Cost–Effectiveness of Newborn Screening for X-Linked Adrenoleukodystrophy in the Netherlands: A Health-Economic Modelling Study
Abstract
1. Introduction
2. Methods
2.1. Model Structure
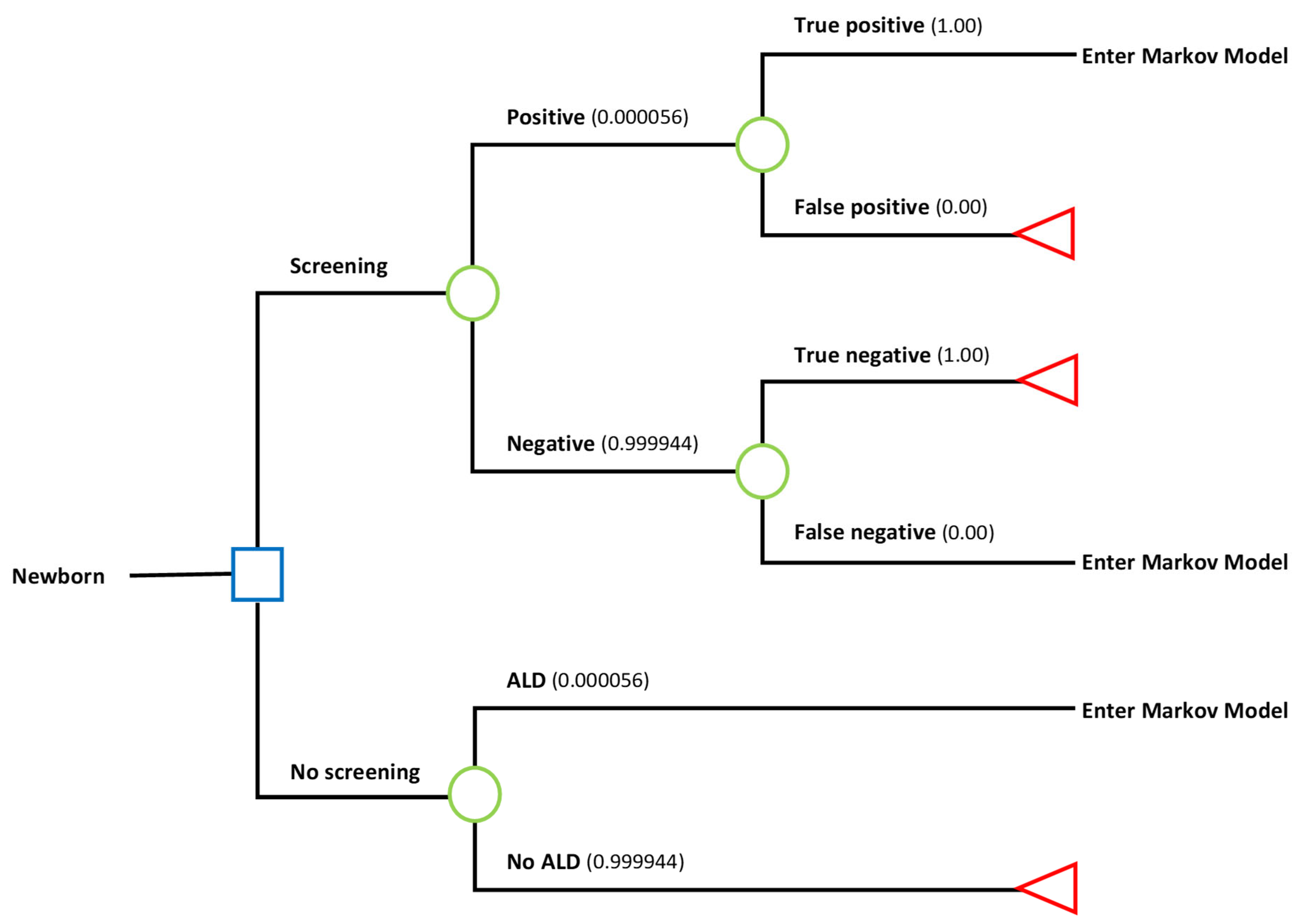
2.2. Decision Tree—ALD Newborn Screening
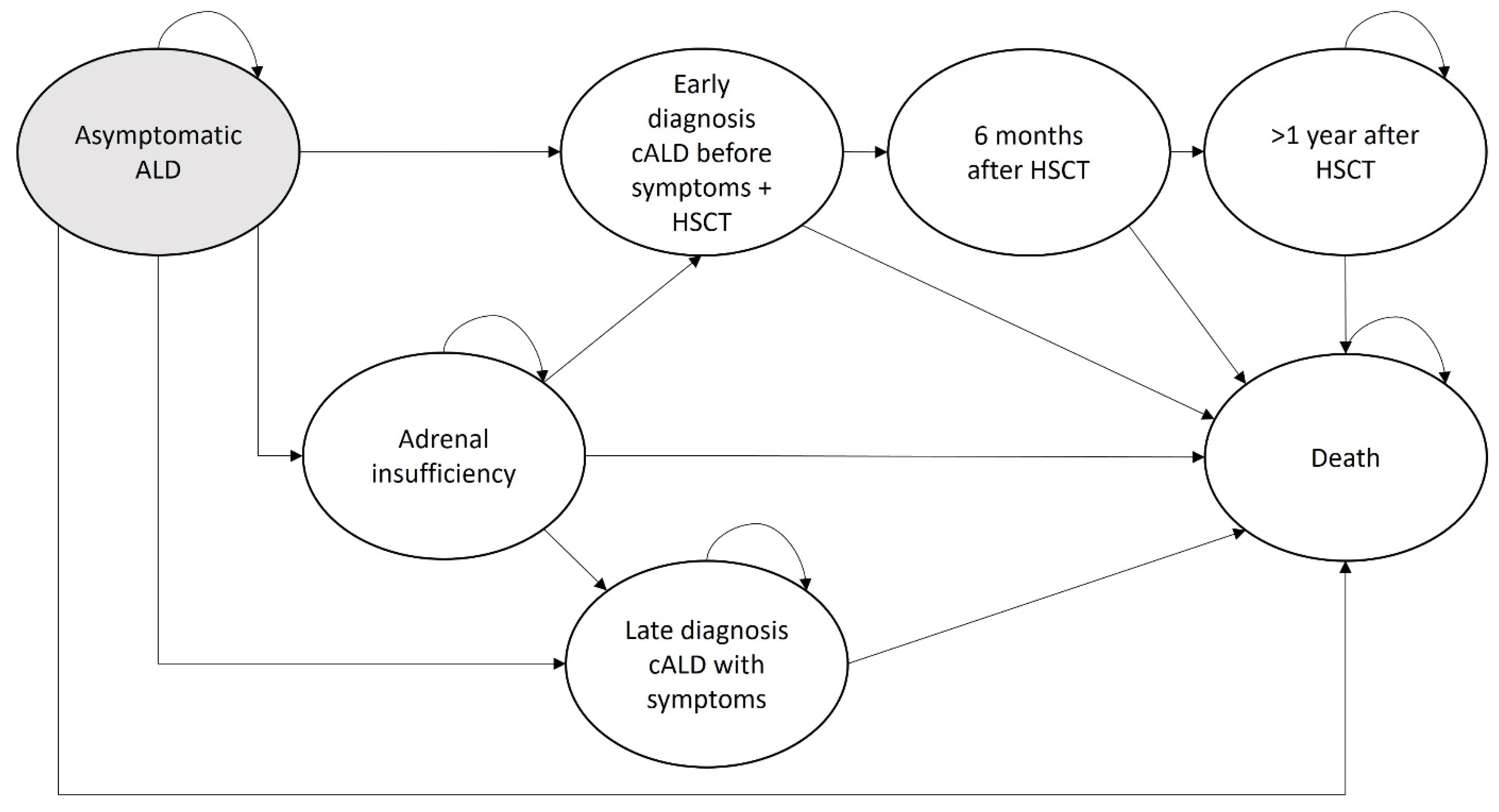
2.3. Markov Model—Disease Progression
2.4. Model Parameters
2.4.1. Transition Probabilities
2.4.2. Costs
2.4.3. Quality-Adjusted Life-Years
2.5. Deterministic Sensitivity Analysis
2.6. Probabilistic Sensitivity Analysis
3. Results
3.1. Screening Costs
3.2. Effectiveness
3.3. Costs
3.4. Cost–Effectiveness
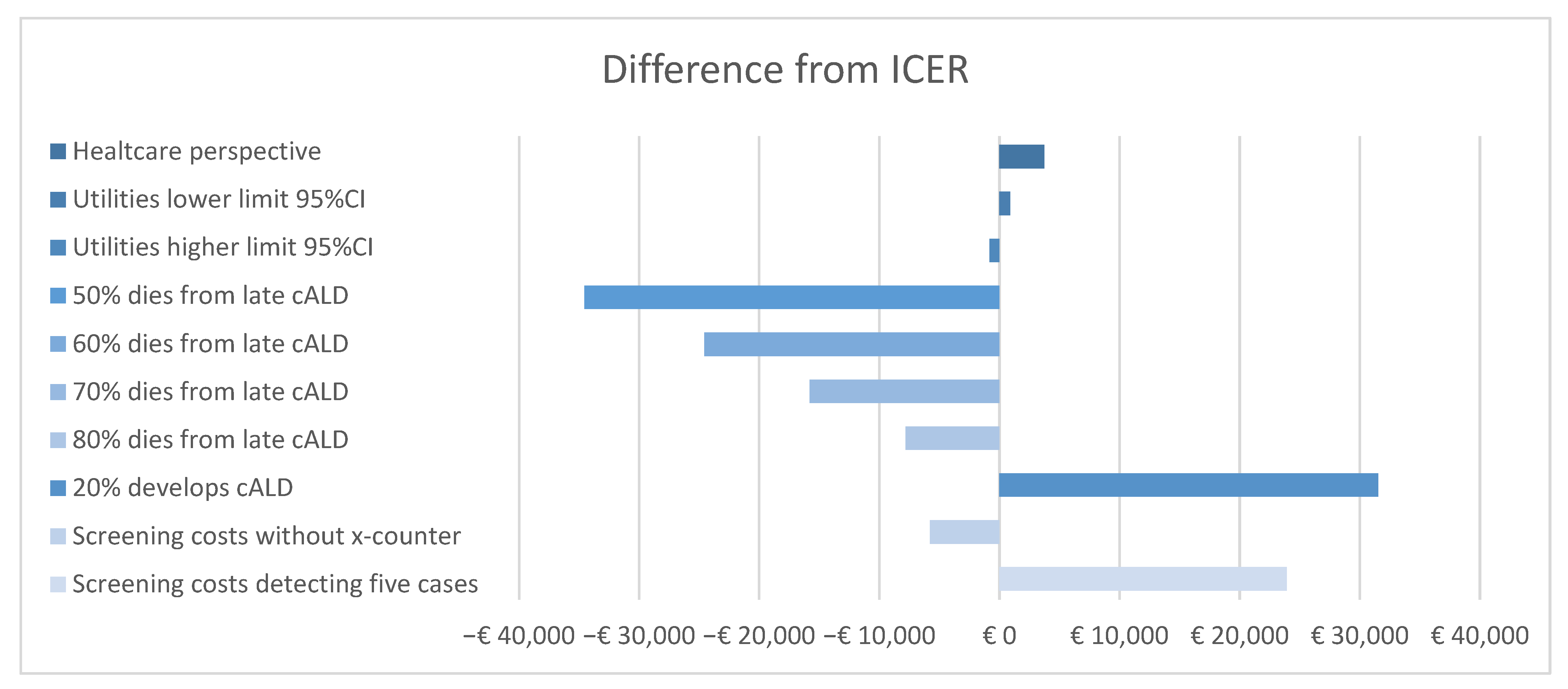
3.5. Deterministic Sensitivity Analysis
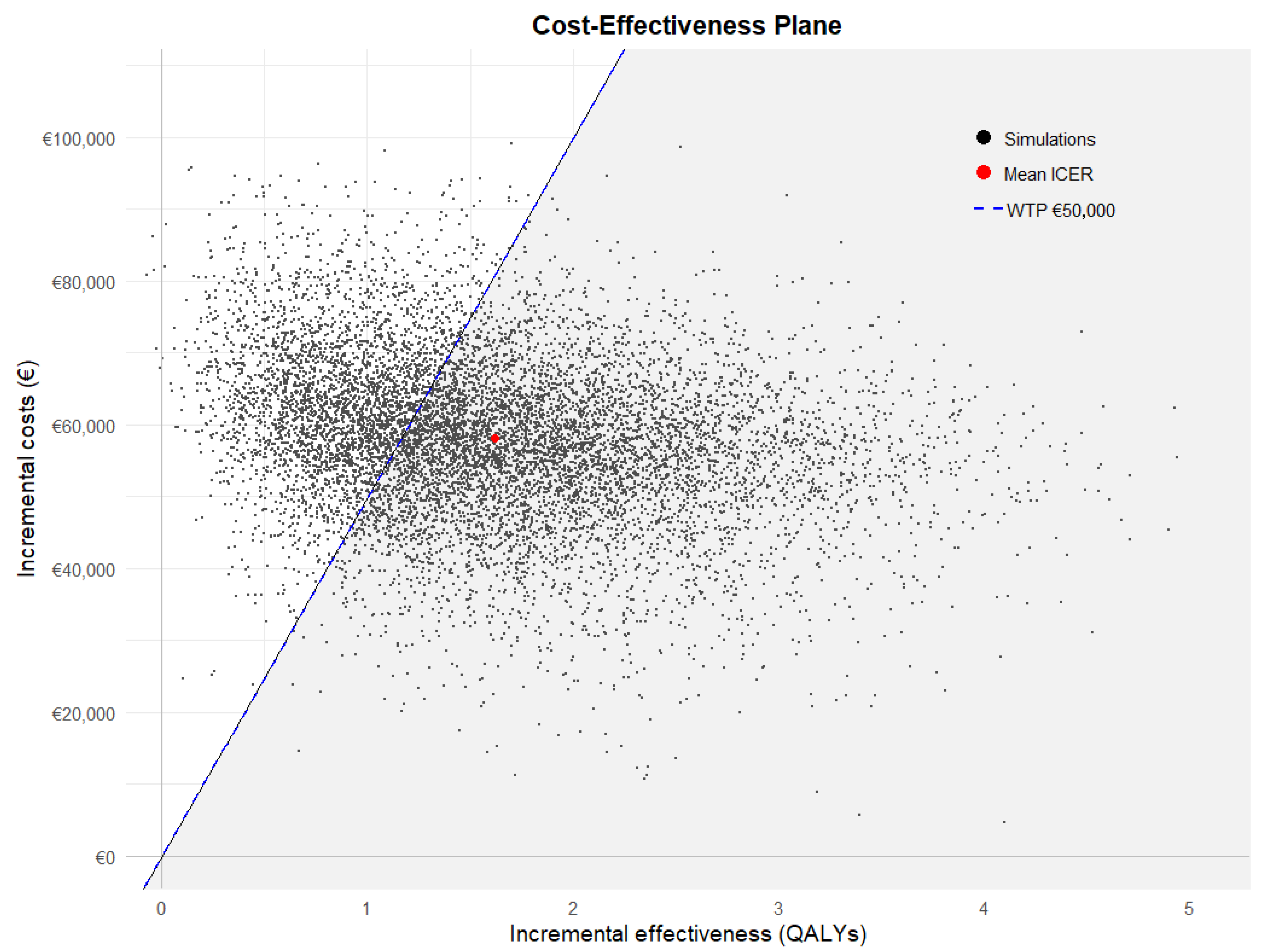
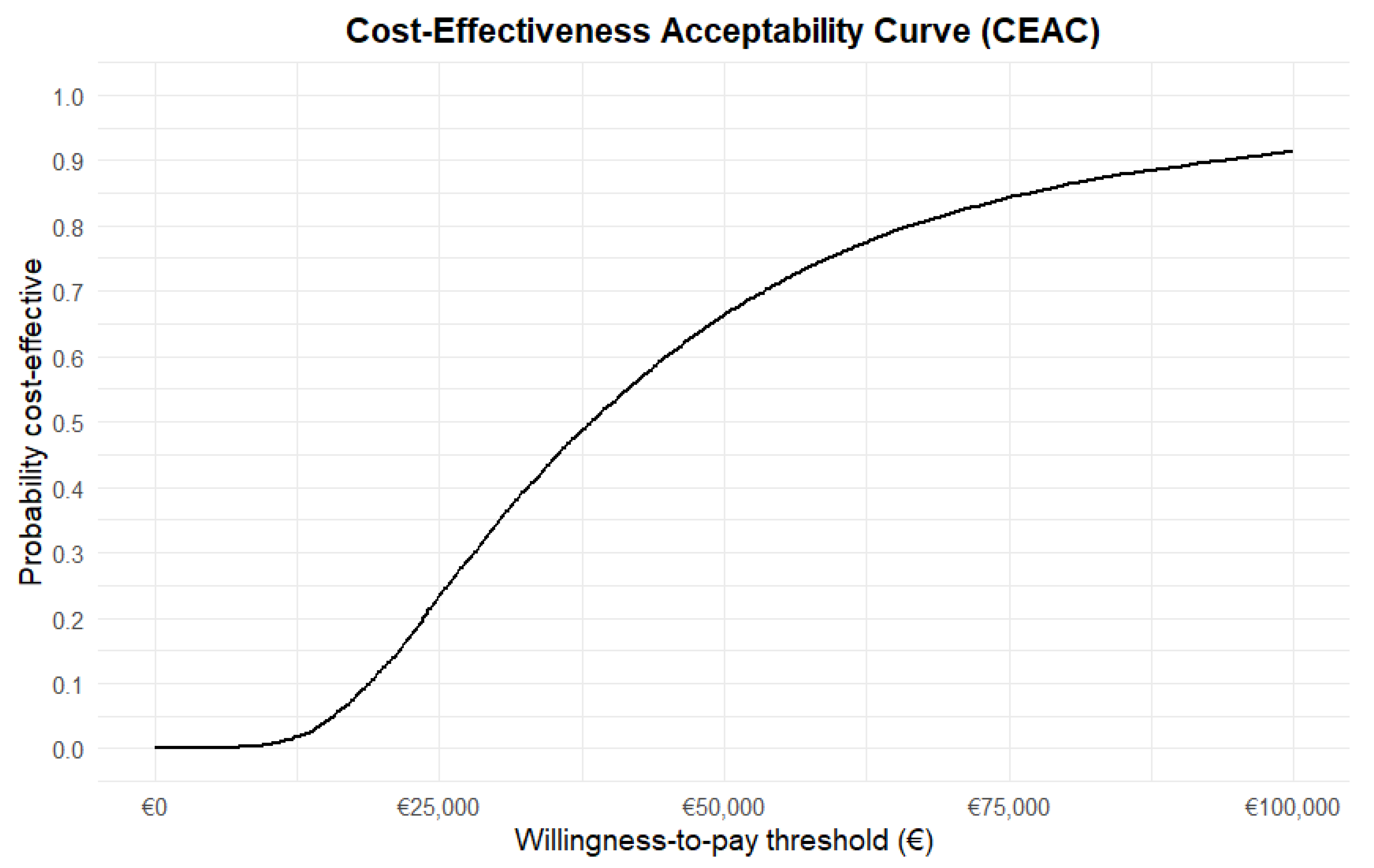
3.6. Probabilistic Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Engelen, M.; Kemp, S.; Poll-The, B.T. X-linked adrenoleukodystrophy: Pathogenesis and treatment. Curr. Neurol. Neurosci. Rep. 2014, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Moser, H.W.; Smith, K.D.; Watkins, P.A.; Powers, J.; Moser, A.B. X-Linked Adrenoleukodystrophy. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Huffnagel, I.C.; Laheji, F.K.; Aziz-Bose, R.; Tritos, N.A.; Marino, R.; Linthorst, G.E.; Kemp, S.; Engelen, M.; Eichler, F. The Natural History of Adrenal Insufficiency in X-Linked Adrenoleukodystrophy: An International Collaboration. J. Clin. Endocrinol. Metab. 2019, 104, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.; Kemp, S.; de Visser, M.; van Geel, B.M.; Wanders, R.J.A.; Aubourg, P.; Poll-The, B.T. X-linked adrenoleukodystrophy (X-ALD): Clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet. J. Rare. Dis. 2012, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Huffnagel, I.C.; Linthorst, G.E.; Wanders, R.J.; Engelen, M. Adrenoleukodystrophy—neuroendocrine pathogenesis and redefinition of natural history. Nat. Rev. Endocrinol. 2016, 12, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Regelmann, M.O.; Kamboj, M.K.; Miller, B.S.; Nakamoto, J.M.; Sarafoglou, K.; Shah, S.; Stanley, T.L.; Marino, R.; Pediatric Endocrine Society Drug and Therapeutics/Rare Diseases Committee. Adrenoleukodystrophy: Guidance for Adrenal Surveillance in Males Identified by Newborn Screen. J. Clin. Endocrinol. Metab. 2018, 103, 4324–4331. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.; van Ballegoij, W.J.C.; Mallack, E.J.; Van Haren, K.P.; Kohler, W.; Salsano, E.; van Trotsenburg, A.S.P.; Mochel, F.; Sevin, C.; Sevin, C.; et al. International Recommendations for the Diagnosis and Management of Patients With Adrenoleukodystrophy: A Consensus-Based Approach. Neurology 2022, 99, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Moser, H.W.; Loes, D.J.; Melhem, E.R.; Raymond, G.V.; Bezman, L.; Cox, C.S.; Lu, S.E. X-Linked adrenoleukodystrophy: Overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics 2000, 31, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Mallack, E.J.; Turk, B.R.; Yan, H.; Price, C.; Demetres, M.; Moser, A.B.; Becker, C.; Hollandsworth, K.; Adang, L.; Vanderver, A.; et al. MRI surveillance of boys with X-linked adrenoleukodystrophy identified by newborn screening: Meta-analysis and consensus guidelines. J. Inherit. Metab. Dis. 2021, 44, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.O.; Raymond, G.; Pierpont, E.I.; Kemp, S.; McIvor, R.S.; Rayannavar, A.; Miller, B.; Lund, T.C.; Orchard, P.J. Treatment of cerebral adrenoleukodystrophy: Allogeneic transplantation and lentiviral gene therapy. Expert Opin. Biol. Ther. 2022, 22, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Raymond, G.V.; Aubourg, P.; Paker, A.; Escolar, M.; Fischer, A.; Blanche, S.; Baruchel, A.; Dalle, J.H.; Michel, G.; Prasad, V.; et al. Survival and Functional Outcomes in Boys with Cerebral Adrenoleukodystrophy with and without Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Charnas, L.R.; Tan, Y.; Ziegler, R.S.; Shapiro, E.G.; DeFor, T.; Grewal, S.S.; Orchard, P.J.; Abel, S.L.; Goldman, A.I.; et al. Cerebral X-linked adrenoleukodystrophy: The international hematopoietic cell transplantation experience from 1982 to 1999. Blood 2004, 104, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Raymond, G.V.; Dubey, P.; Peters, C.; Moser, H.W. Survival analysis of haematopoietic cell transplantation for childhood cerebral X-linked adrenoleukodystrophy: A comparison study. Lancet Neurol. 2007, 6, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.P.; Rothman, S.M.; Nascene, D.; Kivisto, T.; DeFor, T.E.; Ziegler, R.S.; Eisengart, J.; Leiser, K.; Raymond, G.; Lund, T.C.; et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: The largest single-institution cohort report. Blood 2011, 118, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Neonatal Screening: New Recommendations; Health Council of the Netherlands: The Hague, The Netherlands, 2015.
- Albersen, M.; van der Beek, S.L.; Dijkstra, I.M.E.; Alders, M.; Barendsen, R.W.; Bliek, J.; Boelen, A.; Ebberink, M.S.; Ferdinandusse, S.; Goorden, S.M.I.; et al. Sex-specific newborn screening for X-linked adrenoleukodystrophy. J. Inherit. Metab. Dis. 2023, 46, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Barendsen, R.W.; Dijkstra, I.M.E.; Visser, W.F.; Alders, M.; Bliek, J.; Boelen, A.; Bouva, M.J.; van der Crabben, S.N.; Elsinghorst, E.; van Gorp, A.G.M.; et al. Adrenoleukodystrophy Newborn Screening in the Netherlands (SCAN Study): The X-Factor. Front. Cell Dev. Biol. 2020, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Metabolic Disorder ALD Added to Newborn Blood Spot Screening Bilthoven: RIVM. 2023. Available online: https://www.rivm.nl/en/news/metabolic-disorder-ald-added-to-newborn-blood-spot-screening (accessed on 7 November 2024).
- Zorginstituut Nederland. Richtlijn voor Het Uitvoeren van Economische Evaluaties in de Gezondheidszorg; Herziene versie 2024; Zorginstituut Nederland: Diemen, Nederland, 2024.
- Kosteneffectiviteit in de Praktijk, Zorginstituut Nederland. 2015. Available online: https://zoek.officielebekendmakingen.nl/blg-543402.pdf (accessed on 7 November 2024).
- Hakkaart-van Roijen, L.P.S.; Kanters, T. Kostenhandleiding voor Economische Evaluaties in de Gezondheidszorg: Methodologie en Referentieprijzen; Herziene versie 2024; In opdracht van Zorginstituut Nederland: Diemen, Nederland, 2024. [Google Scholar]
- Statline—Werkzame Beroepsbevolking; Arbeidsduur CBS. 2024. Available online: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/85275NED/table?ts=1752577666665 (accessed on 16 September 2024).
- Sanches, S.A.; Wiegers, T.A.; Otten, B.J.; Claahsen-van der Grinten, H.L. Physical, social and societal functioning of children with congenital adrenal hyperplasia (CAH) and their parents, in a Dutch population. Int. J. Pediatr. Endocrinol. 2012, 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.R.; Bacila, I.; Dawson, J.; Mahdi, S.; Alvi, S.; Cheetham, T.D.; Crowne, E.; Das, U.; Dattani, M.T.; Davies, J.H.; et al. Quality of Life in Children and Young People With Congenital Adrenal Hyperplasia—UK Nationwide Multicenter Assessment. J. Clin. Endocrinol. Metab. 2023, 109, e336–e346. [Google Scholar] [CrossRef] [PubMed]
- Feichtl, R.E.; Rosenfeld, B.; Tallamy, B.; Cairo, M.S.; Sands, S.A. Concordance of quality of life assessments following pediatric hematopoietic stem cell transplantation. Psycho Oncol. 2010, 19, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Caocci, G.; Efficace, F.; Ciotti, F.; Roncarolo, M.G.; Vacca, A.; Piras, E.; Littera, R.; Markous, R.S.D.; Collins, G.S.; Ciceri, F.; et al. Prospective Assessment of Health-Related Quality of Life in Pediatric Patients with Beta-Thalassemia following Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2011, 17, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.B.; Miller, W.P.; Dietrich, M.S.; Orchard, P.J. Quality of life among boys with adrenoleukodystrophy following hematopoietic stem cell transplant. Child Neuropsychol. 2018, 24, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Bessey, A.; Chilcott, J.B.; Leaviss, J.; Sutton, A. Economic impact of screening for X-linked Adrenoleukodystrophy within a newborn blood spot screening programme. Orphanet. J. Rare. Dis. 2018, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Shafie, A.A.; Chhabra, I.K.; Wong, J.H.Y.; Mohammed, N.S. Mapping PedsQL™ Generic Core Scales to EQ-5D-3L utility scores in transfusion-dependent thalassemia patients. Eur. J. Health Econ. 2021, 22, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Alcantara, J.; Grant, N.R.; Sethuram, S.; Nagy, A.; Becker, C.; Sahai, I.; Stanley, T.; Halper, A.; Eichler, F.S. Early Detection of Adrenal Insufficiency: The Impact of Newborn Screening for Adrenoleukodystrophy. J. Clin. Endocrinol. Metab. 2023, 108, e1306–e1315. [Google Scholar] [CrossRef] [PubMed]
- Round, J.; Hawton, A. Statistical Alchemy: Conceptual Validity and Mapping to Generate Health State Utility Values. PharmacoEconomics Open 2017, 1, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Orsini, J.J.; Ebberink, M.S.; Engelen, M.; Lund, T.C. VUS: Variant of uncertain significance or very unclear situation? Mol. Genet. Metab. 2023, 140, 107678. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Values | Distribution for PSA | Reference |
|---|---|---|---|
| Six-month transition probabilities | |||
| Screening | |||
| Age 0–4 years | |||
| Asymptomatic to asymptomatic | 0.9846 | 1-rest a | |
| Asymptomatic to adrenal insufficiency | 0.0132 | Beta (α = 16, β = 143) | Huffnagel et al. 2019 [3] |
| Asymptomatic to early cALD | 0.0000 | Beta (α = 0, β = 151) | Huffnagel et al. 2019 [3] |
| Asymptomatic to late cALD | 0.0000 | Beta (α = 0, β = 151) | Huffnagel et al. 2019 [3] |
| Asymptomatic to death | 0.0022 | Fixed | Under-five mortality rate WHO |
| Age 4–18 years | |||
| Asymptomatic to asymptomatic | 0.9620 | 1-rest a | |
| Asymptomatic to adrenal insufficiency | 0.0214 | Beta (α = 67, β = 92) | Huffnagel et al. 2019 [3] |
| Asymptomatic to early cALD | 0.0164 | Beta (α = 52, β = 99) | Huffnagel et al. 2019 [3] |
| Asymptomatic to late cALD | 0.0000 | Beta (α = 0, β = 151) | Huffnagel et al. 2019 [3] |
| Asymptomatic to death | 0.0002 | Fixed | Mortality rate age 5 to 9 and 10 to 14 years WHO |
| Age 0–18 years | |||
| Adrenal insufficiency to adrenal insufficiency | 0.9809 | 1-rest a | |
| Adrenal insufficiency to early cALD | 0.0189 | Beta (α = 46, β = 65) | Huffnagel et al. 2019 [3] |
| Adrenal insufficiency to late cALD | 0.0000 | Beta (α = 0, β = 111) | Huffnagel et al. 2019 [3] |
| Adrenal insufficiency to death | 0.0002 | Fixed | Mortality rate age 5 to 9 and 10 to 14 years WHO |
| No Screening | |||
| Age 0–4 years | |||
| Asymptomatic to asymptomatic | 0.9846 | 1-rest a | |
| Asymptomatic to adrenal insufficiency | 0.0132 | Beta (α = 16, β = 143) | Huffnagel et al. 2019 [3] |
| Asymptomatic to early cALD | 0.0000 | Beta (α = 0, β = 151) | Huffnagel et al. 2019 [3] |
| Asymptomatic to late cALD | 0.0000 | Beta (α = 0, β = 151) | Huffnagel et al. 2019 [3] |
| Asymptomatic to death | 0.0022 | Fixed | Under-five mortality rate WHO |
| Age 4–10 years | |||
| Asymptomatic to asymptomatic | 0.9620 | 1-rest a | |
| Asymptomatic to adrenal insufficiency | 0.0214 | Beta (α = 67, β = 92) | Huffnagel et al. 2019 [3] |
| Asymptomatic to early cALD | 0.0000 | Beta (α = 0, β = 151) | Huffnagel et al. 2019 [3] |
| Asymptomatic to late cALD | 0.0164 | Beta (α = 52, β = 99) | Huffnagel et al. 2019 [3] |
| Asymptomatic to death | 0.0002 | Fixed | Mortality rate age 5 to 9 and 10 to 14 years WHO |
| Age 0–18 years | |||
| Adrenal insufficiency to adrenal insufficiency | 0.9832 | 1-rest a | |
| Adrenal insufficiency to early cALD | 0.0083 | Beta (α = 23, β = 88) | Huffnagel et al. 2019 [3]; Expert opinion |
| Adrenal insufficiency to late cALD | 0.0083 | Beta (α = 23, β = 88) | Huffnagel et al. 2019 [3]; Expert opinion |
| Adrenal insufficiency to death | 0.0002 | Fixed | Mortality rate age 5 to 9 and 10 to 14 years WHO |
| Same for screening and no screening | |||
| Age 0–18 years | |||
| Early cALD to 6 months after HSCT | 0.9623 | 1-rest a | |
| Early cALD to death | 0.0377 | Beta (α = 2, β = 25) | Raymond et al. 2019 [11] |
| 6 months after HSCT to >1 year after HSCT | 0.9623 | 1-rest a | |
| 6 months after HSCT to death | 0.0377 | Beta (α = 2, β = 25) | Raymond et al. 2019 [11] |
| >1 year after HSCT to >1 year after HSCT | 0.9998 | 1-rest a | |
| >1 year after HSCT to death | 0.0002 | Fixed | Raymond et al. 2019 [11] |
| Late cALD to late cALD | 0.6813 | Fixed | Expert opinion |
| Late cALD to death | 0.3187 | Fixed | Expert opinion |
| Cost parameters | |||
| Screening costs initial ALD NBS | |||
| Tier 1 t/m 4 per screened child | EUR 2.21 | Fixed | Market price |
| Positive screening result b | EUR 1694 | Fixed | Reference prices 2022 [21] |
| Extended family screening | EUR 1580 | Fixed | Market price |
| Monitoring costs | |||
| ACTH test + daycare | EUR 410 | Fixed | Market price |
| Brain MRI with anaesthesia | EUR 589 | Fixed | Reference prices 2022 [21] |
| Brain MRI without anaesthesia | EUR 254 | Fixed | Reference prices 2022 [21] |
| Visit paediatrician | EUR 120 | Fixed | Reference prices 2022 [21] |
| Treatment costs | |||
| Hydrocortisone treatment 6 months | EUR 1427 | Fixed | Market price |
| HSCT: HLA characterisation + HSCT | EUR 109,490 | Fixed | Market price |
| Care first year after HSCT | EUR 10,355 | Fixed | Market price |
| Home care late cALD 6 months | EUR 36,280 | Fixed | Reference prices 2022 [21] |
| Visit paediatrician | EUR 120 | Fixed | Reference prices 2022 [21] |
| Societal costs | |||
| Productivity losses parents HSCT 6 months | EUR 30,540 | Fixed | Reference prices 2022 [21]; CBS [22] |
| Productivity losses parents late cALD 6 months | EUR 30,540 | Fixed | Reference prices 2022 [21]; CBS [22] |
| Utility values | |||
| Asymptomatic | 1 | Fixed | |
| Adrenal insufficiency | 0.9 | Beta (α = 19.3, β = 2.1) | Lawrence et al. 2023 [23,24] |
| Early diagnosis cALD and HSCT | 0.85 | Beta (α = 27.5, β = 4.9) | Feichtl et al. 2010 [25,26] |
| 6 months after HSCT | 0.85 | Beta (α = 27.5, β = 4.9 | Feichtl et al. 2010 [25,26] |
| >1 year after HSCT | 0.9 | Beta (α = 19.3, β = 2.1) | Lawrence et al. 2023 [24,27] |
| Late diagnosis cALD | 0.07 | Fixed | Bessey et al. 2018 [28]; Expert opinion |
| Death | 0 | Fixed |
| Type of Costs | Screened | Unscreened |
|---|---|---|
| Costs screening per identified case | EUR 40,630 | EUR - |
| Costs monitoring | EUR 17,157 | EUR 1769 |
| Costs treatment | EUR 46,524 | EUR 38,346 |
| Costs productivity losses | EUR 16,468 | EUR 22,799 |
| Total societal costs | EUR 120,779 | EUR 62,914 |
| Total healthcare costs | EUR 104,311 | EUR 40,115 |
| Analysis | Strategy | Costs | QALYs | Incr Costs | Incr QALYs | ICER |
|---|---|---|---|---|---|---|
| Main analysis (Societal perspective) | Screened | EUR 120,779 | 15.2 | EUR 57,865 | 1.7 | EUR 34,084 |
| Unscreened | EUR 62,914 | 13.5 | ||||
| Healthcare perspective | Screened | EUR 104,311 | 15.2 | EUR 64,196 | 1.7 | EUR 37,813 |
| Unscreened | EUR 40,115 | 13.5 | ||||
| Utilities lower limit 95%CI | Screened | EUR 120,779 | 15.1 | EUR 57,865 | 1.7 | EUR 34,991 |
| Unscreened | EUR 62,914 | 13.5 | ||||
| Utilities higher limit 95%CI | Screened | EUR 120,779 | 15.4 | EUR 57,865 | 1.7 | EUR 33,273 |
| Unscreened | EUR 62,914 | 13.6 | ||||
| 50% die in three years from late cALD | Screened | EUR 120,779 | 15.2 | −EUR 768 | 1.6 | −EUR 480 |
| Unscreened | EUR 121,547 | 13.6 | ||||
| 60% die in three years from late cALD | Screened | EUR 120,779 | 15.2 | EUR 15,888 | 1.7 | EUR 9504 |
| Unscreened | EUR 104,892 | 13.6 | ||||
| 70% die in three years from late cALD | Screened | EUR 120,779 | 15.2 | EUR 30,731 | 1.7 | EUR 18,282 |
| Unscreened | EUR 90,048 | 13.6 | ||||
| 80% die in three years from late cALD | Screened | EUR 120,779 | 15.2 | EUR 44,363 | 1.7 | EUR 26,260 |
| Unscreened | EUR 76,416 | 13.6 | ||||
| 20% progressing to cALD | Screened | EUR 96,208 | 15.4 | EUR 57,219 | 0.9 | EUR 65,626 |
| Unscreened | EUR 38,988 | 14.5 | ||||
| Screening costs without x-counter (tier 2) | Screened | EUR 110,976 | 15.2 | EUR 48,062 | 1.7 | EUR 28,310 |
| Unscreened | EUR 62,914 | 13.5 | ||||
| Screening costs detecting five cases | Screened | EUR 161,409 | 15.2 | EUR 98,495 | 1.7 | EUR 58,015 |
| Unscreened | EUR 62,914 | 13.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, R.C.; Broulikova, H.M.; Engelen, M.; Kemp, S.; Boelen, A.; de Jonge, R.; Bosmans, J.E.; Heijboer, A.C. Cost–Effectiveness of Newborn Screening for X-Linked Adrenoleukodystrophy in the Netherlands: A Health-Economic Modelling Study. Int. J. Neonatal Screen. 2025, 11, 53. https://doi.org/10.3390/ijns11030053
Martens RC, Broulikova HM, Engelen M, Kemp S, Boelen A, de Jonge R, Bosmans JE, Heijboer AC. Cost–Effectiveness of Newborn Screening for X-Linked Adrenoleukodystrophy in the Netherlands: A Health-Economic Modelling Study. International Journal of Neonatal Screening. 2025; 11(3):53. https://doi.org/10.3390/ijns11030053
Chicago/Turabian StyleMartens, Rosalie C., Hana M. Broulikova, Marc Engelen, Stephan Kemp, Anita Boelen, Robert de Jonge, Judith E. Bosmans, and Annemieke C. Heijboer. 2025. "Cost–Effectiveness of Newborn Screening for X-Linked Adrenoleukodystrophy in the Netherlands: A Health-Economic Modelling Study" International Journal of Neonatal Screening 11, no. 3: 53. https://doi.org/10.3390/ijns11030053
APA StyleMartens, R. C., Broulikova, H. M., Engelen, M., Kemp, S., Boelen, A., de Jonge, R., Bosmans, J. E., & Heijboer, A. C. (2025). Cost–Effectiveness of Newborn Screening for X-Linked Adrenoleukodystrophy in the Netherlands: A Health-Economic Modelling Study. International Journal of Neonatal Screening, 11(3), 53. https://doi.org/10.3390/ijns11030053






