Evaluation of the Performance of Newborn Screening for Tyrosinemia Type 1 in The Netherlands: Suggestions for Improvements Using Additional Biomarkers in Addition to Succinylacetone
Abstract
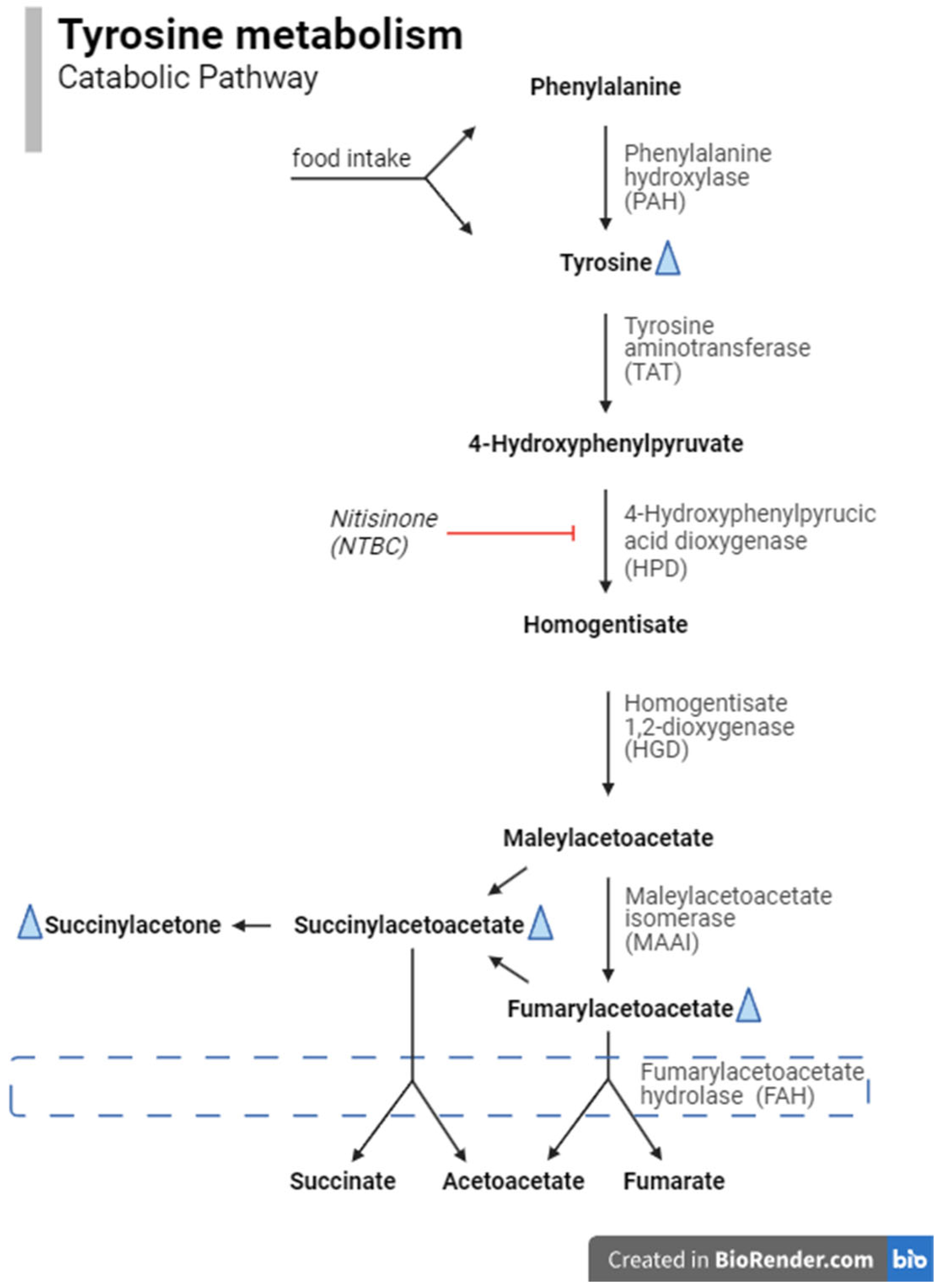
1. Introduction
2. Materials and Methods
2.1. NBS Protocol for TT1 in The Netherlands
2.1.1. NeoBase
2.1.2. NeoBase 2
2.1.3. Data Registration Systems
2.2. Dataset and Study Outcomes
2.3. Performance of the Screening Protocol
2.4. Alternative Screening Protocols
3. Results
3.1. Performance of the Screening Protocol (See Table 1)
3.1.1. NeoBase
3.1.2. NeoBase 2
3.2. Alternative Screening Protocols
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| COV | Cut-off value |
| DBS | Dried blood spot |
| FIA-MS/MS | Flow-injection analysis tandem mass spectrometry |
| FN | False negative |
| FP | False positive |
| HCC | Hepatocellular carcinoma |
| NBS | Newborn screening |
| NGS | Next-generation sequencing |
| NPV | Negative predictive value |
| Phe | Phenylalanine |
| PKU | Phenylketonuria |
| PPV | Positive predictive value |
| QC | Quality control |
| SA | Succinylacetone |
| TP | True positive |
| TT1 | Tyrosinemia type 1 |
| tyr | Tyrosine |
References
- Van Ginkel, W.G.; Jahja, R.; Huijbregts, S.C.J.; Daly, A.; MacDonald, A.; De Laet, C.; Cassiman, D.; Eyskens, F.; Körver-Keularts, I.M.L.W.; Goyens, P.J.; et al. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J. Rare Dis. 2016, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, F.J.; Thomasse, Y.; Smit, G.P.; Leonard, J.V.; Clayton, P.T.; Fidler, V.; Berger, R.; Heymans, H.S. Hereditary tyrosinemia type I: A new clinical classification with difference in prognosis on dietary treatment. Hepatology 1994, 20, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, K.; Dijkstra, A.M.; Bouva, M.J.; van der Krogt, J.; Bijsterveld, K.; van der Sluijs, F.; de Sain-van der Velden, M.G.; Koop, K.; Rossi, A.; Thomas, J.A.; et al. Maleic acid is a biomarker for maleylacetoacetate isomerase deficiency; implications for newborn screening of tyrosinemia type 1. J. Inherit. Metab. Dis. 2023, 46, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.A.; Grompe, M.; Lambert, M.; Tanguay, R.M. Hypertyrosinemia. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D.V., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 1785–1798. [Google Scholar]
- Blackburn, P.R.; Hickey, R.D.; Nace, R.A.; Giama, N.H.; Kraft, D.L.; Bordner, A.J.; Chaiteerakij, R.; McCormick, J.B.; Radulovic, M.; Graham, R.P.; et al. Silent Tyrosinemia Type I Without Elevated Tyrosine or Succinylacetone Associated with Liver Cirrhosis and Hepatocellular Carcinoma. Hum. Mutation 2016, 37, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Castilloux, J.; Laberge, A.-M.; Martin, S.R.; Lallier, M.; Marchand, V. “Silent” Tyrosinemia Presenting as Hepatocellular Carcinoma in a 10-year-old Girl. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Rehsi, P.; Witek, K.; Emmett, E.; Carling, R.; Turner, C.; Dalton, N.; Hutchin, T.; Hadzic, N.; Dhawan, A.; Vara, R. Hereditary tyrosinaemia type 1 in the absence of succinylacetone: 4-oxo 6-hydroxyhepanoate (4OHHA), a putative diagnostic biomarker. JIMD Rep. 2024, 65, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, J.; Alvarez, F.; Bussières, J.F.; Chevalier, I.; Dallaire, L.; Dubois, J.; Faucher, F.; Fenyves, D.; Goodyer, P.; Grenier, A.; et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol. Genet. Metab. 2012, 107, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Imseis, E.M.; Bynon, J.S.; Thornhill, C. Case of hepatocellular carcinoma in a patient with hereditary tyrosinemia in the postnewborn screening era. World J. Hepatol. 2017, 9, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.M.; Evers-van Vliet, K.; Heiner-Fokkema, M.R.; Bodewes, F.A.J.A.; Bos, D.K.; Zsiros, J.; van Aerde, K.J.; Koop, K.; van Spronsen, F.J.; Lubout, C.M.A. A False-Negative Newborn Screen for Tyrosinemia Type 1-Need for Re-Evaluation of Newborn Screening with Succinylacetone. Int. J. Neonatal Screen. 2023, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Lanting, C.I.; Rijpstra, A.; Breuning-Boers, J.M.; Verkerk, P.H. Evaluation of the Neonatal Heel Prick Screening of Babies Born in 2007 [Evaluatie van de Neonatale Hielprikscreening bij Kinderen Geboren in 2007]. KvL/P&Z 2008.119; TNO Kwaliteit van Leven [Behavioural and Societal Sciences—Child Health]. 2008. Available on request: https://www.pns.nl/hielprik/professionals/monitor-en-evaluatie (accessed on 1 September 2023).
- Nawijn, L.; Rijpstra, A.; Breuning-Boers, J.M.; Verkerk, P.H. Evaluation of the Neonatal Heel Prick Screening of Babies Born in 2008 [Evaluatie van de Neonatale Hielprikscreening bij Kinderen Geboren in 2008]. KvL/P&Z 2010.105; TNO Kwaliteit van Leven [Behavioural and Societal Sciences—Child Health]. 2010. Available on request: https://www.pns.nl/hielprik/professionals/monitor-en-evaluatie (accessed on 1 September 2023).
- Laeremans, H.; Turner, C.; Andersson, T.; Angel Cocho De Juan, J.; Gerrard, A.; Heiner-Fokkema, M.R.; Herebian, D.; Janzen, N.; la Marca, G.; Rudebeck, M. Inter-laboratory analytical improvement of succinylacetone and nitisinone quantification from dried blood spot samples. JIMD Rep. 2020, 53, 90–102. [Google Scholar] [CrossRef] [PubMed]
- RIVM. Prenatal and Newborn Screening—Monitoring and Evaluation. Available online: https://www.pns.nl/hielprik/professionals/monitor-en-evaluatie (accessed on 1 September 2023).
- van der Ploeg, K.; van der Mast, O.; Huizing, A.; Verkerk, P.H. The Newborn Blood Spot Screening in the Netherlands—Monitor 2022; TNO 2024 R10057. 2024. Available online: https://www.pns.nl/hielprik/professionals/monitor-en-evaluatie (accessed on 1 September 2023).
- Kuypers, A.M.; Bouva, M.J.; Loeber, J.G.; Boelen, A.; Dekkers, E.; Petritis, K.; Pickens, C.A.; The ISNS Representatives; van Spronsen, F.J.; Heiner-Fokkema, M.R. Evaluation of Neonatal Screening Programs for Tyrosinemia type 1 Worldwide. Int. J. Neonatal Screen. 2024, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Pickens, C.A.; Sternberg, M.; Seeterlin, M.; De Jesús, V.R.; Morrissey, M.; Manning, A.; Bhakta, S.; Held, P.K.; Mei, J.; Cuthbert, C.; et al. Harmonizing Newborn Screening Laboratory Proficiency Test Results Using the CDC NSQAP Reference Materials. Int. J. Neonatal Screen. 2020, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Dorley, M.C.; Dizikes, G.J.; Pickens, C.A.; Cuthbert, C.; Basheeruddin, K.; Gulamali-Majid, F.; Hetterich, P.; Hietala, A.; Kelsey, A.; Klug, T.; et al. Harmonization of Newborn Screening Results for Pompe Disease and Mucopolysaccharidosis Type I. Int. J. Neonatal Screen. 2023, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Laboratory Integrated Reports. Available online: https://clir.mayo.edu (accessed on 1 September 2023).
- Weidler, S.; Stopsack, K.H.; Hammermann, J.; Sommerburg, O.; Mall, M.A.; Hoffmann, G.F.; Kohlmüller, D.; Okun, J.G.; Macek, M.; Votava, F.; et al. A product of immunoreactive trypsinogen and pancreatitis-associated protein as second-tier strategy in cystic fibrosis newborn screening. J. Cyst. Fibros. 2016, 15, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Schanzer, A.; Basek, P.; Bauer, V.; Eber, E.; Ellemunter, H.; Kallinger, M.; Riedler, J.; Thir, C.; Wadlegger, F.; et al. Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters. Diagnostics 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Hypersuccinylacetonaemia and normal liver function in maleylacetoacetate isomerase deficiency. J. Med. Genet. 2017, 54, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stroek, K.; Visser, A.; van der Ploeg, C.P.B.; Zwaveling-Soonawala, N.H.; Heijboer, A.C.; Bosch, A.M.; van Trotsenburg, A.S.P.; Boelen, A.; Hoogendoorn, M.; de Jonge, R. Machine learning to improve false-positive results in the Dutch newborn screening for congenital hypothyroidism. Clin. Biochem. 2023, 116, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.I.; van Haeringen, M.; Bouva, M.J.; den Elzen, W.P.J.; Bruinstroop, E.; van der Ploeg, C.P.B.; van Trotsenburg, A.S.P.; Zwaveling-Soonawala, N.; Heijboer, A.C.; Bosch, A.M.; et al. Optimizing the Dutch newborn screening for congenital hypothyroidism by incorporating amino acids and acylcarnitines in a machine learning-based model. Eur. Thyroid. J. 2023 12, e230141. [CrossRef]
- Veldman, A.; Sikkema-Raddatz, B.; Derks, T.G.J.; van Karnebeek, C.D.M.; Kiewiet, M.B.G.; Mulder, M.F.; Nelen, M.R.; Rubio-Gozalbo, M.E.; Sinke, R.J.; de Sain-van der Velden, M.G.; et al. Newborn Screening by DNA-First: Systematic Evaluation of the Eligibility of Inherited Metabolic Disorders Based on Treatability. Int. J. Neonatal Screen. 2025, 11, 1. [Google Scholar] [CrossRef] [PubMed]

| Period | 1 January 2018/ 31 December 2021 | 1 July 2009/ 31 December 2017 | 1 October 2008/ 30 June 2009 | 1 January 2007/ 28 February 2007 |
| Analyzer | Waters Xevo TQD | Waters Quattro Micro/Premier | Waters Quattro Micro/Premier | Waters Quattro Micro |
| Assay | Revvity NeoBase 2 non-derivatized kit | Revvity NeoBase non-derivatized kit | Revvity NeoBase non-derivatized kit | Revvity NeoGram non-derivatized kit |
| COV | SA ≥ 0.60 µmol/L blood * | SA ≥ 1.2 µmol/L blood | SA ≥ 1.5 µmol/L blood | Tyr ≥ 500 µmol/L blood |
| Screened newborns | 693,821 | 1,506,935 | 136,644 | 34,111 |
| TN | 693,798 | 1,506,912 | 136,641 | 34,099 |
| FN | 0 | 1 | 0 | 0 |
| TP | 2 | 8 | 1 | 0 |
| FP | 21 | 14 | 2 | 12 |
| Referral rate | 0.0033% | 0.0015% | 0.0022% | 0.0352% |
| Sensitivity | 100% | 89% | 100% | - |
| Specificity | 99.997% | 99.999% | 99.999% | 99.965% |
| PPV | 9% | 36% | 33% | 0% |
| Prevalence | 1:346,910 | 1:167,437 | 1:68,322 $ | - |
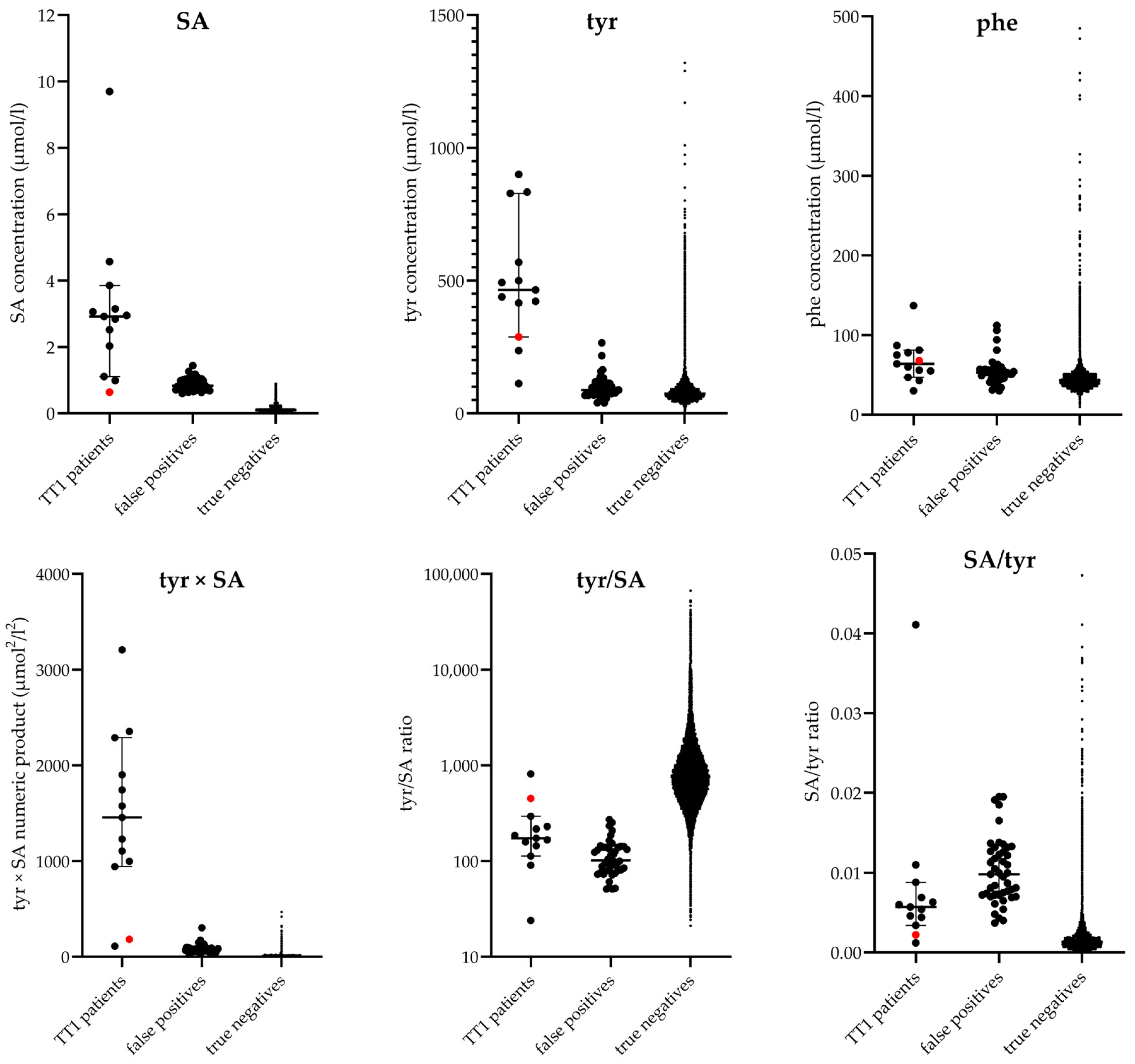
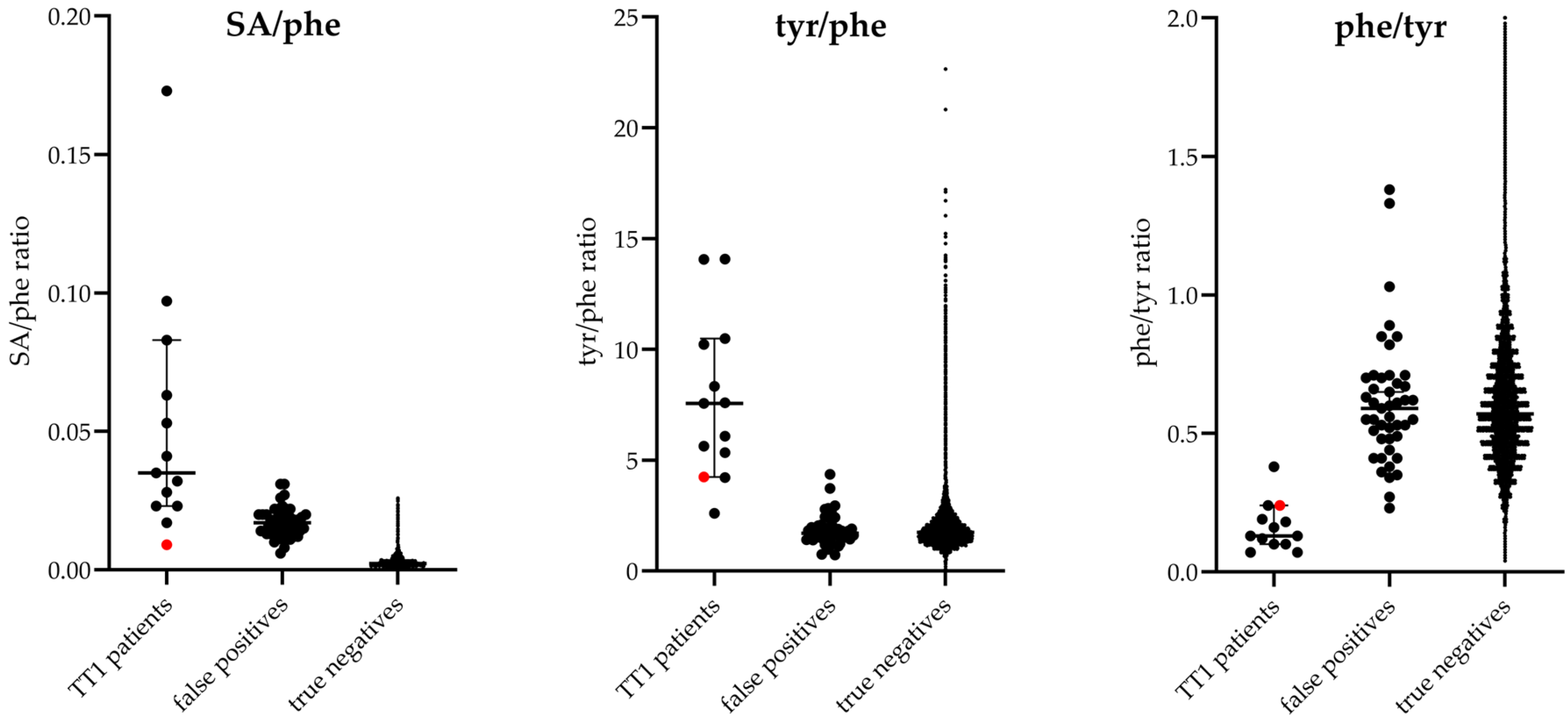
| Screening Assay | SA Cut-Off Value µmol/L | SA µmol/L | Converted SA # µmol/L | tyr µmol/L | phe µmol/L | tyr × SA # µmol2/L2 | tyr/SA # | SA #/tyr | SA #/phe | tyr/phe | phe/tyr | Original Screening Result | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NeoBase | 1.50 | 4.93 | 2.84 | 829 | 81 | 2354 | 292 | 0.003 | 0.035 | 10.23 | 0.10 | Positive |
| 2 | NeoBase | 1.20 | 4.37 | 2.52 | 439 | 78 | 1106 | 174 | 0.006 | 0.032 | 5.63 | 0.18 | Positive |
| 3 | NeoBase | 1.20 | 1.08 | 0.64 | 288 | 68 | 184 | 450 | 0.002 | 0.009 | 4.24 | 0.24 | Negative |
| 4 | NeoBase | 1.20 | 6.70 | 3.85 | 833 | 137 | 3207 | 216 | 0.005 | 0.028 | 6.08 | 0.16 | Positive |
| 5 | NeoBase | 1.20 | 7.96 | 4.57 | 416 | 55 | 1901 | 91 | 0.011 | 0.083 | 7.56 | 0.13 | Positive |
| 6 | NeoBase | 1.20 | 5.13 | 2.95 | 493 | 47 | 1454 | 167 | 0.006 | 0.063 | 10.49 | 0.10 | Positive |
| 7 | NeoBase | 1.20 | 5.48 | 3.15 | 500 | 60 | 1575 | 159 | 0.006 | 0.053 | 8.33 | 0.12 | Positive |
| 8 | NeoBase | 1.20 | 1.90 | 1.11 | 900 | 64 | 999 | 811 | 0.001 | 0.017 | 14.06 | 0.07 | Positive |
| 9 | NeoBase | 1.20 | 16.93 | 9.70 | 236 | 56 | 2289 | 24 | 0.041 | 0.173 | 4.21 | 0.24 | Positive |
| 10 | NeoBase | 1.20 | 5.32 | 3.06 | 569 | 75 | 1741 | 186 | 0.005 | 0.041 | 7.59 | 0.13 | Positive |
| 11 | NeoBase 2 | 0.60 | 0.99 | N/A | 112 | 43 | 111 | 113 | 0.009 | 0.023 | 2.60 | 0.38 | Positive |
| 12 | NeoBase 2 | 0.60 | 2.92 | N/A | 422 | 30 | 1232 | 145 | 0.007 | 0.097 | 14.07 | 0.07 | Positive |
| 13 | NeoBase 2 | 0.60 | 2.03 | N/A | 465 | 87 | 944 | 229 | 0.004 | 0.023 | 5.34 | 0.19 | Positive |
| Protocols and Cut-Off Values | TN | FN | TP | FP | Referral Rate (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| Current protocol SA ≥ 0.60 µmol/L | 653,396 | 0 | 13 | 127 | 0.0214 | 100 | 99.981 | 9 | 100 |
| Alternative L SA ≥ 0.60 µmol/L Tyr ≥ 100 µmol/L Tyr × SA ≥ 100 µmol2/L2 Tyr/Phe ≥ 2.5 | 653,515 | 0 | 13 | 8 | 0.0032 | 100 | 99.999 | 62 | 100 |
| Alternative N SA ≥ 0.60 µmol/L Tyr ≥ 100 µmol/L Tyr × SA ≥ 110 µmol2/L2 Phe/Tyr ≤ 0.5 | 653,516 | 0 | 13 | 7 | 0.0031 | 100 | 99.999 | 65 | 100 |
| Alternative O SA ≥ 0.60 µmol/L Tyr ≥ 100 µmol/L Tyr × SA ≥ 110 µmol2/L2 Tyr/Phe ≥ 2.5 | 653,518 | 0 | 13 | 5 | 0.0028 | 100 | 99.999 | 72 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouva, M.J.; Kuypers, A.M.; Kemper, E.A.; Maase, R.E.; Bosch, A.M.; van Spronsen, F.J.; Heijboer, A.C.; Heiner-Fokkema, M.R.; Heil, S.G.; Boelen, A. Evaluation of the Performance of Newborn Screening for Tyrosinemia Type 1 in The Netherlands: Suggestions for Improvements Using Additional Biomarkers in Addition to Succinylacetone. Int. J. Neonatal Screen. 2025, 11, 35. https://doi.org/10.3390/ijns11020035
Bouva MJ, Kuypers AM, Kemper EA, Maase RE, Bosch AM, van Spronsen FJ, Heijboer AC, Heiner-Fokkema MR, Heil SG, Boelen A. Evaluation of the Performance of Newborn Screening for Tyrosinemia Type 1 in The Netherlands: Suggestions for Improvements Using Additional Biomarkers in Addition to Succinylacetone. International Journal of Neonatal Screening. 2025; 11(2):35. https://doi.org/10.3390/ijns11020035
Chicago/Turabian StyleBouva, Marelle J., Allysa M. Kuypers, Evelien A. Kemper, Rose E. Maase, Annet M. Bosch, Francjan J. van Spronsen, Annemieke C. Heijboer, M. Rebecca Heiner-Fokkema, Sandra G. Heil, and Anita Boelen. 2025. "Evaluation of the Performance of Newborn Screening for Tyrosinemia Type 1 in The Netherlands: Suggestions for Improvements Using Additional Biomarkers in Addition to Succinylacetone" International Journal of Neonatal Screening 11, no. 2: 35. https://doi.org/10.3390/ijns11020035
APA StyleBouva, M. J., Kuypers, A. M., Kemper, E. A., Maase, R. E., Bosch, A. M., van Spronsen, F. J., Heijboer, A. C., Heiner-Fokkema, M. R., Heil, S. G., & Boelen, A. (2025). Evaluation of the Performance of Newborn Screening for Tyrosinemia Type 1 in The Netherlands: Suggestions for Improvements Using Additional Biomarkers in Addition to Succinylacetone. International Journal of Neonatal Screening, 11(2), 35. https://doi.org/10.3390/ijns11020035






