Incidental Detection of Classical Galactosemia through Newborn Screening for Phenylketonuria: A 10-Year Retrospective Audit to Determine the Efficacy of This Approach
Abstract
:1. Introduction
- Affected individuals show symptoms of galactosaemia at an average of 7 days of age—this is equal to or quicker than the point at which NBS results are available;
- An accurate screening test is not available and carries an unacceptable risk of misdiagnosis (false positive rate)—including identifying cases of uncertain clinical significance;
- It is unclear if early treatment in diagnosed individuals changes the long-term outcome (despite the observational evidence apparent from the GalNet registry).
- assess the efficacy of identifying incidental cases of CG within UK NBS laboratories using the current ODS pathway;
- determine the proportion of individuals with CG diagnosed in the UK through the NBS pathway as compared to clinical presentation;
- provide an updated estimate of CG incidence in UK live births.
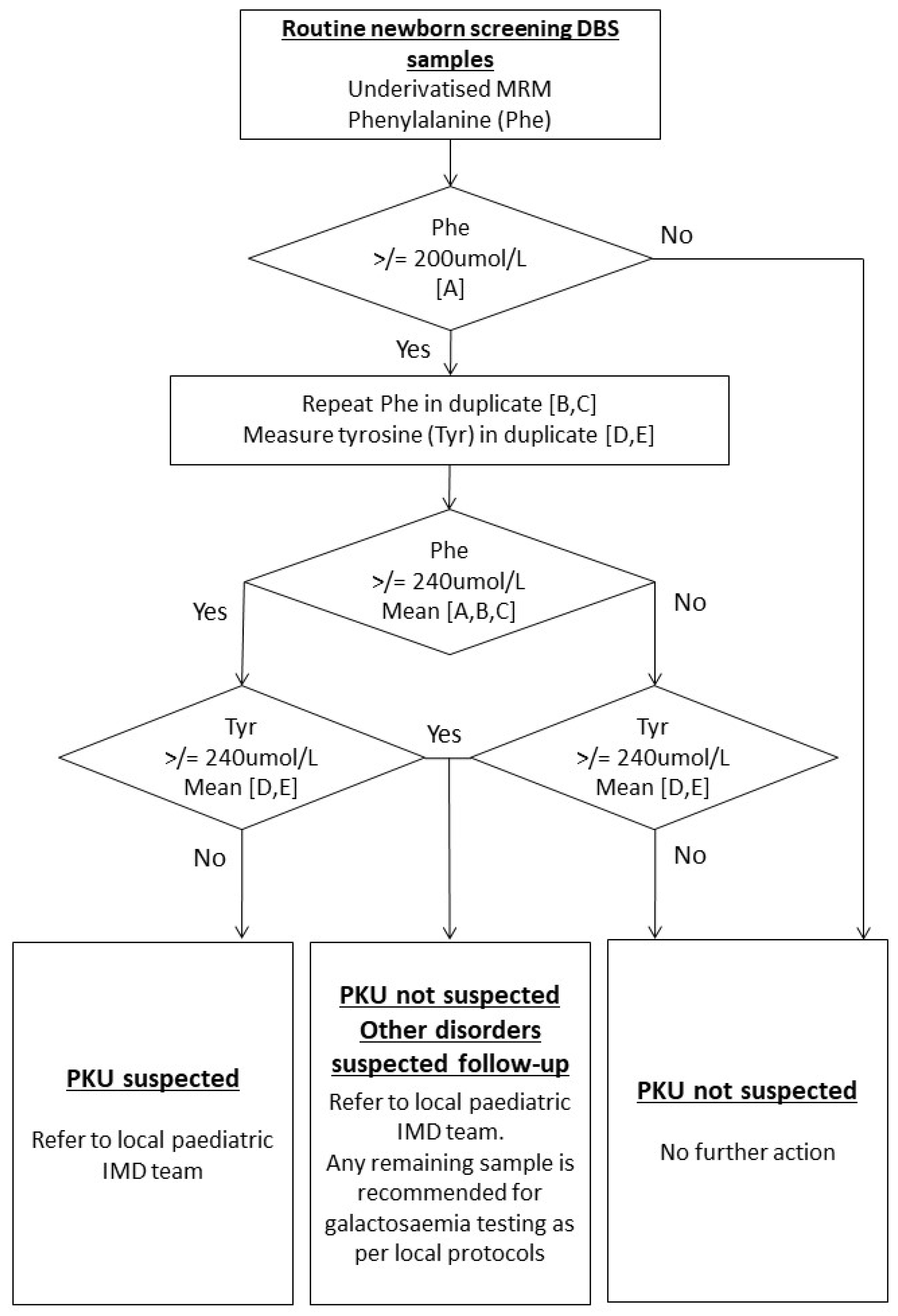
2. Materials and Methods
- number of NBS referrals made using the ODS pathway from PKU screening and the final diagnosis from that referral, where known;
- number of cases of CG diagnosed by the laboratory and the mechanism by which they were diagnosed (e.g., clinical presentation vs. NBS).
- CG;
- other IMD;
- liver disease/liver immaturity;
- not known/not stated;
- clinically presenting;
- NBS;
- pre-symptomatic testing due to a family history of CG;
- not known.
3. Results
3.1. Incidental Diagnosis of CG from ODS PKU Pathway
3.2. Pathway for Diagnosing Cases of CG Using PKU Screening Pathway and Incidence Calculation
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downing, M.; Pollitt, R. Newborn bloodspot screening in the UK—Past, present and future. Ann. Clin. Biochem. 2008, 45 Pt 1, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, R.J.; Worthy, E.; Green, A. Galactosaemia detection as a bonus from screening for phenylketonuria. J. Inherit. Metab. Dis. 1982, 5 (Suppl. 1), 51–52. [Google Scholar] [CrossRef]
- Shakespeare, L.; Downing, M.; Allen, J.; Casbolt, A.-M.; Ellin, S.; Maloney, M.; Race, G.; Bonham, J. Elevated phenylalanine on newborn screening: Follow-up testing may reveal undiagnosed galactosaemia. Ann. Clin. Biochem. 2010, 47 Pt 6, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Kotb, M.A.; Mansour, L.; Shamma, R.A. Screening for galactosemia: Is there a place for it? Int. J. Gen. Med. 2019, 12, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Pyhtila, B.M.; Shaw, K.A.; Neumann, S.E.; Fridovich-Keil, J.L. Newborn screening for galactosemia in the United States: Looking back, looking around, and looking ahead. JIMD Rep. 2015, 15, 79–93, Erratum in JIMD Rep. 2015, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Coss, K.P.; Doran, P.P.; Owoeye, C.; Codd, M.B.; Hamid, N.; Mayne, P.D.; Crushell, E.; Knerr, I.; Monavari, A.A.; Treacy, E.P. Classical Galactosaemia in Ireland: Incidence, complications and outcomes of treatment. J. Inherit. Metab. Dis. 2013, 36, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gozalbo, M.E.; Haskovic, M.; Bosch, A.M.; Burnyte, B.; Coelho, A.I.; Cassiman, D.; Couce, M.L.; Dawson, C.; Demirbas, D.; Derks, T.; et al. The natural history of classic galactosemia: Lessons from the GalNet registry. Orphanet J. Rare Dis. 2019, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- UK National Screening Committee. Galactosaemia—UK National Screening Programme, UKNSC 2021. Available online: https://view-health-screening-recommendations.service.gov.uk/galactosaemia (accessed on 24 September 2022).
- NHS Newborn Blood Spot Screening Programme. “A Laboratory Guide to Newborn Blood Spot Screening for Inherited Metabolic Diseases,” UK NSC 2017. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/642333/IMD_laboratory_handbook_2017.pdf (accessed on 24 September 2022).
- Office for National Statistics. Births in England and Wales; Summary Tables, ONS 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsummarytables (accessed on 3 November 2023).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lema, L.; Paz-Valinas, L.; Atienza-Merino, G.; Zubizarreta-Alberdi, R.; Villares, R.V.; López-García, M. Appropriateness of newborn screening for classic galactosaemia: A systematic review. J. Inherit. Metab. Dis. 2016, 39, 633–649. [Google Scholar] [CrossRef] [PubMed]
| Clinically Presenting | Newborn Screening (NBS) | Family | |
|---|---|---|---|
| Number of cases (%) with data for the day of diagnosis | 80/85 (94.1%) | 27/52 (51.9%) | 8/17 (47.1%) |
| Age of diagnosis—days | 10 (7–16) | 8 (7–11) | 2 (1–4) |
| Number of cases with data for Phe and Tyr concentrations | 45/85 (52.9%) | 43/52 (82.7%) | 10/17 (58.8%) |
| Phenylalanine concentration—μmol/L | 150 (75–213) | 305 (239–405) | 73 (67–85) |
| Tyrosine concentration—μmol/L | 371 (128–808) | 878 (405–1072) | 133 (103–177) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantley, N.W.P.; Barski, R.; Kemp, H.; Hogg, S.L.; Wu, H.Y.T.; Bowron, A.; Collingwood, C.; Cundick, J.; Hart, C.; Shakespeare, L.; et al. Incidental Detection of Classical Galactosemia through Newborn Screening for Phenylketonuria: A 10-Year Retrospective Audit to Determine the Efficacy of This Approach. Int. J. Neonatal Screen. 2024, 10, 2. https://doi.org/10.3390/ijns10010002
Cantley NWP, Barski R, Kemp H, Hogg SL, Wu HYT, Bowron A, Collingwood C, Cundick J, Hart C, Shakespeare L, et al. Incidental Detection of Classical Galactosemia through Newborn Screening for Phenylketonuria: A 10-Year Retrospective Audit to Determine the Efficacy of This Approach. International Journal of Neonatal Screening. 2024; 10(1):2. https://doi.org/10.3390/ijns10010002
Chicago/Turabian StyleCantley, Nathan W. P., Robert Barski, Helena Kemp, Sarah L. Hogg, Hoi Yee Teresa Wu, Ann Bowron, Catherine Collingwood, Jennifer Cundick, Claire Hart, Lynette Shakespeare, and et al. 2024. "Incidental Detection of Classical Galactosemia through Newborn Screening for Phenylketonuria: A 10-Year Retrospective Audit to Determine the Efficacy of This Approach" International Journal of Neonatal Screening 10, no. 1: 2. https://doi.org/10.3390/ijns10010002
APA StyleCantley, N. W. P., Barski, R., Kemp, H., Hogg, S. L., Wu, H. Y. T., Bowron, A., Collingwood, C., Cundick, J., Hart, C., Shakespeare, L., Preece, M. A., Aitkenhead, H., Smith, S., Carling, R. S., & Moat, S. J. (2024). Incidental Detection of Classical Galactosemia through Newborn Screening for Phenylketonuria: A 10-Year Retrospective Audit to Determine the Efficacy of This Approach. International Journal of Neonatal Screening, 10(1), 2. https://doi.org/10.3390/ijns10010002






