Advancing Newborn Screening Long-Term Follow-Up: Integration of Epic-Based Registries, Dashboards, and Efficient Workflows
Abstract
1. Introduction
2. Materials and Methods
2.1. Registry Optimization
2.2. Capturing External Data from Health Information Exchanges
2.3. Incorporating Evidence-Based Guidelines and Decision Support
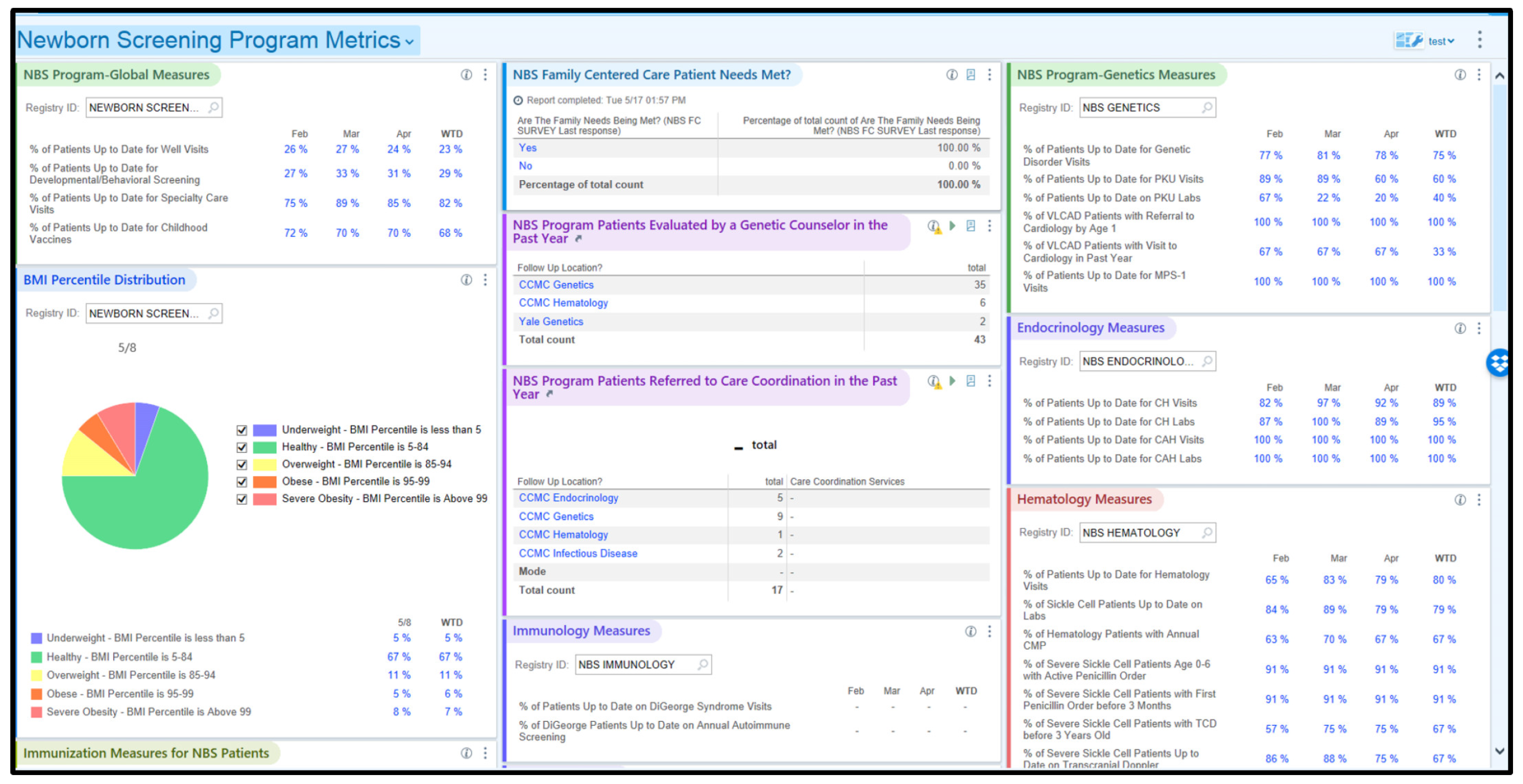
2.4. Selection of Quality Measures and Dashboards
2.5. Attention to Workflows and Training
2.6. Quantitative Methods
2.7. Quantitative Analysis Methods
2.8. Qualitative Methods
3. Results
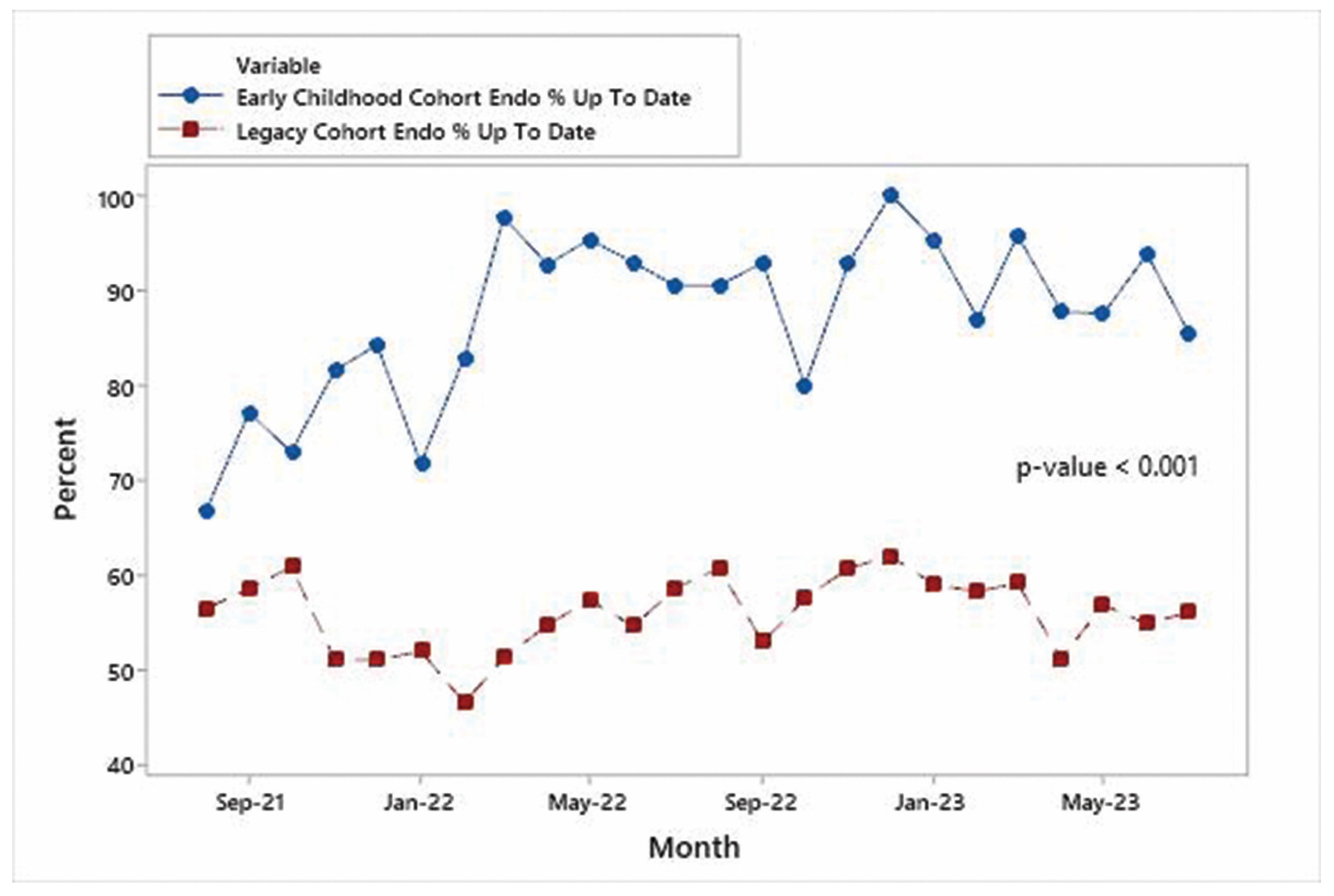
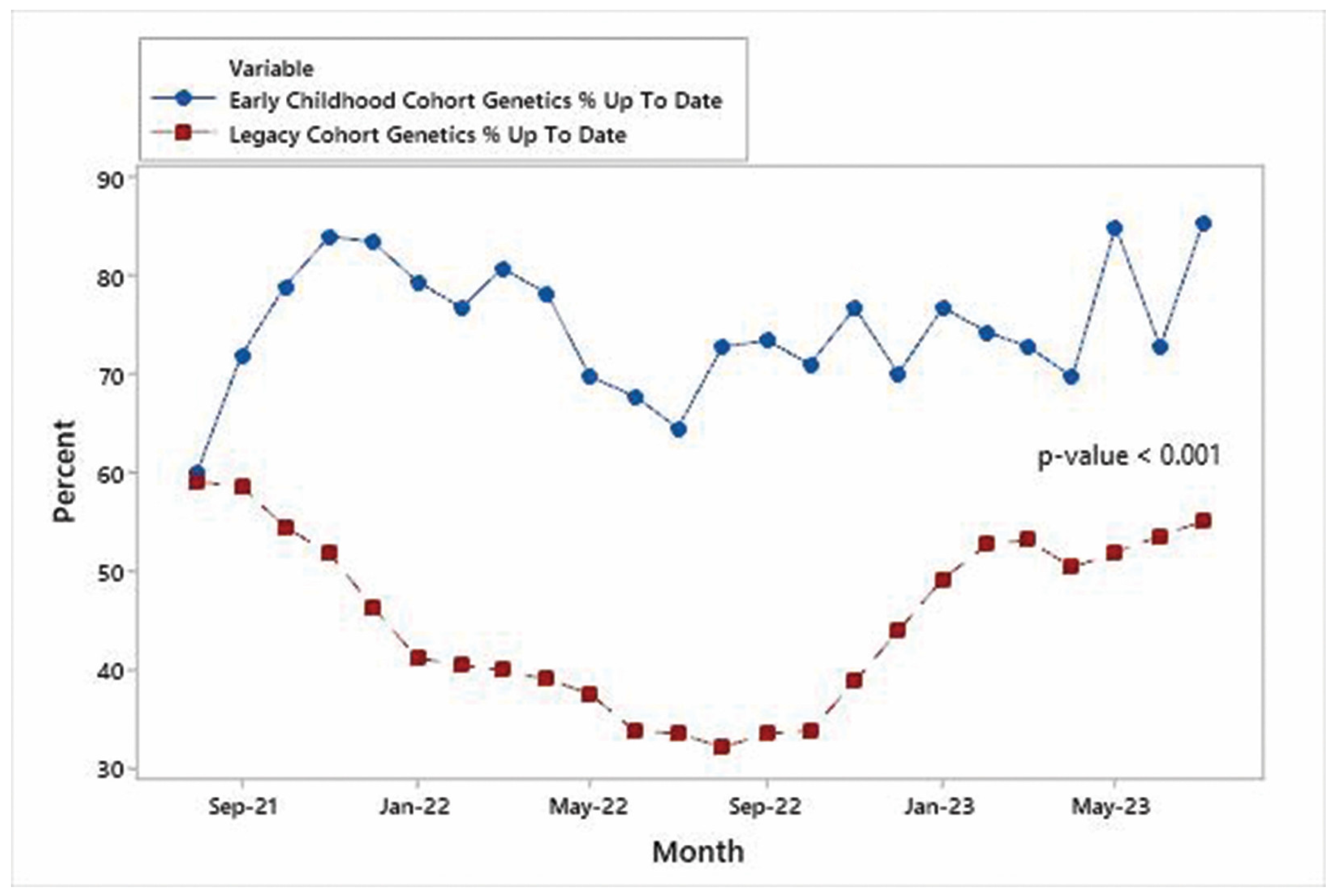
3.1. Trends in %UTD on Visits
3.2. Comparison of %UTD on Visits between Early Childhood and Legacy Cohort
3.3. Trends in Additional KPMs
3.4. Qualitative Results
4. Discussion
4.1. Four Core Components
4.2. Mitigating Challenges
4.3. Quantitative Evaluation
4.4. Qualitative Evaluation
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gliklich, R.E.; Dreyer, N.A.; Leavy, M.B. Registries for Evaluating Patient Outcomes: A User’s Guide, 3rd ed.; Apr. Report No.: 13(14)-EHC111; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2014.
- McIntyre, K.; Bertrand, D.P.; Rault, G. Using registry data to improve quality of care. J. Cyst. Fibros. 2018, 17, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Boyle, C.A.; Brosco, J.P.; Grosse, S.D. Ensuring the Life-Span Benefits of Newborn Screening. Pediatrics 2019, 144, e20190904. [Google Scholar] [CrossRef] [PubMed]
- Hoff, T.; Hoyt, A.; Therrell, B.; Ayoob, M. Exploring barriers to long-term follow-up in newborn screening programs. Genet. Med. 2006, 8, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Boyle, C.A.; Aceves, J.; Dougherty, D.; Figge, J.; Fisch, J.L.; Hinman, A.R.; Greene, C.L.; Kus, C.A.; Miller, J.; et al. Long-term follow-up after diagnosis resulting from newborn screening: Statement of the US Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children. Genet. Med. 2008, 10, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Hinton, C.F.; Feuchtbaum, L.; Kus, C.A.; Kemper, A.R.; Berry, S.A.; Levy-Fisch, J.; Luedtke, J.; Kaye, C.; Boyle, C.A. What questions should newborn screening long-term follow-up be able to answer? A statement of the US Secretary for Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children. Genet. Med. 2011, 13, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Hinton, C.F.; Homer, C.J.; Thompson, A.A.; Williams, A.; Hassell, K.L.; Feuchtbaum, L.; Berry, S.A.; Comeau, A.M.; Therrell, B.L.; Brower, A.; et al. A framework for assessing outcomes from newborn screening: On the road to measuring its promise. Mol. Genet. Metab. 2016, 118, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kannan, V.; Fish, J.S.; Mutz, J.M.; Carrington, A.R.; Lai, K.; Davis, L.S.; Youngblood, J.E.; Rauschuber, M.R.; Flores, K.A.; Sara, E.J.; et al. Rapid Development of Specialty Population Registries and Quality Measures from Electronic Health Record Data*. An Agile Framework. Methods Inf. Med. 2017, 56, 74–83. [Google Scholar]
- Dowding, D.; Randell, R.; Gardner, P.; Fitzpatrick, G.; Dykes, P.; Favela, J.; Hamer, S.; Whitewood-Moores, Z.; Hardiker, N.; Borycki, E.; et al. Dashboards for improving patient care: Review of the literature. Int. J. Med. Inform. 2015, 84, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Twohig, P.A.; Rivington, J.R.; Gunzler, D.; Daprano, J.; Margolius, D. Clinician dashboard views and improvement in preventative health outcome measures: A retrospective analysis. BMC Health Serv. Res. 2019, 19, 475. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Lagha, R.R.; Duford-Hutchinson, B.; Callaway-Lane, C.; Sauer, B.C.; Halwani, A.S.; Bell, J.; Huynh, T.; Douglas, J.R.; Kramer, B.J. Developing the VA Geriatric Scholars Programs’ Clinical Dashboards Using the PDSA Framework for Quality Improvement. Appl. Clin. Inform. 2022, 13, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Dagliati, A.; Sacchi, L.; Tibollo, V.; Cogni, G.; Teliti, M.; Martinez-Millana, A.; Traver, V.; Segagni, D.; Posada, J.; Ottaviano, M.; et al. A dashboard-based system for supporting diabetes care. J. Am. Med. Inform. Assoc. 2018, 25, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Dolley, S. Big Data’s Role in Precision Public Health. Front. Public Health 2018, 6, 68. [Google Scholar] [CrossRef] [PubMed]





| Specialty/ Population | Metric Evaluated 1 | Aug ‘21 (Baseline) | Feb ‘22 | Aug ‘22 | Feb ‘23 | Aug ‘23 |
|---|---|---|---|---|---|---|
| Endocrinology/ Early Childhood Cohort with CH | %UTD on TSH and Free T4 labs | 82% (27/33) | 100% (38/38) | 100% (39/39) | 91% (40/44) | 95% (42/44) |
| Genetics/ Early Childhood Cohort with VLCADD | % with cardiology referral in place by age 1 yr | 50% (1/2) | 100% (3/3) | 100% (3/3) | 100% (2/2) | 100% (2/2) |
| Hematology/ Early Childhood Cohort with severe SCD aged 2 yr–4 yr | %UTD on TCD 2 | 43% (3/7) | 75% (6/8) | 90% (9/10) | 73% (8/11) | 92% (12/13) |
| Hematology/ Early Childhood Cohort with severe SCD | % currently prescribed or offered hydroxyurea | 80% (8/10) | 100% (11/11) | 100% (11/11) | 85% (11/13) | 100% (13/13) |
| Hematology/ Early Childhood Cohort with severe SCD | % with first penicillin order before age 3 mo | 50% (5/10) | 91% (10/11) | 91% (10/11) | 85% (11/13) | 100% (13/13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raboin, K.; Ellis, D.; Nichols, G.; Hughes, M.; Brimacombe, M.; Rubin, K. Advancing Newborn Screening Long-Term Follow-Up: Integration of Epic-Based Registries, Dashboards, and Efficient Workflows. Int. J. Neonatal Screen. 2024, 10, 27. https://doi.org/10.3390/ijns10020027
Raboin K, Ellis D, Nichols G, Hughes M, Brimacombe M, Rubin K. Advancing Newborn Screening Long-Term Follow-Up: Integration of Epic-Based Registries, Dashboards, and Efficient Workflows. International Journal of Neonatal Screening. 2024; 10(2):27. https://doi.org/10.3390/ijns10020027
Chicago/Turabian StyleRaboin, Katherine, Debra Ellis, Ginger Nichols, Marcia Hughes, Michael Brimacombe, and Karen Rubin. 2024. "Advancing Newborn Screening Long-Term Follow-Up: Integration of Epic-Based Registries, Dashboards, and Efficient Workflows" International Journal of Neonatal Screening 10, no. 2: 27. https://doi.org/10.3390/ijns10020027
APA StyleRaboin, K., Ellis, D., Nichols, G., Hughes, M., Brimacombe, M., & Rubin, K. (2024). Advancing Newborn Screening Long-Term Follow-Up: Integration of Epic-Based Registries, Dashboards, and Efficient Workflows. International Journal of Neonatal Screening, 10(2), 27. https://doi.org/10.3390/ijns10020027








