L.D.; Pasquali, M.; Longo, N. Effect of dietary lysine restriction and arginine supplementation in two patients with pyridoxine-dependent epilepsy. Mol. Genet. Metab. 2016, 118, 167–172. [Google Scholar] [CrossRef]
- Al Teneiji, A.; Bruun, T.U.; Cordeiro, D.; Patel, J.; Inbar-Feigenberg, M.; Weiss, S.; Struys, E.; Mercimek-Mahmutoglu, S. Phenotype, biochemical features, genotype and treatment outcome of pyridoxine-dependent epilepsy. Metab. Brain Dis. 2017, 32, 443–451. [Google Scholar] [CrossRef]
- Falsaperla, R.; Vari, M.S.; Toldo, I.; Murgia, A.; Sartori, S.; Vecchi, M.; Suppiej, A.; Burlina, A.; Mastrangelo, M.; Leuzzi, V.; et al. Pyridoxine-dependent epilepsies: An observational study on clinical, diagnostic, therapeutic and prognostic features in a pediatric cohort. Metab. Brain Dis. 2018, 33, 261–269. [Google Scholar] [CrossRef] [PubMed]
- van Karnebeek, C.D.; Tiebout, S.A.; Niermeijer, J.; Poll-The, B.T.; Ghani, A.; Coughlin, C.R., 2nd; Van Hove, J.L.; Richter, J.W.; Christen, H.J.; Gallagher, R.; et al. Pyridoxine-Dependent Epilepsy: An Expanding Clinical Spectrum. Pediatr. Neurol. 2016, 59, 6–12. [Google Scholar] [CrossRef]
- Mills, P.B.; Footitt, E.J.; Mills, K.A.; Tuschl, K.; Aylett, S.; Varadkar, S.; Hemingway, C.; Marlow, N.; Rennie, J.; Baxter, P.; et al. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency). Brain 2010, 133, 2148–2159. [Google Scholar] [CrossRef]
- Mercimek-Mahmutoglu, S.; Horvath, G.A.; Coulter-Mackie, M.; Nelson, T.; Waters, P.J.; Sargent, M.; Struys, E.; Jakobs, C.; Stockler-Ipsiroglu, S.; Connolly, M.B. Profound neonatal hypoglycemia and lactic acidosis caused by pyridoxine-dependent epilepsy. Pediatrics 2012, 129, e1368–e1372. [Google Scholar] [CrossRef]
- Dowa, Y.; Shiihara, T.; Akiyama, T.; Hasegawa, K.; Inoue, F.; Watanabe, M. A case of pyridoxine-dependent epilepsy with novel ALDH7A1 mutations. Oxf. Med. Case Rep. 2020, 2020, omaa008. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, C.R., 2nd; Tseng, L.A.; van Karnebeek, C.D.M. A case for newborn screening for pyridoxine-dependent epilepsy. Mol. Case Stud. 2022, 8, a006197. [Google Scholar] [CrossRef] [PubMed]
- Hess-Homeier, D.L.; Cunniff, C.; Grinspan, Z.M. Priorities for Newborn Screening of Genetic Epilepsy. Pediatr. Neurol. 2019, 101, 83–85. [Google Scholar] [CrossRef]
- Wempe, M.F.; Kumar, A.; Kumar, V.; Choi, Y.J.; Swanson, M.A.; Friederich, M.W.; Hyland, K.; Yue, W.W.; Van Hove, J.L.K.; Coughlin, C.R., 2nd. Identification of a novel biomarker for pyridoxine-dependent epilepsy: Implications for newborn screening. J. Inherit. Metab. Dis. 2019, 42, 565–574. [Google Scholar] [CrossRef]
- Jung, S.; Tran, N.T.; Gospe, S.M., Jr.; Hahn, S.H. Preliminary investigation of the use of newborn dried blood spots for screening pyridoxine-dependent epilepsy by LC-MS/MS. Mol. Genet. Metab. 2013, 110, 237–240. [Google Scholar] [CrossRef]
- Mathew, E.M.; Moorkoth, S.; Lewis, L.; Rao, P. Biomarker Profiling for Pyridoxine Dependent Epilepsy in Dried Blood Spots by HILIC-ESI-MS. Int. J. Anal. Chem. 2018, 2018, 2583215. [Google Scholar] [CrossRef]
- Engelke, U.F.; van Outersterp, R.E.; Merx, J.; van Geenen, F.A.; van Rooij, A.; Berden, G.; Huigen, M.C.; Kluijtmans, L.A.; Peters, T.M.; Al-Shekaili, H.H.; et al. Untargeted metabolomics and infrared ion spectroscopy identify biomarkers for pyridoxine-dependent epilepsy. J. Clin. Investig. 2021, 131, e148272. [Google Scholar] [CrossRef] [PubMed]
- Damiano, R.; Della Bona, M.; Procopio, E.; Guerrini, R.; Bettiol, A.; la Marca, G. Inclusion of pyridoxine dependent epilepsy in expanded newborn screening programs by tandem mass spectrometry: Set up of first and second tier tests. Clin. Chem. Lab. Med. 2025, 63, 1344–1353. [Google Scholar] [CrossRef]
- Pauly, K.; Woontner, M.; Abdenur, J.E.; Chaudhari, B.P.; Gosselin, R.; Kripps, K.A.; Thomas, J.A.; Wempe, M.F.; Gospe, S.M., Jr.; Coughlin, C.R., 2nd. Feasibility of newborn screening for pyridoxine-dependent epilepsy. Mol. Genet. Metab. 2025, 144, 109002. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.P.; Chevalier, N.; Marie, S.; Veiga-da-Cunha, M. DBS are suitable for 1,5-anhydroglucitol monitoring in GSD1b and G6PC3-deficient patients taking SGLT2 inhibitors to treat neutropenia. Mol. Genet. Metab. 2023, 140, 107712. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.U.; Blake, J.; Harrison, J.R.; Gerace, R.; Ranieri, E.; Fletcher, J.M.; Johnson, D.W. Quantification of glutamine in dried blood spots and plasma by tandem mass spectrometry for the biochemical diagnosis and monitoring of ornithine transcarbamylase deficiency. Clin. Chem. 2003, 49, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, Y.; Liu, Y.; Ma, Y.; Song, J.; Wang, Q.; Yang, Y. Five Chinese patients with 5-oxoprolinuria due to glutathione synthetase and 5-oxoprolinase deficiencies. Brain Dev. 2015, 37, 952–959. [Google Scholar] [CrossRef]
- Mills, P.B.; Footitt, E.J.; Ceyhan, S.; Waters, P.J.; Jakobs, C.; Clayton, P.T.; Struys, E.A. Urinary AASA excretion is elevated in patients with molybdenum cofactor deficiency and isolated sulphite oxidase deficiency. J. Inherit. Metab. Dis. 2012, 35, 1031–1036. [Google Scholar] [CrossRef]

| Intra-Day Variation (Precision) | Inter-Day Variation (Reproducibility) | ||||||
|---|---|---|---|---|---|---|---|
| [µmol/L] | Mean | SD | CV (%) | Mean | SD | CV (%) | |
| 2-OPP | 0.25 (L) | 0.35 | 0.04 | 11.7 | 0.34 | 0.04 | 10.3 |
| 1 (M) | 1.13 | 0.10 | 8.4 | 1.13 | 0.13 | 11.9 | |
| 5 (H) | 5.76 | 0.34 | 5.9 | 5.6 | 0.51 | 9.1 | |
| Oxo-PIP | 0.5 (L) | 0.35 | 0.01 | 3.8 | 0.37 | 0.04 | 9.2 |
| 2 (M) | 0.9 | 0.11 | 11.7 | 1.03 | 0.18 | 17.9 | |
| 10 (H) | 4.09 | 0.24 | 5.8 | 4.38 | 0.59 | 13.4 | |
| PIP | 22.5 (L) | 51.3 | 1.73 | 3.4 | 46 | 6.65 | 14.5 |
| 112.5 (M) | 143 | 17.1 | 11.9 | 156 | 9.93 | 6.4 | |
| 225 (H) | 284 | 22.9 | 8.1 | 507 | 52.0 | 10.2 | |
| Patient | Genotype | Sex | Age at 1st Seizure | Age at Pyridoxine | 2-OPP (µmol/L) P99.5th: 0.31 P99.9th: 0.48 | Oxo-PIP (µmol/L) P99.5th: 0.84 P99.9th: 1.13 | PIP (µmol/L) P99.5th: 201 P99.9th: 266 | Age of DBS at Time of Testing |
|---|---|---|---|---|---|---|---|---|
| 1 | c.834G>A (p.Val278 =) (P/LP); c.1279G>C (p.Glu427Gln) (P/LP) | F | 5 days | 28 days | 1.90 | 0.90 | 126 | 2.5 years |
| 2 | c.902A>T (p.Asn301Ile) (P/LP), c.1279G>C (p.Glu427Gln) (P/LP) | M | 5 days | 16 days | 0.45 | 0.63 | 125 | 4 years |
| 3 | c.902A>T (p.Asn301Ile) (P/LP); c.1279G>C (p.Glu427Gln) (P/LP) | F | No seizure | 0 day | 0.82 | 1.35 | 147 | 3 years |
| 4 | c.690A-1095_716 delinsG; r.690_787del (exon 9) (LP); c.612–502 G>C; r.612-541_506ins LP) | M | No seizure | 0 day | 0.71 | 0.85 | 148 | 3 years |
| 5 | c.1061A >G (p.Tyr 354 Lys) (P); c.1061A>G (p.Tyr 354 Lys) (P) | M | 1 day | 2 days | 1.43 | 0.49 | 124 | 3 years |
| 6 | c.1588del (p.Leu530Phefs*21) (LP); c.1588del (p.Leu530Phefs*21) (LP) | M | 2 days | 2 days | 2.85 | 1.19 | 122 | 9 months |
| 7 | c.1279G>C (p.Glu427Gln) (P/LP); c.1279G>C (p.Glu427Gln) (P/LP) | M | H 1 | 1 day * | 1.13 | 1.46 | 147 | 5 years |
| 8 | c.916T>A (p.Phe306Ile) (LP); c.1279G>C (p.Glu427Gln) (P/LP) | M | 5 days | 6 days | 3.57 | 0.97 | 125 | 3.5 years |
| 9 | c.650 + 260_695 + 950del (P); c.774–1095_801del (LP) | F | 3 days | 6 days | 7.90 | 1.81 | 158 | 23 months |
| Biomarker 1 and Threshold Used in Percentiles | Biomarker 2 and Threshold Used in Percentiles | Sensitivity | Specificity | Positive Predictive Value |
|---|---|---|---|---|
| 2-OPP (P99.9th) | N/A | 88.9% (CI:56.5–99.4) | 99.98% (CI:99.9–100) | 80.0% |
| Oxo-PIP (P99.5th) | N/A | 77.8% (CI:45.26–96.05) | 99.6% (CI:99.42–99.69) | 14.9% |
| 2-OPP (P99.5th) | N/A | 100% (CI:70.1–100) | 99.6% (CI:99.5–99.7) | 18.4% |
| 2-OPP (P99.89th) | Oxo-PIP (P85.0th) | 100% (CI:70.1–100) | 100% (CI:99.96–100) | 100% |
| 2-OPP (P99.89th) | PIP (P85.0th) | 100% (CI:70.1–100.0) | 100% (CI:99.96–100) | 100% |
| 2-OPP (P99.5th) | Oxo-PIP (P85.0th) | 100% (CI:70.1–100) | 99.9% (CI:99.8–99.9) | 45.0% |
| 2-OPP (P99.5th) | PIP (P85.0th) | 100% (CI:70.1–100) | 99.9% (CI:99.86–99.97) | 60.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donge, M.; Marie, S.; Pochet, A.; Marcelis, L.; Luis, G.; Boemer, F.; Prouteau, C.; Mesli, S.; Cuykx, M.; Nguyen-Khoa, T.; et al. Advancing Neonatal Screening for Pyridoxine-Dependent Epilepsy-ALDH7A1 Through Combined Analysis of 2-OPP, 6-Oxo-Pipecolate and Pipecolate in a Butylated FIA-MS/MS Workflow. Int. J. Neonatal Screen. 2025, 11, 59. https://doi.org/10.3390/ijns11030059
Donge M, Marie S, Pochet A, Marcelis L, Luis G, Boemer F, Prouteau C, Mesli S, Cuykx M, Nguyen-Khoa T, et al. Advancing Neonatal Screening for Pyridoxine-Dependent Epilepsy-ALDH7A1 Through Combined Analysis of 2-OPP, 6-Oxo-Pipecolate and Pipecolate in a Butylated FIA-MS/MS Workflow. International Journal of Neonatal Screening. 2025; 11(3):59. https://doi.org/10.3390/ijns11030059
Chicago/Turabian StyleDonge, Mylène, Sandrine Marie, Amandine Pochet, Lionel Marcelis, Geraldine Luis, François Boemer, Clément Prouteau, Samir Mesli, Matthias Cuykx, Thao Nguyen-Khoa, and et al. 2025. "Advancing Neonatal Screening for Pyridoxine-Dependent Epilepsy-ALDH7A1 Through Combined Analysis of 2-OPP, 6-Oxo-Pipecolate and Pipecolate in a Butylated FIA-MS/MS Workflow" International Journal of Neonatal Screening 11, no. 3: 59. https://doi.org/10.3390/ijns11030059
APA StyleDonge, M., Marie, S., Pochet, A., Marcelis, L., Luis, G., Boemer, F., Prouteau, C., Mesli, S., Cuykx, M., Nguyen-Khoa, T., Guénet, D., Empain, A., Barth, M., Dauriat, B., Laroche-Raynaud, C., De Laet, C., Verloo, P., Jonckheere, A. I., Schiff, M., ... Dewulf, J. P. (2025). Advancing Neonatal Screening for Pyridoxine-Dependent Epilepsy-ALDH7A1 Through Combined Analysis of 2-OPP, 6-Oxo-Pipecolate and Pipecolate in a Butylated FIA-MS/MS Workflow. International Journal of Neonatal Screening, 11(3), 59. https://doi.org/10.3390/ijns11030059






