Consistency in the Assessment of Dried Blood Spot Specimen Size and Quality in U.K. Newborn Screening Laboratories
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of the Consistency in DBS Specimen Acceptance/Rejection in UK NBS Laboratories Using the 2015 Specimen Acceptability Criteria
2.2. Establishment of the U.K. DBS Quality Group
2.2.1. Development of a Standard Operating Procedure (SOP) for DBS Specimen Acceptance/Rejection
2.2.2. Assessment of the Impact of Moving to a Minimum DBS Diameter Size of ≥8 mm for Specimen Rejection on the AVRR in UK NBS Laboratories
2.2.3. Assessment of the Impact of Utilizing the National SOP Using the Existing Minimum DBS Diameter of ≥7 mm to Guide Specimen Acceptance/Rejection in UK NBS Laboratories
3. Results
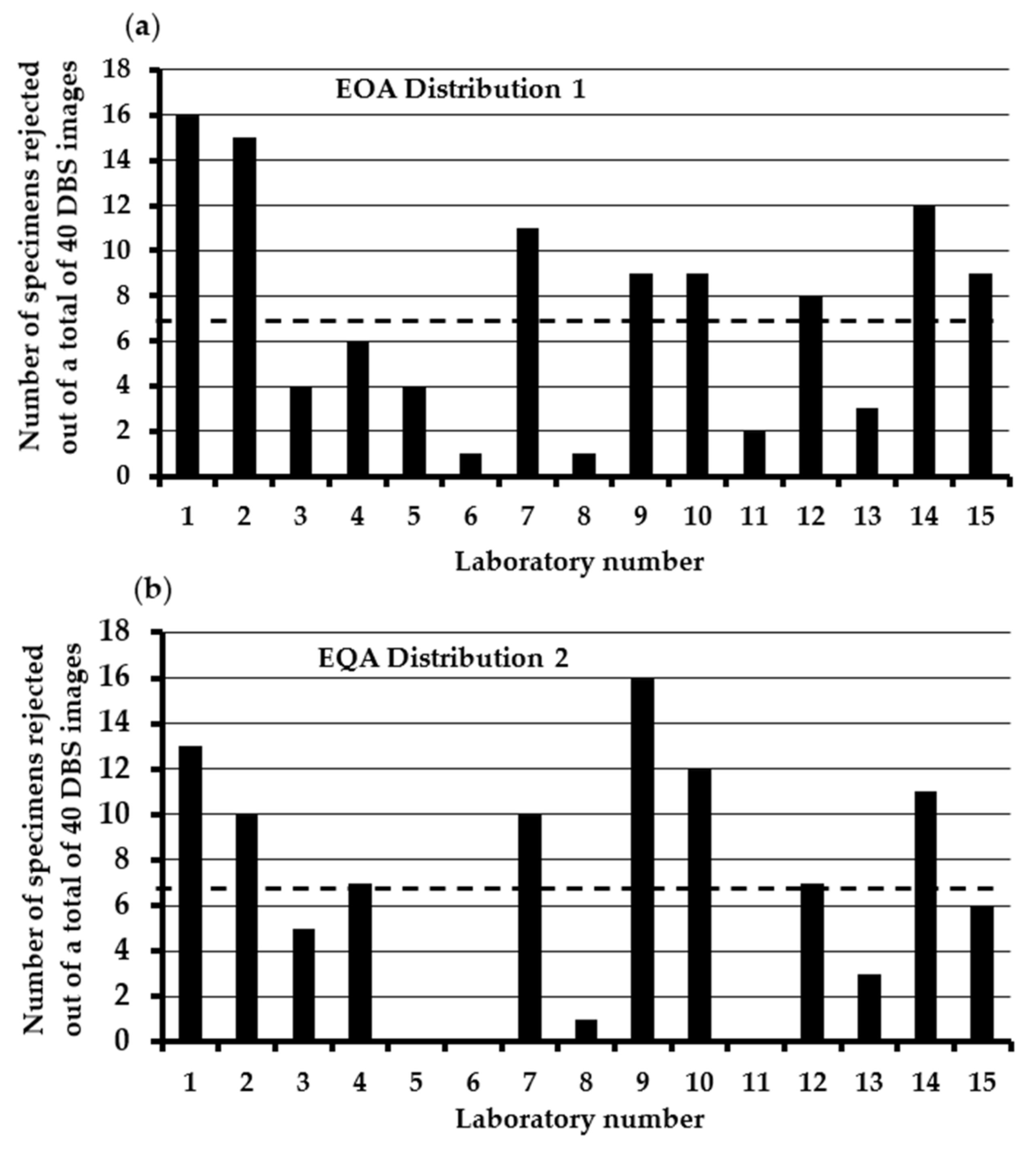
3.1. Assessment of the Consistency in DBS Specimen Acceptance/Rejection in U.K NBS Laboratories Using the 2015 Specimen Acceptability Criteria
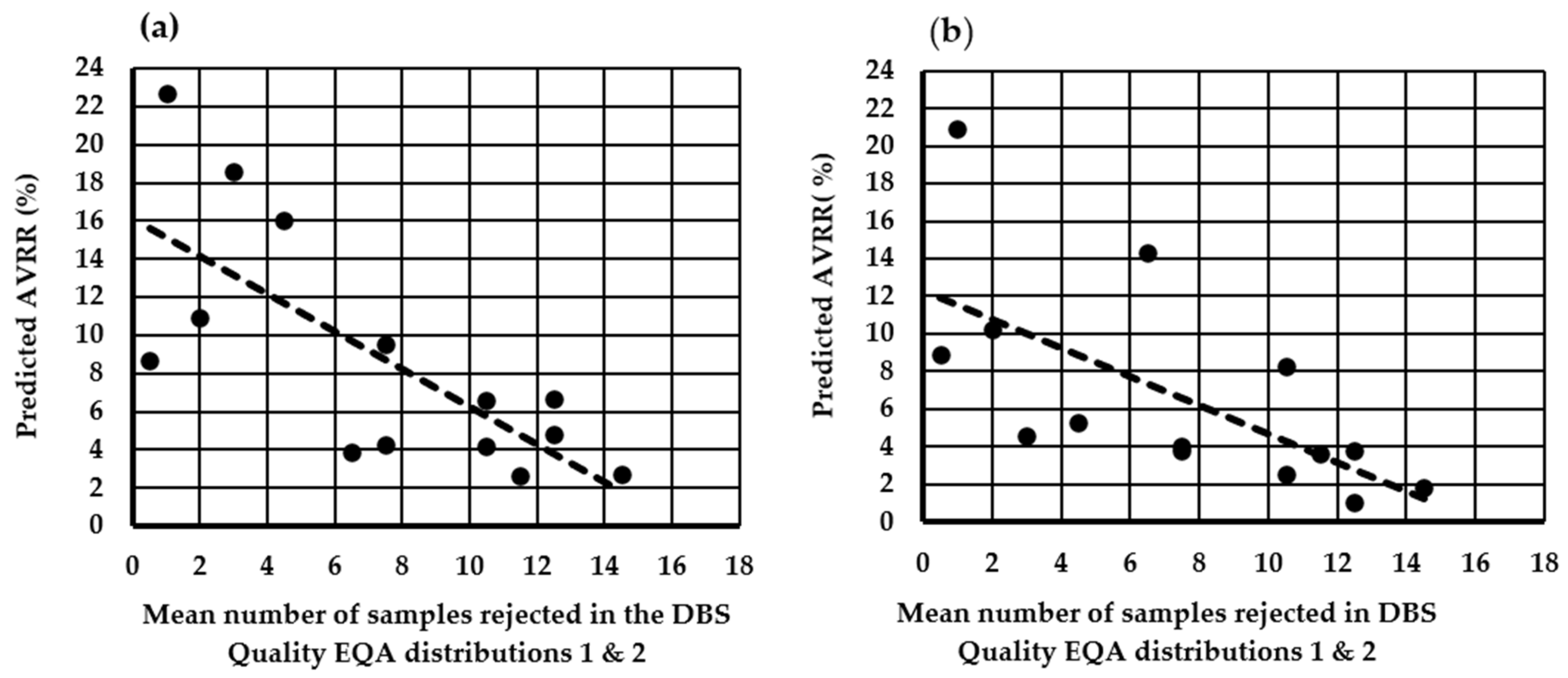
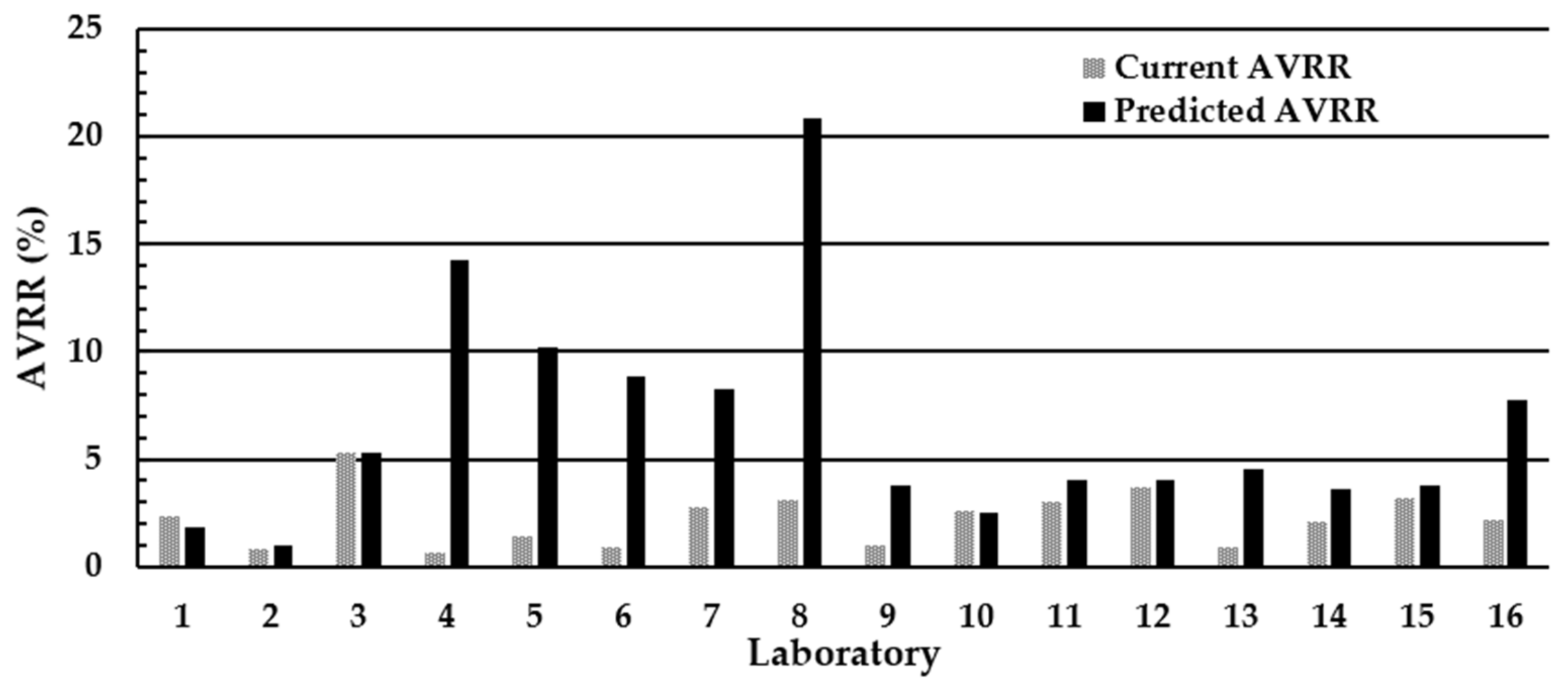
3.2. Assessment of the Impact of Using ≥8 mm as the Minimum Acceptable DBS Diameter on the AVRR in the U.K. NBS Laboratories
3.3. Assessment of the Impact of the National Standard Operating Procedure (SOP) and Using ≥7 mm as the Minimum Acceptable Diameter on the AVRR in the U.K. NBS Laboratories
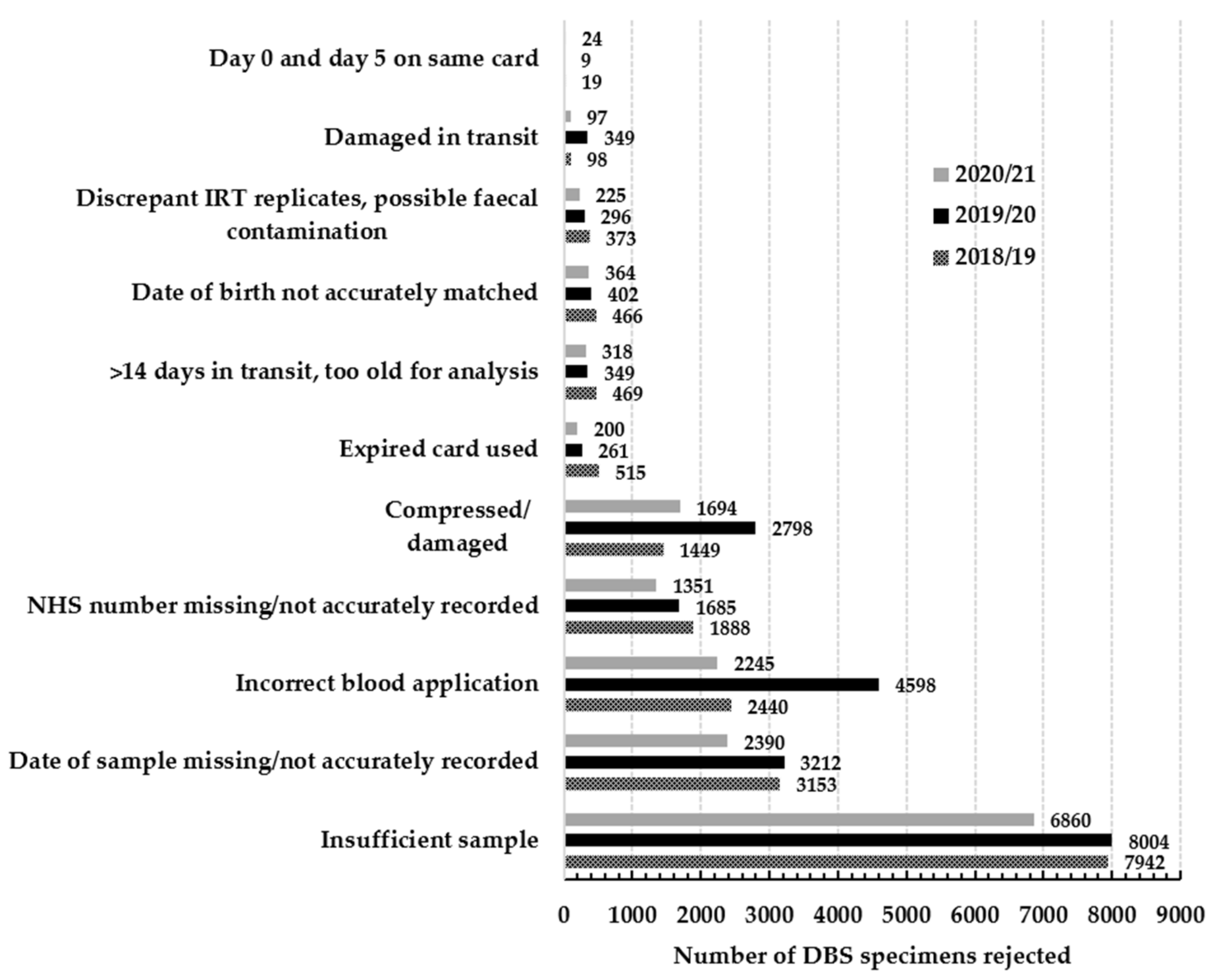
4. Discussion
- Multi-spotting of blood to fill the circle on the filter paper.
- Poor application of blood leading to insufficient blood to fully saturate filter paper but large enough to allow two 3.2 mm sub-punches to be taken (Figure S1).
- Contaminated/water damaged specimens.
- Ridged/wrinkled specimens.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawson, A.J.; Bernstone, L.; Hall, S.K. Newborn screening blood spot analysis in the UK: Influence of spot size, punch location and haematocrit. J. Med. Screen. 2016, 23, 7–16. [Google Scholar] [CrossRef] [PubMed]
- George, R.S.; Moat, S.J. Effect of dried blood spot quality on newborn screening analyte concentrations and recommendations for minimum acceptance criteria for sample analysis. Clin. Chem. 2016, 62, 466–475. [Google Scholar] [CrossRef] [PubMed]
- U.K. Government. Newborn Blood Spot Screening Data Collection and Performance Analysis Report 1 April 2018 to 31 March 2019. Newborn Blood Spot Screening Data Collection and Performance Analysis Report 1 April 2018 to 31 March 2019—GOV.UK. Available online: https://www.gov.uk (accessed on 27 January 2024).
- Moat, S.J.; Dibden, C.; Tetlow, L.; Griffith, C.; Chilcott, J.; George, R.; Hamilton, L.; Wu, T.H.; MacKenzie, F.; Hall, S.K. Effect of blood volume on analytical bias in dried blood spots prepared for newborn screening external quality assurance. Bioanalysis 2020, 12, 22–109. [Google Scholar] [CrossRef] [PubMed]
- Winter, T.; Lange, A.; Hannemann, A.; Nauck, M.; Müller, C. Contamination of dried blood spots—An underestimated risk in newborn screening. Clin. Chem. Lab. Med. 2018, 56, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; George, R.S.; Carling, R.S. Use of Dried Blood Spot Specimens to Monitor Patients with Inherited Metabolic Disorders. Int. J. Neonatal Screen. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Flores, S.; De Jesús, V. Influence of Hematocrit and Total-Spot Volume on Performance Characteristics of Dried Blood Spots for Newborn Screening. Int. J. Neonatal Screen. 2015, 1, 69–78. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard NBS01; Blood Collection on Filter Paper for Newborn Screening Programs. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2013.
- Mei, J.V.; Alexander, J.R.; Adam, B.W.; Hannon, W.H. Use of filter paper for the collection and analysis of human whole blood specimens. J. Nutr. 2001, 131, 1631S–1636S. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.V.; Zobel, S.D.; Hall, E.M.; De Jesus, V.R.; Adam, B.W.; Hannon, W.H. Performance properties of filter paper devices for whole blood collection. Bioanalysis 2010, 2, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Adam, B.W.; Hall, E.M.; Sternberg, M.; Lim, T.H.; Flores, S.R.; O’Brien, S.; Simms, D.; Li, L.X.; De Jesus, V.R.; Hannon, W.H. The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin. Biochem. 2011, 44, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Higgins, R.; Lim, M.; Lin, K.; Yang, J.; Borchers, C.H. Short-term stabilities of 21 amino acids in dried blood spots. Clin. Chem. 2017, 64, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Golbahar, J.; Altayab, D.D.; Carreon, E. Short-Term Stability of Amino acids and Acylcarnitines in the Dried Blood Spots Used to Screen Newborns for Metabolic Disorders. J. Med. Screen. 2014, 21, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Strnadova, K.A.; Holub, M.; Muhl, A.; Heinze, G.; Ratschmann, R.; Mascher, H.; Stockler-Ipsiroglu, S.; Waldhauser, F.; Votava, F.; Lebl, J.; et al. Long-term stability of amino acids and acylcarnitines in dried blood spots. Clin. Chem. 2007, 53, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, R.; Ensenauer, R.; Röschinger, W.; Arnecke, R.; Olgemöller, B.; Roscher, A.A. Stability of acylcarnitines and free carnitine in dried blood samples: Implications for retrospective diagnosis of inborn errors of metabolism and neonatal screening for carnitine transporter deficiency. Anal. Chem. 2009, 81, 3571–3575. [Google Scholar] [CrossRef] [PubMed]
- U.K. Government. Newborn Blood Spot Screening Data Collection and Performance Analysis Report 1 April 2019 to 31 March 2020. Newborn Blood Spot Screening: Data Collection and Performance Analysis Reports—GOV.UK. Available online: https://www.gov.uk (accessed on 27 January 2024).
- U.K. Government. Newborn Blood Spot Screening Data Collection and Performance Analysis Report 1 April 2020 to 31 March 2021. Newborn Blood Spot Screening: Data Collection and Performance Analysis Reports—GOV.UK. Available online: https://www.gov.uk (accessed on 27 January 2024).
- Doull, I.; Course, C.W.; Hanks, R.E.; Southern, K.W.; Forton, J.T.; Thia, L.P.; Moat, S.J. Cystic fibrosis newborn screening: The importance of bloodspot sample quality. Arch. Dis. Child. 2021, 106, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, J.; Waibren, S.E. A review of the psychosocial effects of false positive results on parents and current communication practices in newborn screening. J. Inherit. Metab. Dis. 2006, 29, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Gurian, E.A.; Kinnamon, D.D.; Henry, J.J.; Waisbren, S.E. Expanded newborn screening for biochemical disorders: The effect of a false-positive result. Pediatrics 2006, 117, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.; Moat, S.J.; Hogg, S.L. A computer vision approach to the assessment of dried blood spot size and quality in newborn screening. Clin. Chim. Acta. 2023, 547, 117418. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moat, S.J.; Bonham, J.R.; Cavanagh, C.; Birch, M.; Griffith, C.; Shakespeare, L.; Le Masurier, C.; Manfredonia, C.; Hird, B.; Goddard, P.; et al. Consistency in the Assessment of Dried Blood Spot Specimen Size and Quality in U.K. Newborn Screening Laboratories. Int. J. Neonatal Screen. 2024, 10, 60. https://doi.org/10.3390/ijns10030060
Moat SJ, Bonham JR, Cavanagh C, Birch M, Griffith C, Shakespeare L, Le Masurier C, Manfredonia C, Hird B, Goddard P, et al. Consistency in the Assessment of Dried Blood Spot Specimen Size and Quality in U.K. Newborn Screening Laboratories. International Journal of Neonatal Screening. 2024; 10(3):60. https://doi.org/10.3390/ijns10030060
Chicago/Turabian StyleMoat, Stuart J., James R. Bonham, Christine Cavanagh, Margaret Birch, Caroline Griffith, Lynette Shakespeare, Clare Le Masurier, Claire Manfredonia, Beverly Hird, Philippa Goddard, and et al. 2024. "Consistency in the Assessment of Dried Blood Spot Specimen Size and Quality in U.K. Newborn Screening Laboratories" International Journal of Neonatal Screening 10, no. 3: 60. https://doi.org/10.3390/ijns10030060
APA StyleMoat, S. J., Bonham, J. R., Cavanagh, C., Birch, M., Griffith, C., Shakespeare, L., Le Masurier, C., Manfredonia, C., Hird, B., Goddard, P., Smith, S., Wainwright, L., Carling, R. S., Cundick, J., Jenkinson, F., Collingwood, C., Flynn, N., Taj, N., Mirzazadeh, M., ... Tetlow, L. (2024). Consistency in the Assessment of Dried Blood Spot Specimen Size and Quality in U.K. Newborn Screening Laboratories. International Journal of Neonatal Screening, 10(3), 60. https://doi.org/10.3390/ijns10030060






